Abstract
Objectives: To evaluate the cost-effectiveness of switching to biphasic insulin aspart (BIAsp 30) from human premix insulin for type 2 diabetes patients in the United States (US) setting.
Methods: The previously published and validated IMS Core Diabetes Model was used to project life expectancy, quality-adjusted life expectancy (QALE) and costs over 30 years. Patient characteristics and treatment effects were based on Canadian patients included the IMPROVE observational study (n = 311). Mean glycohaemoglobin (HbA1c) was 8.4%, duration of diabetes 16 years and prevalence of complications high at baseline. Simulations were conducted from the perspective of a third-party payer, with costs accounted in 2008 US dollars ($).
Results: BIAsp 30 was projected to improve life expectancy by 0.202 years and QALE by 0.301 quality-adjusted life-years (QALYs), due to a reduced incidence of most diabetes-related complications. BIAsp 30 was associated with increased lifetime direct medical costs ($76,517 vs. 67,518) and an incremental cost-effectiveness ratio of $29,870 per QALY gained. Long-term outcomes were sensitive to the impact of BIAsp 30 on hypoglycaemia and changes in HbA1c.
Conclusions: BIAsp 30 may represent a cost-effective treatment option in the US setting for advanced type 2 diabetes patients experiencing poor glycaemic control or hypoglycaemia on human premix insulin.
Limitations: The application of treatment effect data derived from a Canadian cohort to the US setting was a limitation of the cost-effectiveness analysis. The findings of this cost-effectiveness analysis are not applicable to insulin-naïve diabetes patients.
Introduction
The aim of diabetes care is to prevent microvascular and macrovascular complications; it has been shown in type 2 diabetes that achieving good glycaemic control is central to this aimCitation[1–3]. In 2009 the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) issued an updated and detailed treatment algorithm for type 2 diabetes that takes into account the characteristics of individual interventions, their synergies, and expenseCitation[4]. In short, the consensus recommends transition to intensive insulin therapy to achieve glycaemic control (glycohaemoglobin [HbA1c] <7%), after failure with oral antidiabetic medications (OADs) with or without a basal insulinCitation[4].
The National Health and Nutrition Examination Survey (NHANES) of 2003–2004 showed that in the US approximately 64% of patients with diabetes taking a combination of OADs and insulin failed to achieve HbA1c < 7%Citation[5]. Despite the availability of evidence based guidelines and a range of insulin based regimens, patients and physicians are often reluctant to initiate intensive insulin therapy for a number of reasons including fear of hypoglycaemia, potential weight gain and the requirement for multiple daily injectionsCitation[6–8]. For patients with type 2 diabetes there are a number of insulin based regimens available that allow physicians to individualise treatment according to insulin requirements and patient preference, including a basal-only regimen, twice-daily premixed insulin regimen, prandial insulin regimen, basal-bolus regimen and insulin pump therapyCitation[9]. In routine clinical practice failure to achieve adequate glycaemic control may be due to physiological changes or psychological and behavioural barriers to effective implementation. The challenge is to match the regimen to the patient and this may require switching between similar insulin regimensCitation[10].
Pre-mixed insulins containing a rapid-acting insulin and a protaminated, intermediate-acting insulin, provide prandial and basal insulin coverage and have been shown in randomised trials to improve glycaemic control in many patients with type 2 diabetesCitation[11–13]. Of importance when transitioning patients to more intensive insulin regimens, such as basal-bolus, pre-mixed insulin is associated with fewer daily injections and reduces the likelihood of dosing errors compared to self-mixed insulinCitation[14–16]. A recent systematic review of randomised controlled trials (RCTs) assessing the safety and effectiveness of pre-mixed insulin analogues in type 2 diabetes concluded that they decrease HbA1c more effectively than long-acting insulin analogues but are associated with an increased incidence of hypoglycaemiaCitation[13].
Pre-mixed insulin analogues provide a more physiological insulin profile compared to premixed human insulin, which can be speculated to account for improved postprandial glucose control with the formerCitation[13],Citation[17]. A systematic review of 16 RCTs concluded that pre-mixed insulin analogues are associated with similar reductions in HbA1c and incidences of major and minor hypoglycaemia compared to pre-mixed human insulinCitation[13]. However, it was also noted that because most of the studies excluded patients with diabetic complications or other comorbidities the findings could not be generalised to all diabetes patients. Well-designed observational studies complement RCTs by providing information regarding efficacy within routine clinical practice, with cohorts including patients with a diverse range of clinical backgroundsCitation[18].
