Abstract
Objective: To develop a model to predict the length of time before patients with Alzheimer's disease (AD) of varying severity require full-time care (FTC).
Methods: A predictive model (equation) of time to FTC (defined as being institutionalised or dependent) was developed based on the London and South-East Region (LASER–AD) epidemiological study using a discrete time representation of the Cox continuous time proportional hazards model and complementary log–log specification.
Results: Of the 117 pre-FTC patients, 68 (58.1%) patients progressed to FTC during the 54-month follow-up period. Analysis of potential predictors showed that baseline cognitive state, impairment of activities of daily living (ADL) and neuropsychiatric symptoms were strong predictors of time to FTC. In addition, the rate of cognitive and ADL decline predicted time to FTC. The final model predicted 88.2% of observations.
Conclusion: The model simulates and predicts progression of pre-FTC AD patients until the need for FTC based on assessments for cognitive, functional and behavioural domains. The main application of the model is to assess the cost effectiveness of AD therapies as potential adjuncts to a background AD treatment including disease-modifying treatments. The applicability of the predictive model to a specific setting should be carefully assessed, i.e. the patient population being examined should have similar characteristics as patients in the LASER–AD cohort.
Introduction
The escalating financial burden on healthcare systems requires cost containment measures and therefore economic appraisals of health technologies. Economic evaluations of chronic progressive diseases, such as Alzheimer's disease (AD), typically rely on modelling techniques because clinical trials are often too short to measure long-term outcomes of treatment alternatives.
A number of modelling approaches have been used to assess the cost effectiveness of pharmacological treatments in AD. For example, disease progression has been modelled on patient's cognitive decline corresponding to severity states using Markov modelsCitation[1–3], or by employing survival analysis to predict the time at which patients reach a more severe stateCitation[4]. These approaches assume that disease severity is determined entirely by cognitive deterioration. While cognitive impairment is a major clinical feature, on its own it is a poor predictor of disease severity. Other manifestations of AD progression, such as the worsening of a patient's ability to perform day-to-day activities and behavioural disturbances are also important, resulting in a greater need for long-term institutional care and increased healthcare expenditureCitation[5–9]. Ignoring these symptoms can distort the consistency of the model because comorbid health conditions and the complexity of AD are insufficiently accounted for, and this impedes accurate assessment of the impact of an intervention.
More recent attempts to model disease progression in AD have attempted to address this limitation. For instance, some Markov models have defined severity states according to a Clinical Dementia Rating (CDR) scaleCitation[10],Citation[11], which is a global measure of patient functioningCitation[12] and locus of careCitation[13]. Other models have simulated a course of AD taking into account AD severity, residential setting, and patient's dependency statusCitation[14–17]. Together these approaches have provided a more comprehensive assessment of worsening of AD symptoms, by factoring in the degree of cognitive impairment using the Mini-Mental State Examination (MMSE), the ability to perform activities of daily living using the Alzheimer's Disease Cooperative Study Group – Activities of Daily Living (ADCS–ADL) scale, and the patient's care setting (community vs. institutions). However, despite the improvements in assessing disease progression with these models they are associated with several limitations. Firstly, the thresholds for defining health states by severity are often arbitrary. Secondly, the estimation of the model input parameters (e.g., transition probabilities) are based on a smaller sample size because of a greater number of model states. Both may increase uncertainty around the model outputs and conclusions.
An alternative approach to modelling AD progression and the cost-effectiveness of pharmacological treatments for AD is based on a patient's need for full-time care (FTC). FTC is defined in terms of the amount of supervision and care required by a patient on a daily basis, regardless of the locus of care and who the caregiver is. This framework was developed and implemented in 1997 using a cohort of patients in the US with mild-to-moderate AD. The estimation of time to FTC was based on multiple patient's characteristics, such as cognitive score as measured by the modified Mini Mental State Examination (mMMSE), presence of extrapyramidal and psychotic symptoms, duration of illness, and early onset of the diseaseCitation[18]. This work was further extended in 2001 by modelling the influence of timeCitation[19].
