Abstract
Objective: The Bowel Function Index (BFI) is a clinician-administered, patient-reported, 3-item questionnaire to evaluate opioid-induced constipation in cancer and non-cancer chronic pain patients. The objective of the present analysis was to evaluate the psychometric characteristics of the BFI using data from clinical studies of oral prolonged release (PR) oxycodone/naloxone.
Methods: OXN2401 was a multicenter, controlled, randomized, double-blind, parallel-group study including oral PR oxycodone combined with oral PR naloxone as well as oral PR oxycodone combined with corresponding naloxone placebo. OXN3401 and OXN3001 were 12-week multicenter, controlled, randomized, double-blind, parallel-group studies of a fixed combination of oral PR oxycodone/naloxone versus PR oxycodone. In addition, a placebo group was included in study OXN3401. BFI psychometric characteristics (reliability, reproducibility, convergent/known groups validity, and responsiveness) were evaluated.
Results: Demographic data (n=985) were comparable and analyses indicated a high degree of internal consistency (Cronbach's alpha >0.7). Change of less than 5 points in BFI was indicative of high reproducibility. Correlations between BFI item and total scores to stool frequency were statistically significant and in the low-to-moderate range (OXN2401 –0.23 to –0.29, p < 0.001; OXN3401 range –0.26 to –0.40, p < 0.001; OXN3001 –0.14 to –0.15, p < 0.05). Data indicate that a BFI score change of ≥12 points represents a clinically meaningful change in constipation.
Limitations: This publication for validation of BFI only includes data from three clinical trials. However, another publication of an additional specifically designed cross-sectional validation study is in preparation.
Conclusion: The BFI is a valid and reliable instrument for the assessment of opioid-induced constipation in chronic pain patients. Psychometric analyses from clinical trials support the BFI's psychometric properties.
Introduction
Opioids are established for the treatment of chronic pain patients. However, opioid therapy is frequently related with opioid-induced bowel dysfunction (OIBD)Citation1, the most significant and therefore treatment-limiting adverse side-effect. In fact, a recent study shows that approximately 30% of patients reduce or terminate treatment with opioids as a result of OIBDCitation2,3.
Implying a spectrum of opioid induced gastrointestinal (GI) effects, OIBD is a consequence of opioid binding to μ- and κ-opioid receptorsCitation4 resulting in reduced, dyscoordinated muscle contraction and reduced GI secretionsCitation5–8. As a result, OIBD comprises hard dry stools, straining, bloating, abdominal cramping, abdominal distension, increased gastric reflux, incomplete bowel evacuation and as the most distressing lead symptom opioid-induced constipation (OIC)Citation1,2. Whereas many of the other known side-effects of opioids (e.g., nausea, sedation or itching) subside with long-term use, OIC often persists and therefore has to be managed to ensure and improve patients´ compliance and quality of lifeCitation3,9.
Traditional management strategies of OIC have been nonspecific and often ineffectiveCitation10 as they were not targeting the underlying opioid-receptor mediated mechanismsCitation1,3. A typical treatment option is laxativesCitation11,12, but all are associated with side-effects and treat the consequences of opioid therapy rather than the cause, therefore failing optimal treatment resultsCitation1. To overcome OIC, a range of compounds have been developed that target peripheral opioid receptors in the GI tract including alvimopanCitation13,14, methylnaltrexoneCitation15 and oral prolonged release (PR) naloxone combined with the oral PR opioid oxycodoneCitation15–17. The lack of validated outcome measures has been a limitation when evaluating the efficacy of treatments that alleviate symptoms associated with OICCitation18. Similarly, it has been difficult to quantify GI side-effects of opioids.
Hence, it is very important to have an easy-to-use patient-reported outcome (PRO) tool to evaluate the severity and impact of OIC. A PRO is a measure of a patient's health status that comes directly from the patient, without interpretation by a clinician. In clinical trials, a PRO instrument is used to measure the impact of an intervention on aspects of patients’ health status, and can be unidimensional (e.g., measuring symptoms such as headache or constipation) or multi-domain concepts with physical, psychological, and social componentsCitation19. Data generated by a PRO instrument can provide evidence of a treatment benefit from the patient perspective, but requires evaluation to ensure it is a reliable and valid measure.