Biphasic insulin aspart (BIAsp 30; NovoLog Mix 70/30; Novo Nordisk Inc.) is a pre-mixed insulin analogue consisting of 30% soluble rapid-acting insulin aspart and 70% protaminated insulin aspart. A large, single-arm, 26-week, observational study, IMPROVE, has provided insights into the safety and efficacy of BIAsp 30 when used in routine clinical settings among patients with varying duration of type 2 diabetes and complication statusCitation[19–22]. Briefly, patients were enrolled if they were considered by their treating physician to be in need of insulin treatment and the decision to initiate treatment with BIAsp 30 was made. Conducted in 11 countries and enrolling a total of 52,419 patients, the primary aim of IMPROVE was to compare baseline rates of major hypoglycaemia associated with various antidiabetic interventions, relative to values reported after 26 weeks of BIAsp 30 treatmentCitation[19]. Secondary end-points included HbA1c reduction and weight change.
A subgroup analysis from the IMPROVE study evaluated outcomes for 3,856 patients switched from human premix insulin, often known as biphasic human insulin 30 (BHI 30), to BIAsp 30Citation[22]. BHI 30 contains a fixed soluble human insulin component (30% of the formulation) and neutral protamine Hagedorn insulin (70% of the formulation). Based on data from 11 countries, switching to BIAsp 30 was associated with significant reductions in HbA1c, and a reduced incidence of severe, minor and nocturnal hypoglycaemia. The aim of the current analysis was to evaluate the long-term clinical and cost-related impact of switching patients to BIAsp 30 from BHI 30 in the US setting. To project outcomes relevant to the US diabetes patient population, the current study was based on safety and efficacy outcomes corresponding to the 311 patients included in the Canadian arm of IMPROVE and switching from BHI 30 to BIAsp 30. As IMPROVE was not conducted in US patients, it was assumed in this analysis that the characteristics of inadequately controlled Canadian patients using human premix insulin did not differ from those of US patients.
Methods
The lifetime cost-effectiveness of switching to BIAsp 30 from BHI 30 was modelled in the US setting based on treatment effects observed in the Canadian cohort of IMPROVE and US-specific costs, using a computer simulation model of diabetes.
Model
The widely published and validated IMS Core Diabetes Model (Version 6.5) was used for this analysisCitation[23],Citation[24]. In brief, the model is an interactive computer simulation model of diabetes (type 1 and type 2), comprising 15 inter-dependent sub-models accounting for the complications of angina, myocardial infarction (MI), congestive heart failure (CHF), stroke, peripheral vascular disease (PVD), diabetic retinopathy, macula oedema (ME), cataract, hypoglycaemia, ketoacidosis, lactic acidosis, nephropathy and end-stage renal disease (ESRD), neuropathy, foot ulcer and amputation, in addition to other-cause mortality. Each Markov sub-model uses time-, state-, and diabetes type-dependent probabilities derived from published sources. To obtain projected outcomes relevant to specific patient groups and country settings of interest, patient cohorts can be defined in terms of age, gender, baseline risk factors and pre-existing complications, and disease management components and costs can be altered to reflect country specific patterns of care and cost.
Simulation cohorts
A hypothetical cohort for simulation was defined based on the characteristics of patients enrolled in IMPROVE in Canada (Novo Nordisk Inc.; Data on file), supplemented with data from US-specific diabetes publicationsCitation[19],Citation[22]. Where data regarding patient baseline characteristics were unavailable from IMPROVE data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study of intensive diabetes treatment were usedCitation[25]. This was considered appropriate because the ACCORD study was conducted in US and Canadian centres and enrolled patients at similar disease severity (in terms of age and duration of diabetes) to those in IMPROVE.