This approach captures the progression of AD without relying on arbitrary thresholds of clinical assessment, encompasses all management modalities for dependent patients, and has been widely used to assess the cost effectiveness of acetylcholinesterase inhibitors (AChEIs) in the treatment of patients with mild and moderate ADCitation[20–26].
However, there remain a number of limitations. For example, due to an absence of functional assessment of patients in the original cohort, the predictions of patients’ deterioration are made on two out of the three core clinical domains of AD (cognition and behavioural disturbances). However, patients’ functional level and, therefore, their degree of independence is a key feature of AD progression. In part, this can be explained by the limitations of the original study on which predictive equations were derived, since the majority of patients participating in the study had mild AD. The suitability of these equations for more advanced stages of AD, however, remains unclear.
This article presents an alternative predictive equation for determining the length of time required to FTC based on a more representative AD population and a more complete set of clinical assessments. It also investigates the potential influence of the speed of patients’ decline on the length of time required to FTC. The overall aim is to develop a predictive equation suitable for wider application in the treatment of AD.
Methods
Data source
The patient-level data required to develop the predictive equation were obtained from the London and South-East Region (LASER–AD) longitudinal epidemiological study which followed-up 224 patients with possible or probable AD over 4.5 yearsCitation[5]. Patients were selected on the basis of gender, residential setting, and severity of disease using independent quotas methods to representatively reflect the wider AD population. The patient population comprised mild (30%), moderate (40%) and severe (30%) levels of severity. The severity of AD was defined in terms of cognitive deficit using MMSE scores according to a standard definitionCitation[27]. Patients were assessed by trained personnel at baseline and after 6, 18, 30, 42 and 54 months. The assessments included all standard AD end-points for cognition, functioning, behavioural disturbances and global status including AD Assessment Scale–Cognitive subscale (ADAS–cog)Citation[28], the Severe Impairment Battery (SIB)Citation[29], Cooperative Study–Activities of Daily Living Scale (ADCS–ADL)Citation[30], Neuropsychiatric Inventory (NPI)Citation[31], Clinician Interview-Based Impression of Change with caregiver input (CIBIC–Plus)Citation[32],Citation[33], and the Rating Scale for Geriatric Patients (BGP) scaleCitation[34]. Data on utilisation of information on formal and informal services were collected using the Client Service Receipt Inventory (CSRI)Citation[35] as well as a recording of the care setting for each patient at each visit.
Defining a need for FTC in patients with AD
Building on the conceptual framework mentioned above and described in detail elsewhereCitation[18], here a need for full-time care, later referred to as FTC, was defined based on patient's dependency status and locus of care. The classification of dependency from the LASER–AD study identified three disability clusters: dependent, independent, and non-dependent but with instrumental functional disability according to the ADCS–ADL domains (basic activities, domestic activities and communication)Citation[5]. For the purpose of this analysis, patients who were dependent and/or institutionalised were considered to require FTC; non-dependent and non-institutionalised patients were assumed to be pre-FTC.
Estimating the observed transition from pre-FTC to FTC
Data for pre-FTC patients were used to assess the risk of requiring FTC over the 54-month period. The risk (i.e., hazard) of FTC at each time interval was described using the life-table methodCitation[36]. The time intervals were defined by the schedule of visits in the study, at 6, 18, 30, 42 and 54 months after baseline. Based on estimates of hazard of FTC, a proportion of patients remaining pre-FTC (for instance, staying alive, non-dependent or non-institutionalised) was calculated over time.
A life-table method is similar to the Kaplan–Meier approach, but is designed for discrete (grouped) data. Since the exact point in time when patients required FTC could not be continuously monitored between scheduled visits, assuming that FTC occurred at the time of assessment (as with Kaplan–Meier methods), a systematic overestimation of the length of time required for FTC would result. This error was avoided by only considering the time interval during which the transition to FTC occurred (without assuming an exact time for the event) using discrete (grouped) data methods.