Indeed, there are already objective measurements that can partly detect parameters like stool frequency and stool consistencyCitation20, but from the patient's point of view the quality aspects like severity and impact are not captured through these parameters. For a comprehensive assessment, this points out the need to draw on objective and even more important subjective criteria. Although respective subjective rating scales for OIC are already describedCitation21,22, there is still a lack of validated easy-to-use methods to specifically assess OIC. To have a subject assessment for bowel function with focus on OIC, a specific rating scale the Bowel Function Index (BFI) has been developed.
The development of the BFI was based on established criteria of known assessment tools for OIC. It is a subjective rating scale consisting of three items captured by a numeric analogue scale (NAS; 0–100) an important point as the main target group is chronic pain patients who are in general familiar with NAS for the assessment of pain. Along with the fact that the BFI is focused on the three main concepts for measuring OIC, it is an easy-to-use tool contributing to the simplification and harmonization of symptom assessment in this patient group.
The symptoms ease of defecation (straining), incomplete bowel evacuation and the general judgment of constipation are also established in the Rome criteriaCitation23 and other questionnaires like the PAC-SYM (Patient Assessment of Constipation Symptoms)Citation21,22, although use of these tools may not be practical for daily use in the clinical setting.
The Bowel Function Index (BFI) has been developed to assess clinical symptoms of opioid-induced constipation. It is patient-assessed and clinician-administered and intended to be easy to use to reduce burden on patients. In this analysis the BFI was evaluated using data from three clinical trials for the combination of PR oxycodone and PR naloxone. For validation purpose of the BFI, selected subjective (e.g., PAC-SYM) and also clinical characteristics like stool consistency, stool frequency, and in particular complete spontaneous bowel movements (CSBM) were used. The objective was to evaluate the psychometric characteristics of the BFI in patients with opioid-induced constipation. We report on the reliability and validity and responsiveness of the BFI total and item scores.
Methods
Data to psychometrically evaluate the BFI scale were derived from three clinical studies of oral PR oxycodone administered with oral PR naloxoneCitation17,24–26. All studies were multicenter, prospective, randomized, double-blind, parallel-group studies. Each study was conducted in accordance with the Declaration of Helsinki (1964) and its successors (Edinburgh, 2000 and Washington, 2002) and complied with the principles of Good Clinical Practice (GCP) set by the International Conference on Harmonization (ICH) and applicable German regulatory requirements. Written informed consent was obtained from patients at screening. Male and female patients aged more than 18 years were eligible to take part in the studies if they were experiencing moderate-to-severe chronic pain, as assessed by the investigator. Exclusion criteria included current alcohol or drug abuse, current acute pancreatitis, current severe cardiovascular or respiratory diseases (e.g., lung cancer or metastases), current severe renal or liver impairment (transaminase levels three times above normal range), liver or renal carcinoma or metastases. Details of the design of these studies, including statistical analyses and overview of patient populations, can be found elsewhereCitation17,24–26.
Study designs
The purpose of the clinical studies was to assess the efficacy of PR naloxone combined with PR oxycodone. The purpose of the two studies OXN2401 and OXN3001 was to assess the efficacy of naloxone combined with oxycodone on bowel function as measured by the BFI. In addition, study OXN3401 was also drawn on for this purpose. Although the primary focus was the evaluation of analgesic efficacy, the assessment of the BFI was one of the secondary endpoints. The psychometric analyses described in this publication were not designed to evaluate the treatment effects of PR oxycodone plus oral PR naloxone compared with placebo. This report only describes the methods and results of a blinded evaluation of the psychometric qualities of the BFI assessed during the screening and double-blind phase of the three clinical trials.
Each study had three phases: pre-randomization (which consisted of screening and titration/run-in), a double-blind treatment period (maintenance phase), and an open-label phase (–).
Figure 1. Overview of study design. (a) Study OXN2401: six study visits (V1–V6) were undertaken over the course of up to 10 weeks. (b) Study OXN3401. (c) Study OXN3001 (R = Randomization).