At baseline the simulation cohort had a mean age of 64.2 years, mean HbA1c of 8.4%, mean body mass index (BMI) 32.1 kg/m2, mean duration of diabetes of 16.4 years and mean systolic blood pressure of 136.4 mmHg (). At baseline 27% of patients had a history of MI, 8% a history of stroke, 14% had PVD and 27% had microalbuminuria. The mean dose of BHI 30 was 59.96 IU at baseline and the mean dose of BIAsp 30 at study end was 69.81 IU.
Table 1. Baseline demographics, complications, relevant concomitant mediations and management of patients in the simulated cohort.
Intervention effects
Based on results from IMPROVE, treatment with BIAsp 30 was associated with a change in HbA1c of −0.58%-points. The occurrence of major hypoglycaemia was reduced from 30.9 events per 100 patient-years (baseline event rate with BHI 30) to 7.7 events per 100 patient-years (). Switching to BIAsp 30 was associated with moderate weight gain; an increase in BMI by 0.28 kg/m2 relative to baseline.
Table 2. Treatment associated effects with BIAsp 30 and BHI 30.
Perspective, costs and time horizon
The study was conducted from the perspective of a third-party healthcare payer in the US setting. Direct costs comprised the sum of treatment, complication and medications costs as derived from published sources, and were inflated to year 2008 values ()Citation[26],Citation[27]. Annual costs for BIAsp 30 and BHI 30 amounted to approximately $2,361 and $899, respectively. Total treatment costs also included costs for other antidiabetic agents based on reported mean baseline and end-of-study use in IMPROVE. In line with recommendations for the US, future clinical and cost outcomes were discounted at the rate of 3% per annumCitation[28]. In recognition of the chronic nature of type 2 diabetes a 30-year time horizon was used for the base-case analysis.
Table 3. Cost data for treatment and hospitalisation of type 2 diabetes complications and patient management in the US for 2008.
Health state utilities
Health state utilities for type 2 diabetes and its complications were derived wherever possible from the UKPDSCitation[29], supplemented with data from other published sourcesCitation[30],Citation[31]. Disutility values for major and minor hypoglycaemic events were −0.0118 and −0.0035, respectively, based on data published by Currie and colleagues (2006)Citation[32].
Sensitivity analyses
The impact of key assumptions made for the base-case analysis was assessed in sensitivity analyses. To assess the impact of the time horizon on outcomes, this was varied between 5 and 25 years. The impact of discount rates of 0% and 6% were assessed (base-case value of 3%). Sensitivity to variation in the costs of diabetes-related complications was assessed by assuming a 20% increase or 20% decrease in event and follow-up costs assigned in the base case. The impact of treatment-associated changes in HbA1c and hypoglycaemia, both minor and major events, was further investigated by assuming either no change, or reducing the impact of treatment to 50% of that observed in the Canadian subgroup of IMPROVE in four separate one-way sensitivity analyses. Because response to treatment may vary between patients, a probabilistic sensitivity analysis was conducted by sampling from the distributions surrounding patient baseline values () and treatment-associated changes in HbA1c and body weight ().
Statistical methodology
Monte Carlo simulation techniques were used to calculate cost-effectiveness outcomes, capturing individual patient history (such as prior events and pre-existing complications), without the need to define an unwieldy number of Markov health states. A cohort of 1000 patients was run through the model 1000 times for each simulation, with mean values and standard deviations generated using a non-parametric bootstrapping approachCitation[33]. Resultant mean values of incremental cost and incremental effectiveness corresponding to the 1000 simulations were plotted on the cost-effectiveness plane, and these data were then used to generate an acceptability curve by calculating the proportion of points below a range of willingness-to-pay thresholds.
Results
Life expectancy and quality-adjusted life expectancy
In the base-case analysis, treatment with BIAsp 30 was associated with increased life expectancy compared to remaining on a regimen of BHI 30 (). Undiscounted life expectancy improved by 0.202 years and discounted life expectancy by 0.124 years for patients who switched to BIAsp 30 from BHI 30. When quality of life was taken into account, BIAsp 30 was associated with an improvement of 0.301 quality-adjusted life-years (QALYs) compared to BHI 30.
Table 4. Summary of base-case results for BIAsp 30 versus BHI 30.