Developing a basic predictive equation for time to FTC
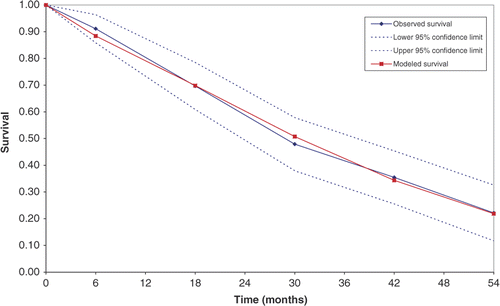
A basic equation was developed to approximate the observed data on a time vector. The complementary log–log specification (cloglog), a discrete time representation of the Cox continuous time proportional hazards model, was used to estimate whether each patient at each time interval required FTC This approach defines the proportion of patients remaining free of FTC (S), where j = time interval and p = hazard function.Different parametric specifications of function of time (in months) at the end of each time interval were tested in the cloglog equation. The observed data were used to test the relationship between time and hazard rate of FTC. Goodness-of-fit of the model was assessed by the Likelihood and the Wald tests associated with the time parameter. The best parameterisation of the time function was chosen using the Akaike information criteria (the smaller, the better) and the count RCitation[2] (the higher, the better), or in the case of nested models, the likelihood ratio test. The fit of data to the chosen model was also presented and examined graphically by plotting the predicted survivor function against the observed survivor function and its 95% confidence interval produced in the life-table analysisCitation[36].
Complementing the basic equation by adding baseline predictors
Baseline variables that could potentially affect time to FTC were individually tested in the basic equation. Variables that were significant in the univariate models were combined in a single model to select the best predictors using a stepwise selection algorithm. The goodness-of-fit of the modified equation was then tested in the same way as the basic equation.
The baseline variables analysed included: disease severity defined by MMSE score, cognition (ADAS–cog), functional disability (ADCS–ADL), and behavioural disturbances (total and sub-item scores of NPI), global evaluations (CIBIC–plus and BGP), dependency status, socio-demographic characteristics of the patient (age, gender, education, ethnicity, language, living arrangements, marital status, and country of birth) and caregiver (age, gender, living with the patient, relation to patient), AD background treatment (AChEI and antipsychotics), and medical history (coronary artery bypass graft, atrial fibrillation, cerebrovascular event, myocardial infarction, hypertension, diabetes, and vascular dementia), caregiver depression and anxiety level measured with the Hospital Anxiety and Depression Scale (HADS)Citation[37] and caregiver time spent for basic and instrumental tasks and providing supervision collected with Resource Utilisation in Dementia (RUD) questionnaireCitation[38].
Finalising the equation by adding predictors of disease progression
To finalise the equation, disease progression factors, such as decline in cognitive, functional and behavioural domains, were considered as possible predictors of time to FTC. These variables were analysed as monthly rates of change (slope) of the assessment scales, in keeping with regulatory and expert recommendationsCitation[39],Citation[40]. The same algorithm was applied to find the best predictors. The fit characteristics were compared with those of the basic and modified equations.
Examining the predictive equation for unobserved factors
Other unobserved factors can also affect progression to FTC in some individuals. This possibility (termed frailty) was assessed as:where u = log(v), X = identified predictive factors and v follows a gamma distribution. The impact of unobserved factors on the predictive equation was assessed by determining the percentage change in coefficients for each of the static and dynamic predictive parameters and by testing the significance of the heterogeneity term. All the analyses were conducted using SAS 9.1.3 and Stata.SE version 8.2.