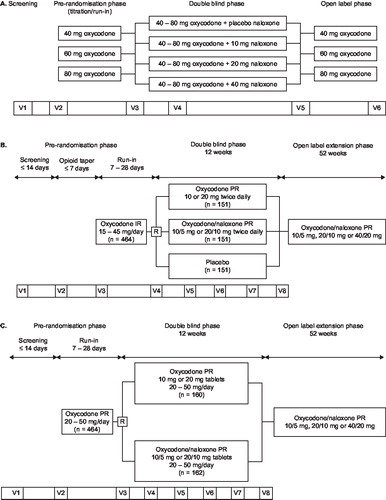
The OXN2401 clinical trial was a multicenter, prospective, controlled, randomized, double-blind, four parallel group phase II study of oral PR oxycodone plus oral PR naloxone (three different dose groups) and oral PR oxycodone plus naloxone placebo in subjects with severe chronic pain ()Citation17. The OXN3401 phase III trial was a 12-week multicenter, randomized, double-blind, placebo- and active-controlled, double-dummy parallel group trial of the safety and efficacy of PR oxycodone/naloxone tablets in subjects with moderate-to-severe, chronic non-malignant low back pain ()Citation25. The OXN3001 clinical trial was a phase III, 12-week multicenter, randomized, double-blind, double-dummy, parallel-group clinical trial evaluating PR oxycodone/naloxone tablets compared to PR oxycodone alone in patients with moderate-to-severe, chronic, non-malignant pain reporting the presence of opioid-induced constipation ()Citation26.
Bowel Function Index (BFI)
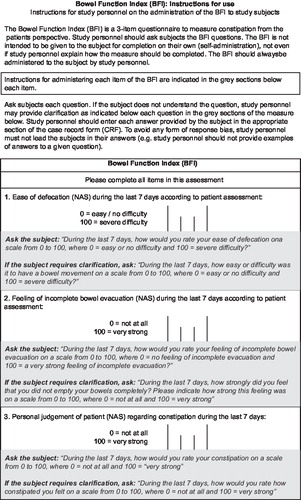
The BFI is a clinician administered, patient-reported 3-item questionnaire measuring the level of bowel function focusing on constipation a patient is experiencing (). It evaluates constipation from the patient's perspective. The patient assesses the severity of each component using a 0–100 numerical rating scale where 0 = no problems, and 100 = most severe problems ().
The components of the BFI are:
| • | Ease of defecation | ||||
| • | Feeling of incomplete bowel evacuation | ||||
| • | Patient's personal judgment of constipation | ||||
| • | The patient rates his/her perception of each component from his/her experience over the preceding 7 days | ||||
Figure 2. The Bowel Function Index (BFI) tool. The BFI is a 3-item questionnaire to measure constipation from the patient's perspective. It is intended to be used by healthcare professionals to question patients and should not be used for patient self-administration.
All randomized patients who received study medication and completed any of the three BFI questions were evaluated. An overview of data collection points is shown in –. For each study, the sociodemographic data were collected at visit 1 (–) and pre-treatment data were collected at visit 3. Data collected at visit 5 (OXN2401), visit 6 (OXN3001), or visit 8 (OXN3401) compared with the baseline values at visit V3 (OXN2401), V4 (OXN3401) and V3 (OXN3001) were used as endpoints assessing bowel efficacy (–). In studies OXN2401 and OXN3401, data from the period between visit 5 and visit 6 were used as retest data; in study OXN3001 data from visit 1 to visit 2 were used. In all cases it was assumed that data stability at these points would be greatest due to stability of interventions in the studies at these time points.
Table 1a. Overview of patient visits and questionnaire completion by participants in study OXN2401.
Table 1b. Overview of patient visits and questionnaire completion by participants in study OXN3401.
Table 1c. Overview of patient visits and questionnaire completion by participants in study OXN3001.
Bowel function measures
In all three studies, the bowel function measures were completed by patients in daily diaries. The bowel function measures included stool frequency assessed by number of complete spontaneous bowel movements (CSBM in OXN3001), or bowel evacuations per day (OXN2401, OXN3401), stool consistency as measured by the Bristol Stool Form Scale (OXN3001) and the Likert Scale (OXN2401, OXN3401), the frequency of laxative use (OXN3001), the number of days with laxative use (OXN2401) or the number of days of laxative use prior to each bowel movement (OXN3401), and stool completion (OXN3401 and OXN3001). – give an overview of the study designs of the three studies including the bowel function measures. The data were used to validate the BFI.
Data sets analyzed
All patients randomized in each study who received study medication and completed any of the three BFI questions were evaluated. Baseline data (visit 3 in OXN2401 and OXN3001, visit 4 in OXN3401) and endpoint data (visit 5 in OXN2401, visit 6 in OXN3001, visit 8 in OXN3401) were used (–). Data from the period between visit 5 and visit 6 (study OXN2401 and OXN3401) and visit 1 and visit 2 (study OXN3001) were used as test–retest data as it was assumed that data stability at this point would be greatest because of the consistency of therapeutic interventions at these points – double-blind phase in studies OXN2401 and study OXN3401 and opioid run-in phase for study OXN3001.