Cumulative incidence of diabetes-related complications
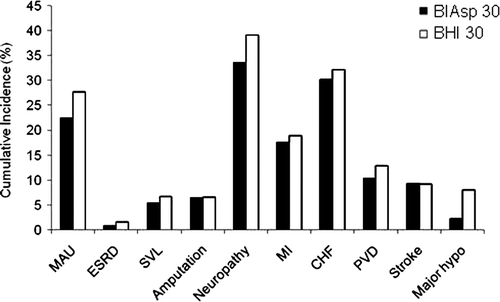
Over patient lifetimes treatment with BIAsp 30 was associated with a lower cumulative incidence of most diabetes-related complications including cardiovascular and renal events (). Notably, switching to BIAsp 30 was associated with a reduced cumulative incidence of ESRD (0.83 vs. 1.55%), PVD (10.34 vs. 12.82%), and severe vision loss (5.44 vs. 6.67%) compared to BHI 30. In line with the reduced incidence of major hypoglycaemia observed in IMPROVE, the lifetime cumulative incidence of severe hypoglycaemia was lowered in the simulations (2.24% with BIAsp 30 vs. 7.95% with BHI 30).
Figure 1. Cumulative incidence of diabetes-related complications for BIAsp 30 versus BHI 30. BIAsp 30, biphasic insulin aspart 30; BHI 30, biphasic human insulin; MAU, microalbuminuria; ESRD, end-stage renal disease; SVL, severe vision loss; MI, myocardial infarction; CHF, congestive heart failure; PVD, peripheral vascular disease; Major hypo, major hypoglycaemia.
Lifetime costs and incremental cost-effectiveness
Switching to BIAsp 30 was associated with an increase in lifetime direct medical costs of $8,998 per patient compared to BHI 30 (). The estimated lifetime direct costs for BIAsp 30 were $76,517 compared to $67,518 per patient with BHI 30, and treatment costs accounted for most of this difference ($26,583 vs. 15,030 per patient for BIAsp 30 and BHI 30, respectively). The higher cost of BIAsp 30 treatment was partially offset by a reduction in complication-related costs. For example, cardiovascular-related costs were reduced by $303 per patient on BIAsp 30 ($35,732 vs. 36,035 per patient), renal costs were reduced by $690 per patient ($768 vs. 1458 per patient), and hypoglycaemia-related costs were reduced by $1315 per patient ($512 vs. 1600 per patient). Given the increased medical costs of $8,998 per patient and an improvement in quality-adjusted life expectancy for BIAsp 30 of 0.301 QALYs, the projected incremental cost-effectiveness ratio (ICER) was within the range considered to represent good value for money in the US setting at $29,870 per QALY gained versus BHI 30.
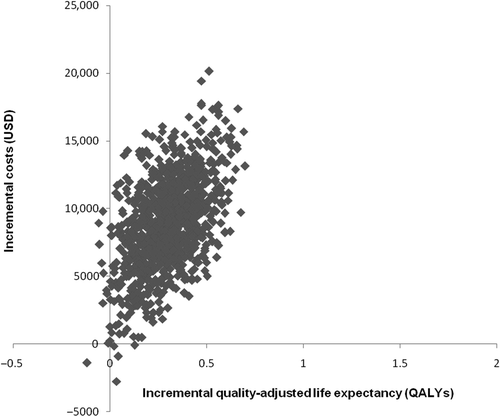
Incremental cost-effectiveness scatter plot
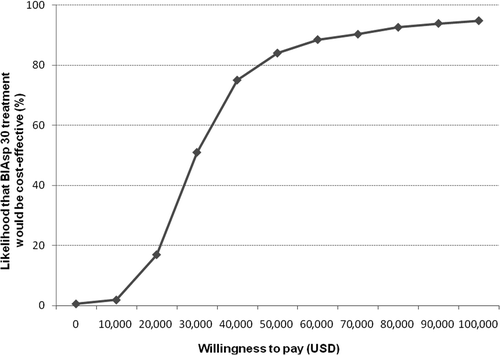
Most points in the incremental cost-effectiveness scatter plot () were located within the upper-right quadrant, indicating that treatment with BIAsp 30 was more effective and more costly than treatment with BHI 30. When converted to an acceptability curve, it was evident that at a willingness-to-pay threshold of $50,000 per QALY gained, BIAsp 30 had an 84% likelihood of being considered cost-effective compared to BHI 30 ().