Results
Patient population
The LASER cohort of 224 AD patients provided the basis for developing the predictive equation. From this cohort, 107 (47.8%) patients were classified as requiring FTC (dependent, institutionalised or both) at baseline and were therefore excluded from the analysis. A total of 33 (14.7%) patients were dependent, 23 (10.3%) patients institutionalised and 51 (22.8%) patients both dependent and institutionalised. Data for the remaining 117 pre-FTC patients, covering a 54-month follow-up period, were used for this analysis (). Mean (SD) age of the 117 patients was 79.8 (7.5) years. There were 36 men and 81 women; 83.5% of the group were white British. There was a mix of AD severity with 59.0% of the patients with mild AD, 36.8% with moderate AD and 4.3% with severe AD. Mean score on the ADAS–cog, ADCS–ADL and NPI scales (SD) at baseline were 27.2 (21.2), 52.3 (13.6) and 15.7 (12.8), respectively. In all, 72% of patients received treatment with AChEIs. Forty one (35.0%) patients died during the study period.
Table 1. Baseline characteristics of the cohort included in the analysis.
Observed transition from pre-FTC to FTC
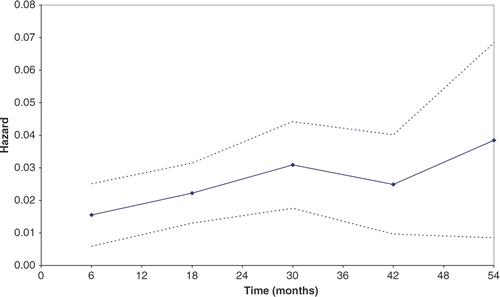
Of the 117 pre-FTC patients, 68 (58.1%) progressed to FTC during the 54-month follow-up period. A total of 38 (32.5%) patients became dependent, 13 (11.1%) patients were institutionalised and 17 (14.5%) patients became dependent and institutionalised during the same time interval. The hazard of FTC was observed to be an increasing function over time ().
The basic predictive equation
What are referred to as the increasing functions of time (the linear, logarithmic, exponential, square, cube, and square root functions) were tested in the cloglog model. The exponential and cubic functions were found to be insignificant. The predictive equation, using logarithm of time, can be considered a discrete-time analogue to the Weibull parameterisation of a continuous time model. The goodness-of-fit of this basic predictive equation to the observed data for the proportion of patients remaining free of FTC is shown in .
The modified predictive equation including baseline predictors
The potential predictors of time to FTC were further tested to improve the fit of the basic equation to the observed data. When entered individually in the cloglog model with logarithm of time, the following baseline covariates were identified as possible predictors of FTC: AD severity, dependence, ADAS–cog total score, ADCS–ADL total score, NPI total score and NPI domain scores for hallucination, apathy, reduced inhibition and motor behaviour, CIBIC–plus and BGP scores, caregiver living with the patient, caregiver HADS depression score and caregiver time spent for basic tasks. Assessing these static factors in a combined model showed that only the ADAS–cog and ADCS–ADL total scores, and the NPI hallucination score were significant predictors. Incorporating these predictive factors into the basic equation improved the fit to observed data (AIC 270.11) and increased the proportion of correctly predicted observations (RCitation[2] 79.4%).
The final predictive equation including disease-progression factors
To finalise the predictive equation, time-varying (dynamic) factors were tested. Four variables (rate of change of ADAS–cog total score, ADCS–ADL total score, and NPI agitation and euphoria domain scores) were identified as potential predictors of FTC when individually tested in the cloglog model with logarithm of time. Introducing these variables in a combined model with the static factors showed that only the rate of deterioration in ADAS–cog and ADCS–ADL scores remained significant (). Including predictive dynamic and static factors with time gave a hazard function, p, for the time interval, j, of:In the LASER–AD cohort, the assessments were made at 6 months and every 12 months thereafter, generating five intervals. This equation gave the best fit to the observed data (AIC 163.21) and increased the proportion of correctly predicted observations (RCitation[2] 88.2%), as summarised in .
Table 2. Static and dynamic factors included in the predictive equation.
Table 3. Fit characteristics during development of the predictive equation.