Statistics
Descriptive statistics are reported on sociodemographic (age, gender, race) and clinical variables (daily pain intensity [average pain], stool frequency, stool consistency, and number of days of laxative intake). Mean, standard deviation, and range were calculated for continuous variables and frequency was calculated for categorical variables. The sociodemographic and clinical variables were used to characterize the sample and quantify constipation severity. To evaluate the performance of each item of the BFI scale, descriptive statistics of each BFI item and BFI total score were undertaken at visit 3 and visit 5 (study OXN2401)Citation17, visit 6 (OXN3001) and visit 8 (OXN3401)Citation25. Patients were stratified into three severity levels (mild, moderate, severe).
Analyses of BFI item, mean total BFI score and severity of opioid-induced constipation were evaluated using an analysis of co-variance (ANCOVA) with severity being determined using a 4-point response scale in OXN2401 and OXN3401 (hard, solid, semi-solid, watery) in OXN2401 and OXN3401 and the well-established 7-point Bristol Stool Form Scale in OXN3001Citation20.
The Bristol Stool Form Scale was recoded to match the stool consistency data collected in OXN2401 and OXN3401: type 1 for the Bristol Stool Form Scale was ‘hard’ (= 1), types 2 and 3 were ‘solid’ (= 2), types 4 and 5 were ‘semi-solid’ (= 3), and types 6 and 7 ‘watery’ (= 4). If the median was located between two values, the worst case was selected for use in the analysis.
Reliability and validity
Internal consistency was evaluated using Cronbach's alpha for the BFI total score using data from the period before the randomization visit (visit 2 in study OXN2401 and visit 3 in studies OXN3401 and OXN3001)Citation27,28. Values range from 0 to 1.0, and higher values indicate an increasingly reliable, homogeneous instrument with good reliability (i.e., ≥0.7). Inter-item reliability was evaluated using methods of Cohen et al Citation29 as correlations of 0.4 or less are indicative of poor correlation. Test–retest reliability was explored using data from the naloxone plus placebo group and the interval from visit 5 to visit 6 as the retest interval using intra-class correlation (ICC) coefficients, Pearson's correlations and change in scoreCitation30.
In study OXN2401, convergent validity, the relationship between BFI questions and total BFI score and clinical characteristics of stool frequency, number of days of laxative use and global assessment of tolerability, were analyzed using Spearman's rank correlationsCitation31. Known-groups validity was analyzed using ANCOVA models to compare BFI item and total scores based on visit 5 dataCitation32. Patients were stratified into the three severity groups of mild, moderate and severe based on patient diary data and stool consistency data. In studies OXN3401 and OXN3001, convergent validity was evaluated through calculations of Spearman's product-moment range correlationsCitation31,32 between each BFI item and BFI total score and a series of clinical outcomes including stool frequency and consistency, and number of days of laxative intake. In study OXN3001, correlations with the Patient Assessment of Constipation Symptoms (PAC-SYM), a subjective outcome measure, were also undertaken. The PAC-SYM questionnaire is a 12-item self-report instrument divided into abdominal, rectal and stool domainsCitation21,22. All correlations were explored using visit 3 data. In study OXN3401 correlations with complete spontaneous bowel movements (CSBM) were undertaken using visit 8 data.
Responsiveness
Responsiveness refers to the extent to which the BFI scale can detect true changes in patients with opioid-induced constipation as outlined by Hays and Revicki 2005Citation33. In these studies effect size was determined from individual BFI item and total scores by dividing the mean visit 3 to visit 5 (OXN2401), visit 6 (OXN3001) and visit 8 (OXN3401) score change by the standard deviation (SD) of BFI at visit 3. The effect size was characterized as small, moderate or large according to Cohen et al Citation29.
Two further methods for determining responsiveness were undertaken. The first was the standard error of measurement (SEM) which has been proposed as a useful distribution-based statistic for evaluating clinically meaningful change in health-related quality of life measuresCitation34,35. The SEM was calculated for all patients based on visit 3 BFI total score and Cronbach's alpha. The second method was that of half standard deviation (SD)Citation12, to quantify clinically significant BFI score changes at visit 3. The SEM and one-half SD methods are supportive of one another in quantifying clinically meaningful changes.
Results
There were 985 evaluable patients included in this secondary analysis validation study (). Patients were evenly matched in each study in terms of age (ranged from 56 to 59 years). Across all three studies females predominated with over 60% of the sample.
Table 2. Demographic information / clinical parameters of patients included in the study.