Sensitivity analyses
Sensitivity analysis revealed that projected outcomes were most sensitive to changes in HbA1c and hypoglycaemia event rates. In contrast, accounting for variation in patient response to treatment and changes in time horizon, discounting and complication-related costs did not appreciably alter outcomes with ICERs all below $50,000 per QALY gained ().
Table 5. Sensitivity analyses for BIAsp 30 versus BHI 30 on cost-effectiveness outcomes.
When the BIAsp 30-associated change in HbA1c was set to 50% of that observed in the Canadian BHI 30 cohort (from −0.58% to −0.26%-points) the ICER increased moderately to $38,767 per QALY gained (vs. $29,870 per QALY gained in the base case). When no reduction in HbA1c was assumed the improvement in quality-adjusted life expectancy was reduced from the base-case estimate of 0.301 QALYs to a value of 0.166 QALYs, and consequently the ICER increased to $58,462 per QALY gained. HbA1c has a major impact on modelled microvascular events such as blindness and ESRD, and hence on quality of life and costs.
Sensitivity analyses for change in hypoglycaemia event rates had a greater effect on projected outcomes. When the reduction in major hypoglycaemia (events requiring third-party assistance) was assumed as 11.6 events per 100 patient-years (vs. 23.2 events per 100 patient-years in the base case) the improvement in quality-adjusted life expectancy was 0.203 for BIAsp 30 and the ICER for BIAsp 30 versus BHI 30 was $48,396 per QALY gained. When no reduction in hypoglycaemia was assumed for BIAsp 30 in the improvement in quality-adjusted life expectancy was 0.106 QALYs (vs. 0.301 in the base case) and an ICER of $95,819 per QALY gained was calculated for BIAsp 30 versus BHI 30.
Discussion
This analysis has applied data from the IMPROVE observational study to the IMS Core Diabetes Model in estimating the long-term clinical and economic outcomes when switching to BIAsp 30 from BHI 30 in patients with advanced type 2 diabetes. Specifically, in a cohort of patients with a long duration of type 2 diabetes and a high prevalence of complications at baseline, it was projected that switching to BIAsp 30 from BHI 30 was associated with improvements in life expectancy of 0.124 years and quality-adjusted life expectancy of 0.301 QALYs. Additionally, treatment with BIAsp 30 was associated with a reduced incidence of most diabetes-related complications, including major reductions in the cumulative incidence of ESRD (0.83 vs. 1.55%) and PVD (10.3 vs. 12.8%). The increased lifetime treatment costs incurred with switching to BIAsp 30 ($11,553) were partially offset by reductions in complication costs ($2642), including cardiovascular-related ($303), renal-related ($690) and hypoglycaemia-related costs ($1315). The estimated ICER of $29,870 per QALY gained was within the range considered acceptable in the US setting. Extensive sensitivity analyses indicated that long-term outcomes were sensitive to the impact of BIAsp 30 on HbA1c and hypoglycaemia.
The improvements in HbA1c and severe hypoglycaemia observed in the IMPROVE study for patients switching to BIAsp 30 from BHI 30 provide some insight into the effectiveness of pre-mixed insulin analogues in everyday clinical settings. However, a recent systematic review of RCTs by Qayyum and colleagues (2008) indicated that pre-mixed insulin analogues and pre-mixed human insulin are equivalent in terms of HbA1c reductionsCitation[13]. It is likely that the long duration of diabetes and high prevalence of complications in the Canadian subgroup of IMPROVE at baseline resulted in pronounced reductions in HbA1c and hypoglycaemia over 26 weeks. Risk factors for hypoglycaemia include older age, duration of diabetes, presence of comorbidities, and renal impairmentCitation[34]. Therefore, the IMPROVE observational study has highlighted the importance of conducting future RCTs of pre-mixed insulins in patients at increased risk for hypoglycaemia, a need also identified in the aforementioned systematic reviewCitation[13]. Nevertheless, the current modelling analysis used treatment effects based on a large observational study, providing an indication as to the impact of BIAsp 30 in clinical practice. The 2-year RCT conducted by Boehm and colleagues (2004) measured a significant reduction in major hypoglycaemia for BIAsp 30 versus BHI 30 after the first year, in patients with relatively advanced diabetes (disease duration of 14.1 years)Citation[35]. Despite the clinically important reductions in HbA1c experienced by Canadian patients enrolled in IMPROVE, the mean end-of-study value of 7.8% was above ADA and EASD consensus targetsCitation[4]. Cost-effectiveness outcomes for BIAsp 30 would be improved if mean end-of-study HbA1c values were closer to ADA and EASD targets. Failure to reach target might reflect less-aggressive titration in clinical practice; a recent Danish observational study revealed that 28% of non-insulin-naïve patients achieved HbA1c ≤ 7.0% 26 weeks after switching to BIAsp 30Citation[36].