Unobserved factors
Testing for the impact of unobserved factors showed that no major predictor was omitted from the analysis. The addition of a gamma frailty component to the final predictive equation had negligible impact on the estimation of model coefficients: there was a 2.4% change in the estimation of NPI coefficient and <1% change in other coefficients. Heterogeneity was insignificant (p = 0.967).
Discussion
This article presents a predictive equation to predict time to FTC in patients suffering from AD. Measures of AD severity provide an objective summary of formal and informal care-related requirements. Thus, the clinical significance of treatment can be quantified in terms of reducing care requirements based on delaying time to FTC following AD treatment. The accuracy of predictions based on any mathematical model depends on a number of factors, such as content validity, reliability of employed data, and internal consistency of the model.
The predictive model presented here addresses some of the limitations of the original model by Stern et alCitation[18] and its successorCitation[19]. It is more consistent with the clinical course of AD and allows for the inclusion of evidence on all measurable clinical manifestations of AD. Similarly to the previous models, this equation takes into account patient's cognition disability and behavioural symptoms, yet it also includes a functional domain, which determines patient dependency status and thus a need for FTCCitation[5]. The present analysis shows that baseline cognitive impairment, functional disability and behaviour disturbances are found to be the main static predictors of time to FTC.
Despite being based on a patient cohort with predominantly mild AD, the previous models found behavioural symptoms to be a predictor of time to FTC. This may suggest the underlying patient population had rapidly declining ADCitation[41],Citation[42]. The model developed here is based on an epidemiologically representative sample of patients in order to widen its application to other settings. It identifies the speed of patient's deterioration through dynamic predictors and, by extension, reflects differences in disease progression. The analysis shows that speed of cognitive decline and functional impairment are independent predictors of time to FTC. This feature of the equation allows the evaluation of both symptomatic treatments and potential disease-modifying effects. Importantly, a linear decline in disease progression factors is assumed which may not necessarily reflect the natural history of the condition, yet fits with current recommendationsCitation[39], Citation[40]. The model shows excellent goodness-of-fit implying tenability of chosen mathematical functions in the yielded equation.
It is worth noting the difference in outcomes between this modelling exercise and the analysis conducted by Habermann et alCitation[43]. Although both analyses have been performed on the same database the results show some differences. For example, Habermann et al have found that factors, such as more severe cognitive impairment of patients, absence of a primary family carer, lower educational level of caregivers, and less time spent caring for patients by caregivers, are significant predictors of full-time institutionalisation. In contrast, although, the analysis here has confirmed the relationship between cognitive disability and need for full-time care, none of the caregiver-related characteristics are seen to be significant in final multivariate models.
This discrepancy can be explained by a number of factors. Firstly, the two analyses use different definitions for ‘outcome’ or ‘event’. Whilst Habermann et al defined 24-hour care as being institutionalised, the model here uses a combined measure of institutionalisation and dependency. The exploratory analysis demonstrates that institutionalisation may not necessarily imply dependency (and vice versa). During the 54-month follow-up period, the majority of the 68 (55.9%) FTC patients became dependent without being institutionalised with only 13 (19.1%) being institutionalised while still maintaining an independent status, and the remaining 17 (25.0%) becoming both dependent and institutionalised. As highlighted in this article, the predictor of dependency differs from institutionalisation.
Secondly, Habermann et al focused their analysis on identifying independent predictors of institutionalisation. Therefore, due to the high correlation between cognition (MMSE) and functional disability (ADCS–ADL), only one variable (MMSE) was chosen for the final model, allowing multicollinearity to be handled. In contrast, the research here aims to develop predictive models, and to ensure accuracy of predictions, all significant and/or relevant predictors are kept in the model. The fact that two correlated predictors are significant in the multivariate models suggests that each contains unique information not captured by the other, and ignoring one of the two in a predictive model would be an omission of relevant information.