In all three studies, the BFI total scores demonstrated a high internal consistency, with Cronbach's alpha above 0.7 (range 0.87–0.91; ). The highest correlations (range 0.79–0.86) were between ease of defecation and patient personal judgment compared with other BFI items; correlations were slightly lower (range 0.59–0.75) for assessments of feeling of incomplete evacuation ().
Table 3. Correlations between each BFI item and internal consistency across studies OXN2401 (n=202), OXN3401 (n=460) and OXN3001 (n=323).
Reproducibility in studies OXN2401 and OXN3401 was examined using data from patients receiving placebo naloxone from visit 5 to visit 6 (, and secondarily from patients receiving placebo naloxone but who reported no change in symptoms (–). For OXN3001 () the data of the entire sample between visit 1 and visit 2 was used since it is expected that the patients are stable during the respective periods (screening and run-in). It is clear that mean differences in BFI score from visit 5 to visit 6 differ less than 5 points, which was not statistically significant, indicative that BFI scores were stable over a test–retest period. For the secondary analysis of patients receiving placebo naloxone who also experienced no change in symptoms patient numbers are low in study OXN2401 (n=12) which limits interpretation of results. However, in study OXN3401 patients randomized to placebo numbered 135; mean differences from visit 5 to visit 6 were <2 points and were not significantly different, again suggesting stability of the BFI score between the time points. Similarly, in study OXN3001, whilst reproducibility was examined on the entire patient population at visit 1 (screening) and visit 2 (run-in), BFI item and mean total scores remained stable with <2 points difference between the timepoints.
Table 4a. Reproducibility of BFI items visit 5 to visit 6 for patients randomized to (a) placebo.
Table 4b. Reproducibility of BFI items visit 5 to visit 6 for patients randomized to (b) those subjects who had no change in stool frequency between visit 5 and visit 6 in study OXN2401.
Table 4c. Reproducibility of BFI items visit 5 to visit 6 for patients randomized to (c) those subjects who had no change in stool frequency between visit 5 and visit 6 in study OXN3401.
Table 4d. Reproducibility of BFI items visit 5 to visit 6 for patients randomized to (d) entire subject group in study OXN3001 between visit 1 and visit 2.
Stability and reproducibility were further explored across all studies using Pearson's product-moment and ICC correlations. In each study it was clear that the ICC coefficients and Pearson's correlations were in the moderate-to-large range and were statistically significant (p < 0.001).
When patients were stratified according to severity of their constipation it was evident that BFI scores were higher for those classified as severely constipated compared with those classified as moderately constipated (). BFI scores also differed between those patients classified as mildly constipated compared with those classified as severe. BFI in those classified as moderately constipated was not always higher than in those classified as mildly constipated ().
Table 5. Determining known-groups validity of the BFI tool, constipation severity based on stool consistency (ANCOVA analysis).
In all studies, correlations between individual BFI items and mean total score to clinical characteristics of stool frequency/consistency, number of days of laxative use and CSBM were examined using Spearman's rank order correlation (). Correlation between BFI item and total scores to stool frequency were inverse, in the low-to-moderate range and statistically significant (study OXN2401 range –0.23 to –0.29, p < 0.001; study OXN3401 range –0.26 to –0.40, p < 0.001; study OXN3001 using CSBM range –0.27 to –0.45, p < 0.01). Similar observations were seen for stool consistency, albeit slightly lower (). In all studies, the number of days a patient received laxatives positively and moderately correlated with each BFI item and BFI total score. In studies OXN2401 and OXN3401 ease of defecation and personal judgment of constipation demonstrated a statistically significant correlation with the number of days receiving laxatives, exceeding 0.40 (). However, the correlation observed for feelings of incomplete evacuation was lower (r≤0.39), suggesting that perceptions of defecation and personal judgment are more related to laxative intake than feelings of evacuation (). In study OXN3001 BFI score correlated moderately with PAC-SYM data (range 0.38–0.66, p < 0.001).
Table 6. Spearman's rank order correlations of BFI items and clinical data of stool consistency, frequency and number of days the patients received laxatives in studies OXN2401, OXN3401 and OXN3001*.