Projected cost-effectiveness outcomes were sensitive to the impact of BIAsp 30 on reductions in hypoglycaemic events. The present modelling study employed quality of life disutility values for hypoglycaemia derived from diabetes patients with a similar age to that of the simulation cohortCitation[32]. A recent study of Canadian and UK subjects reported disutility for minor hypoglycaemia of −0.0033 per eventCitation[37]. An exploratory sensitivity analysis using this more recent disutility value resulted in an ICER of $34,192 per QALY gained for BIAsp 30, which was still below the willingness-to-pay threshold. Cost-effectiveness outcomes were more sensitive to hypoglycaemic event rates than their associated disutilities.
A limitation of the current modelling analysis was the assumption that outcomes observed in type 2 diabetes patients in a Canadian setting are applicable to US patients. Comparisons of Canadian and US patients with diabetes have reported considerable overlap in patient characteristics despite differences in care delivery patternsCitation[38],Citation[39]. Therefore, until appropriate observational studies of BIAsp 30 have been conducted in the US, data from the Canadian cohort of IMPROVE may provide the most reasonable clinical data for the estimation of likely outcomes. Another limitation of the current analysis was that long-term compliance with insulin therapy was not captured. The net effect of insulin-related savings due to non-compliance and the likely increase in medical costs due to poorer glycaemic control was not determined.
Conclusion
With increasing demands for limited healthcare resources it is important to consider both clinical and economic aspects of a given treatment option. Based on outcomes from the IMPROVE observational study and an analysis using the IMS Core Diabetes Model, for patients with a long duration of diabetes and high prevalence of baseline complications receiving BHI 30, switching to BIAsp 30 may be cost-effective from the perspective of a third-party payer.
Transparency
Declaration of financial/other relationships: JLP and MSK have disclosed that they are current employees of IMS Health, a consultancy that has received fees from Novo Nordisk. MA and TLT have disclosed that they are current employees of Novo Nordisk.
Declaration of funding: This study was funded by Novo Nordisk, Princeton, NJ USA.
Acknowledgments: The authors would like to acknowledge the assistance of Katrina Erny-Albrecht of MedWriters, Inc. in the preparation of this manuscript.