The model developed here aims to provide the basis for the cost-effectiveness assessment of AD treatments with or without potential disease-modifying properties. Therefore a comprehensive evaluation of the AD symptoms is a prerequisite. The analysis considers dynamic evaluation of the disease (slope analysis), explores influence of individual NPI items, and its total score relies on more sensitive AD scales, e.g. ADAS–cog instead of MMSE.
The methodology used in this analysis facilitates extrapolation and provides a robust outcome with the required sensitivity assessments. Discrete methods to analyse the data and produce a predictive model are employed to avoid the overestimating of time to FTC. The model underwent extensive sensitivity analyses, which confirmed that no major predictors had been omitted from the equation. The equation also shows goodness-of-fit and robust predictive properties. External validation of yielded indices and the equation is best established by conducting similar modelling exercises using other longitudinal studies. However, presence of common predictors in the previous model may already be a good indicator. All the results have been reported with full parametric specification including uncertainty on parameters to permit the extrapolation beyond the observation period and enable the robustness of the cost-effectiveness model utilising this equation to be tested.
Despite a number of advantages to the equation presented here, its applicability to a specific setting needs to be carefully assessed. Firstly, the patient population being examined should have similar characteristics to the population used to derive the predictive model, e.g. severity of the disease, co-morbidities, mortality, treatment modalities and disease management. Therefore, the model could not be used to assess an effect of an AChEI on time to FTC, as any impact has already been captured in the equation. Nevertheless, it is suitable for any pre–FTC population, regardless of AD severity, and enables economic evaluation of therapies that are prescribed in addition to standard treatment (with or without AChEI).
Secondly, the accuracy of the prediction may be questioned if AD symptoms or treatment effects are assessed with scales which are different from those used in the equation. The data in the LASER study, however, were collected with a standard set of AD scales typically used in the clinical trials and, therefore, the predictive qualities of the equation are not affected here.
Lastly, this predictive model has been based on a relatively small patient population, 117 of pre-FTC patients, which may impact internal validity of the model. However, the method used in this analysis implies that information is obtained for each patient from each time interval, i.e. that analysis relies on a total of 320 observations. While the statistical technique was chosen to overcome such limitation, modelling time to FTC based on other patient cohorts, e.g. with longer follow-up, from different countries, different management patterns and larger patient population would be valuable contribution to the research efforts in this area as well as provide sought external validation of the current model. In addition, the statistical technique may be refined, if data allows. More accurate predictions will be obtained if the predictive equation is modelled on a shorter interval or employing continuous data instead of a discrete time modelisation as in this research. One should be aware of the data requirement for such analysis – more frequent assessment visit or the exact dates at which patients became FTC.
Conclusion
Evaluating the health benefits and economic consequences of pharmacological interventions for chronic illness is essential for effective and efficient healthcare systems. Empirical data are often unavailable because of time and resource constraints, which compound the difficulties in making informed and effective healthcare decisions. The predictive model presented here enables the effects of pharmacological interventions in AD to be identified and examined over a longer-term perspective. Notwithstanding some of the limitations of the model, predictions on all core domains of AD, regardless of patient's severity, can be made based on the data required for the model is easily available from clinical trials or observational studies as the predictions are based on standard AD scale scores, which are typically reported in the literature. The model is also suitable for evaluating both existing and emerging therapies, including those with potential disease-modifying effect.
Transparency
Declaration of funding: Lundbeck SAS provided financial support for data gathering assistance for LASER-AD study.
Declaration of financial/other relationships: B.R., M.G., J.C. and C.F. have disclosed that they are full time employees of Lundbeck SAS; C.L. has disclosed that she was contracted by Lundbeck SAS to devise the methodology and run the statistical analysis; C.K. has disclosed that he has received grant money from Lundbeck SAS for his role in this study and has also been in receipt of speaker honoraria and consultancy fees from Lundbeck SAS; G.L. has disclosed the she received from Lundbeck SAS the grant money and a gift to UCL for data gathering for this study; M.T. has disclosed that he was a full time employee of Lundbeck SAS at the time of this study. M.L. has disclosed that he has no relevant financial relationships.