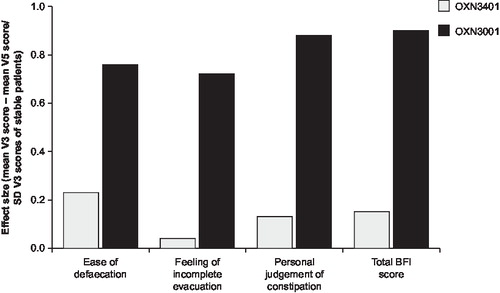
In study OXN2401, improvements in each BFI parameter: defecation, complete evacuation and personal judgment of constipation, with respect to effect size increased with increasing dose of naloxone, indicative of increasing responsiveness (). In this study, effect size changes were typically greater than 0.5 or 0.8 indicative of a moderate or large change as a result of treatment, respectively. Similarly, in study OXN3001 effect sizes were all large (range 0.76–0.88) (). By contrast, the effect sizes in study OXN3401 were all small which is perhaps not surprising as analyses were conducted on the entire study population including patients receiving non-constipating placebo (). In all studies effect size was greatest for the parameters of defecation and personal judgment with a lower effect size for the feeling of incomplete evacuation suggesting slightly less responsiveness to change over time.
Figure 3a. Responsiveness of BFI in study OXN2401 (n =169). Responsiveness of individual BFI items and total BFI score by treatment group.
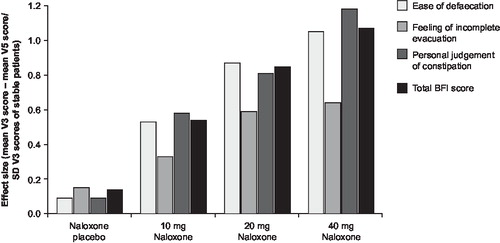
Figure 3b. Responsiveness of BFI in studies OXN3401 and OXN3001. Responsiveness of individual BFI items and total BFI score.
In study OXN2401, the SEM for all patients at visit 3 was 9.01. In study OXN3401, a similar result was observed with an SEM of 7.59 and in study OXN3001 the SEM value was 8.16. These data would suggest that a patient may realize a clinically significant change in bowel function when a change of BFI score between 7.5 and 9 points is observed.
Similarly, across all studies the one-half SD for the total BFI score at visit 3 was observed to be 22.6 (study OXN2401), 25.3 (study OXN3401) and 23.3 (study OXN3001), so half SD ranges from 11.3 to 12.7. This value suggests that changes in BFI total score of ≥12 will be of clinical significance.
Discussion
The BFI has been developed to evaluate patient assessment of constipation. As a patient rating tool it has been used to evaluate the efficacy of prolonged release oxycodone combined with prolonged release naloxone. In a survey by Connell et al Citation36 99% of the included patients (n=1055), which were defined as normal, non-constipated persons seen at health inspections in a factory and in patients seen at a general medical practitioner's surgery reported a stool frequency ranging from three times per week up to three times a day. This indicates that a diagnosis of constipation solely based on stool frequency may be misleadingCitation37. Many patients report a normal stool frequency but experience symptoms of constipation as straining, bloating and the feeling of incomplete bowel evacuationCitation2. This makes clear that subjective measures need to be considered alongside objective measures like stool frequency and stool consistencyCitation38,39.
The BFI was developed to take into account those subjective criteria often reported by patients who feel constipated. Since pain patients are experienced with the use of numerical rating scales to rate the intensity of their pain this kind of instrument was also used to develop the BFI. Three studies investigating the combination of PR oxycodone and PR naloxone in patients with moderate-to-severe chronic pain have been conducted to evaluate analgesia and impact on opioid-induced constipationCitation17,24–26. In all studies effective analgesia was undiminished by the presence of naloxone and an alleviation of constipation was observed. Data from these studies have been used to validate the BFI tool.
The level of missing data across the entire dataset was low (less than 0.5%), suggesting that the BFI tool is easy for patients to use and does not promote confusion or difficulty. The clinician administering the BFI is allowed to clarify the questions once per the BFI instruction sheet. By the end of the maintenance phase mean BFI scores were the lowest for all patients receiving active treatment (see also ). Not surprisingly, BFI scores were highest for those patients with more severe symptoms of constipation and clear significant differences between severe and mild constipation were observed. In one of the three studies, the BFI was not able to discriminate between those with mild and those with moderate constipation although in the other two studies, the BFI was able to discriminate between mild and moderate patients. These data indicate that the BFI tool can discriminate the severity of constipation based on stool consistency which can act as a surrogate marker for constipation severity per se.