References
- Scheen AJ, Paquot N, Lefebvre PJ. [United Kingdom Prospective Diabetes Study (UKPDS): 10 years later]. Rev Med Liege 2008; 63: 624–629
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1988; 352: 854–865
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203
- Hoerger TJ, Segel JE, Gregg EW, et al. Is glycaemic control improving in U.S. adults?. Diabetes Care 2008; 31: 81–86
- Wallace TM, Matthews DR. Poor glycaemic control in type 2 diabetes: a conspiracy of disease, suboptimal therapy and attitude. QJM 2000; 93: 369–374
- Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev 2002; 18: S42–49
- Hayes RP, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract 2008; 62: 860–868
- Crasto W, Jarvis J, Khunti K, et al. New insulins and new insulin regimens: a review of their role in improving glycaemic control in patients with diabetes. Postgrad Med J 2009; 85: 257–267
- Hirsch IB, Vega CP. Optimal initiation of insulin in type 2 diabetes. MedGenMed 2005; 7: 49
- Hermansen K, Colombo M, Storgaard H, et al. Improved postprandial glycaemic control with biphasic insulin aspart relative to biphasic insulin lispro and biphasic human insulin in patients with type 2 diabetes. Diabetes Care 2002; 25: 883–888
- Boehm BO, Home PD, Behrend C, et al. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in Type 1 and Type 2 diabetic patients. Diabet Med 2002; 19: 393–399
- Qayyum R, Bolen S, Maruthur N, et al. Systematic review: comparative effectiveness and safety of premixed insulin analogues in type 2 diabetes. Ann Intern Med 2008; 149: 549–559
- Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care 1992; 15: 1628–1630
- Bell DS, Clements RS, Jr, Perentesis G, et al. Dosage accuracy of self-mixed vs premixed insulin. Arch Intern Med 1991; 151: 2265–2269
- Majumdar S, Barrett E. Newer insulins in search of a niche. Ann Intern Med 2008; 149: 586–588
- Garber AJ, Ligthelm R, Christiansen JS, et al. Premixed insulin treatment for type 2 diabetes: analogue or human?. Diabetes Obes Metab 2007; 9: 630–639
- Ligthelm RJ, Borzi V, Gumprecht J, et al. Importance of observational studies in clinical practice. Clin Ther 2007; 29: 1284–1292
- Valensi P, Benroubi M, Borzi V, et al. The IMPROVE study–a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract 2008; 62: 1809–1819
- Valensi P, Benroubi M, Borzi V, et al. Initiating insulin therapy with, or switching existing insulin therapy to, biphasic insulin aspart 30/70 (NovoMix 30) in routine care: safety and effectiveness in patients with type 2 diabetes in the IMPROVE observational study. Int J Clin Pract 2009; 63: 522–531
- Gumprecht J, Benroubi M, Borzi V, et al. Intensification to biphasic insulin aspart 30/70 (BIAsp 30, NovoMix 30) can improve glycaemic control in patients treated with basal insulins: a subgroup analysis of the IMPROVE observational study. Int J Clin Pract 2009; 63: 966–972
- Shah S, Benroubi M, Borzi V, et al. Safety and effectiveness of biphasic insulin aspart 30/70 (NovoMix 30) when switching from human premix insulin in patients with type 2 diabetes: subgroup analysis from the 6-month IMPROVE observational study. Int J Clin Pract 2009; 63: 574–582
- Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin 2004; 20: S27–40
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004; 20: S5–S26
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559
- Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 2007; 18: 2644–2648
- Minshall ME, Oglesby AK, Wintle ME, et al. Estimating the long-term cost-effectiveness of exenatide in the United States: an adjunctive treatment for type 2 diabetes mellitus. Value Health 2008; 11: 22–33
- Tarn TSM. Pharmacoeconomic Guidelines Around the World. ISPOR Connections 2004; 10: 5–15
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ–5D (UKPDS 62). Med Decis Making 2002; 22: 340–349
- Australian Institute of Health and Welfare. The Burden of Disease and Injury in Australia 2003. Available at http://www.aihw.gov.au/. Accessed January 2009.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000; 38: 583–637
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006; 22: 1523–1534
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997; 6: 327–340
- Amiel SA, Dixon T, Mann R, et al. Hypoglycaemia in Type 2 diabetes. Diabet Med 2008; 25: 245–254
- Boehm BO, Vaz JA, Brøndsted L, et al. Long-term efficacy and safety of biphasic insulin aspart in patients with type 2 diabetes. Eur J Intern Med 2004; 15: 496–502
- Breum L, Almdal T, Lund P, et al. Initiating or switching to biphasic insulin aspart 30/70 therapy in subjects with type 2 diabetes mellitus. An observational study. Rev Diabet Stud 2008; 5: 154–162
- Levy AR, Christensen TL, Johnson JA. Utility values for symptomatic non-severe hypoglycaemia elicited from persons with and without diabetes in Canada and the United Kingdom. Health Qual Life Outcomes 2008; 6: 73
- Klarenbach SW, Jacobs P. International comparison of health resource utilization in subjects with diabetes: an analysis of Canadian and American national health surveys. Diabetes Care 2003; 26: 1116–1122
- Booth GL, Zinman B, Redelmeier DA. Diabetes care in the U.S. and Canada. Diabetes Care 2002; 25: 1149–1153
- Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26: 2300–2304