There was strong internal consistency as measured by Cronbach's alpha for BFI total score and between individual BFI items. The observation of high internal consistency is indicative that each item of the BFI measure variables that are highly related. The highest correlations were observed between items of defecation and personal judgment. Similarly, the item to total score correlations were high, suggesting each item adds to the overall concept of the BFI. The lower correlation between BFI items of defecation and personal judgment with feelings of incomplete evacuation is likely because this variable is distinct from the other two in the BFI tool and suggests that patients may judge feelings of constipation more on the ability to defecate rather than feelings of incomplete evacuation.
This evaluation study has shown that the BFI tool has good test–retest reliability and all correlations undertaken for reproducibility were in the moderate range for studies OXN2401 and OXN3401 and in the large range for study OXN3001. These data are supportive of the expectation that BFI scores will be stable during a period of time.
Patients who expressed a preference for PR oxycodone/naloxone in the maintenance phases had better resolution of constipation symptoms than those expressing a preference during the titration phase. It would be anticipated that these patients were those for whom PR oxycodone/naloxone provided effective analgesia with an acceptable degree of side-effects. In study OXN3001, BFI correlated with the PAC-SYM suggesting that there is overlap between the BFI and PAC-SYM, but not outright redundancy with the stool and rectal scales of the PAC-SYM as these scales correlated more highly with the BFI than did the abdominal scale.
As expected when patients were grouped according to constipation severity, those with more severe symptoms demonstrated a higher BFI value suggesting that the BFI was valid in determining the impact of opioid-induced constipation. A systematic review of 83 studies has shown, that if patients with a chronic disease are asked to identify a minimal relevant change in health-related patient reported outcomes, the estimates fall very close to half a SDCitation12. Therefore it can be concluded that the threshold of discrimination for changes in health-related patient reported outcomes is approximately half a SD.
The remarkable consistency of the half SD criterion results across all studies made this criterion an accepted one in the determination of clinically important changes of health-related outcome measures. The FDA Draft Guidance for Industry on Patient Reported Outcome Measures states that for defining a minimum important difference, a distribution based approach (e.g., defining the minimal important difference as 0.5 times the SD) is acceptable as long as this result is not used in isolationCitation19.
Another distribution based approach, the standard error of measurement (SEM) for evaluating responsiveness was also taken into account, because it has been proposed as a useful statistic for evaluating clinically meaningful individual changes in health-related quality of life (HRQL) measuresCitation34,35.
Analysis of the SEM and one-half SD characteristics of each BFI component indicated that changes in BFI score less than 7.5 points are unlikely to be clinically meaningful, whilst changes in BFI score of ≥12 points are likely to be related to clinically meaningful changes in patient's perception of their bowel habits. It is not known what the clinical relevance will be of BFI score changes between 7.5 and 12 points, score changes within this region would benefit from further evaluation. These values provide a guide to clinicians using the BFI tool with patients in aiding interpretation; however treatment in some patients may still provide a clinically meaningful effect at BFI changes less than 7.5 pointsCitation40.
Although this analysis includes a large sample of patients with OIC with measurements of stool frequency and consistency, and bowel function symptoms, the data are all from clinical trials whose primary focus was the evaluation of analgesic efficacy and bowel function regarding the fixed combination of oral PR oxycodone with oral PR naloxone. Further data for the validation of the BFI have been collected in a cross-sectional, observational, non-interventional, multicenter study in chronic, non-malignant pain patients receiving different opioid treatment. Results from this study will be discussed within another publication which is currently in preparation.
Taken together, the BFI is a valid and reliable instrument for the assessment of constipation evaluating the clinical symptoms in chronic pain patients. The findings of psychometric analyses from the phase II and phase III clinical trials support the BFI's psychometric properties, necessary information for interpretation of any data based on the BFI. Specific BFI score changes can be used for establishing thresholds for clinically meaningful change in constipation.
Acknowledgments
Declaration of interest: This study was supported by an unrestricted educational grant from Mundipharma Research. S. M-L has disclosed that he is currently a medical consultant for Axcan, Boehringer International, Movetis, Mundipharma, Novartis, Procter & Gamble; R.Y. and A.M.R. have disclosed that they have no relevant financial relationships. Editorial support was provided by Susan Allen PhD and Neil McKendrick PhD of BrandFish.
References
- Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 2001;182(5A Suppl):11-18S.
- Bell T, Panchal SJ, Miaskowski C, The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35-42.
- Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs 2003;63:649-71.
- Shahbazian A, Heinemann A, Schmidhammer H, Involvement of μ- and κ-, but not δ-opioid receptors in the peristaltic motor depression caused by endogenous and exogenous opioids in the guinea pig intestine. Br J Pharmacol 2002;135:741-50.
- De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther 1996;69:103-15.
- Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil 2004;16(Suppl 2):17-28.
- Manara L, Bianchi G, Ferretti P, Tavani A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther 1986;237:945-9.
- Grider JR, Makhlouf GM. Role of opioid neurons in the regulation of intestinal peristalsis. Am J Physiol Gastrointest Liver Physiol 1987;253:G226-31.
- Wirz S, Wartenberg HC, Wittman M. Post-operative pain therapy with controlled release tramadol following orthopedic surgery: a prospective, randomized, double-blind investigation. Pain Clinic 2005;17:367-76.
- Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manage 2002;23:48-53.
- Avila JG. Pharmacological treatment of constipation in cancer patients. Cancer Control 2004;11(Suppl 1):10-18.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life. The remarkable university of half a standard deviation. Med Care 2003;41:582-92.
- Bates JJ, Foss JF, Murphy DB. Are peripheral opioid antagonists the solution to opioid side effects? Anesth Analg 2004;98:116-22.
- Paulson D, Kennedy DT, Donovick RA, Alvimopan: an oral, peripherally acting μ-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction – a 21 day treatment-randomized clinical trial. J Pain 2005;6:184-92.
- Holzer P. New approaches to the treatment of opioid-induced constipation. Eur Rev Med Pharmacol Sci 2008;12(Suppl 1):119-27.
- Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett 2004;361:192-5.
- Meissner W, Leyendecker P, Müller-lissner S, A randomised controlled trial with prolonged-release oral oxycodone and naloxone to prevent and reverse opioid-induced constipation. Eur J Pain 2009;13:56-64.
- Müller-Lissner S, Koch G, Talley NJ, Subjects global assessment of relief: an appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J Clin Epidemiol 2003;56:310-16.
- Draft Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Food and Drug Administration, 2006.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920-4.
- Frank L, Kleinman L, Farup C, Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol 1999;34:870-7.
- Slappendel R, Simpson K, Dubois D, Keininger DL. Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur J Pain 2006;10:209-17.
- Rome Foundation. Guidelines– Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J Gastrointestin Liver Dis 2006;15:307-12.
- Nadstawek J, Leyendecker P, Hopp M, Patient assessment of a novel therapeutic approach for the treatment of severe, chronic pain. Int J Clin Pract 2008;62:1159-67.
- Vondrackova D, Leyendecker P, Meissner W, Analgesic efficacy and safety for oxycodone in combination with naloxone as prolonged release tablets in patients with moderate to severe chronic pain. J Pain 2008;9:1144-54.
- Simpson KH, Leyendecker P, Hopp M, Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate to severe noncancer pain. Curr Med Res Opin 2008;24,3503-12.
- Cronbach L. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16:297-334.
- Hays RD, Anderson RT, Revicki DA. Assessing reliability and validity of measurement in clinical trials . In: Staquet MJ, Hays RD, Fayers PM , eds. Quality of Life Assessment in Clinical Trials: Methods and Practice. Oxford: Oxford University Press, 1998.
- Cohen J. Statistical power analyses for the behavioral sciences, 2nd edn. Hillsdale NJ: Lawrence Erlbaum, 1988.
- Deyo RA, Dieher P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials 1991;12:142-58S.
- Myers JL., Well AD. Research Design and Statistical Analysis, 2nd edn. Hillsdale, NJ: Lawrence Erlbaum, 2003:508.
- Mc Donald JH. Handbook of Biological Statistics. Baltimore, MD: Sparky House Publishing, 2008. [URL: http://udel.edu/∼mcdonald/statanovamodel.html ] Accessed April 2009.
- Hays RD, Revicki DA. Reliability and validity (including responsiveness). In: Fayers P, Hays RD , eds. Assessing Quality of Life in Clinical Trials, 2nd edn. New York: Oxford University Press, 2005.
- Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care 1999;37:469-78.
- Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52:861-73.
- Connell AM, Hilton C, Irwine G, Variation in bowel habits in two population samples. Br Med J 1965;2:1095-9.
- McMillan SC. Assessing and managing opioid-induced constipation in adults with cancer. Cancer Control 2004;11(Suppl 1):3-9.
- Müller-Lissner S, Beil W. Moderne Therapie mit Laxanzien. Bremen: UniMed Verlag, 2006.
- Sweeney MA. Constipation – diagnosis and treatment. Home Care Provid 1997;2:250-5.
- Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness or evaluative trials. Br Med J 1998;316:690-3