References
- Stewart A, Phillips R, Dempsey G. Pharmacotherapy for people with Alzheimer's disease: a Markov-cycle evaluation of five years' therapy using donepezil. Int J Geriatr Psychiatry 1998; 13: 445–453
- Jönsson L, Lindgren P, Wimo A, et al. The cost-effectiveness of donepezil therapy in Swedish patients with Alzheimer's disease: a Markov model. Clin Ther 1999; 21: 1230–1240
- Teipel SJ, Ewers M, Reisig V, et al. Long-term cost-effectiveness of donepezil for the treatment of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci 2007; 257: 330–336
- Fenn P, Gray A. Estimating long-term cost savings from treatment of Alzheimer's disease. A modelling approach. Pharmacoeconomics 1999; 16: 165–174
- Livingston G, Katona C, Roch B, et al. A dependency model for patients with Alzheimer's disease: its validation and relationship to the costs of care – the LASER–AD Study. Curr Med Res Opin 2004; 20: 1007–1016
- Small GW, McDonnell DD, Brooks RL, et al. The impact of symptom severity on the cost of Alzheimer's disease. J Am Geriatr Soc. 2002; 50: 321–327
- Jönsson L, Jönhagen ME, Kilander L, et al. Determinants of costs of care for patients with Alzheimer's disease. Int J Geriatr Psychiatry 2006; 21: 449–459
- Rozzini L, Cornali C, Chilovi BV, et al. Predictors of institutionalization in demented patients discharged from a rehabilitation unit. J Am Med Dir Assoc 2006; 7: 345–349
- Zhu CW, Scarmeas N, Torgan R, et al. Clinical features associated with costs in early AD: baseline data from the Predictors Study. Neurology 2006; 66: 1021–1028
- Ikeda S, Yamada Y, Ikegami N. Economic evaluation of donepezil treatment for Alzheimer's disease in Japan. Dement Geriatr Cogn Disord 2002; 13: 33–39
- López-Bastida J, Hart W, García-Pérez L, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer's disease. J Alzheimers Dis 2009; 16: 399–407
- Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140: 566–572
- Neumann PJ, Hermann RC, Kuntz KM, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer's disease. Neurology 1999; 52: 1138–1145
- François C, Sintonen H, Sulkava R, et al. Cost effectiveness of memantine in moderately severe to severe Alzheimer's disease: a Markov model in Finland. Clin Drug Investig 2004; 24: 373–384
- Jones RW, McCrone P, Guilhaume C, et al. Cost effectiveness of memantine in Alzheimer's disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Aging 2004; 21: 607–620
- Jönsson L. Cost-effectiveness of memantine for moderate to severe Alzheimer's disease in Sweden. Am J Geriatr Pharmacother 2005; 3: 77–86
- Gagnon M, Rive B, Hux M, et al. Cost-effectiveness of memantine compared with standard care in moderate-to-severe Alzheimer disease in Canada. Can J Psychiatry 2007; 52: 519–526
- Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA 1997; 277: 806–812
- Caro JJ, Getsios D, Migliaccio-Walle K, et al. Assessment of health economics in Alzheimer's disease (AHEAD) based on need for full-time care. Neurology 2001; 57: 964–971
- Getsios D, Caro JJ, Caro G, et al. AHEAD Study Group. Assessment of health economics in Alzheimer's disease (AHEAD): galantamine treatment in Canada. Neurology 2001; 57: 972–978
- Migliaccio-Walle K, Getsios D, Caro JJ, et al. Economic evaluation of galantamine in the treatment of mild to moderate Alzheimer's disease in the United States. Clin Ther 2003; 25: 1806–1825
- Ward A, Caro JJ, Getsios D, et al. Assessment of health economics in Alzheimer's disease (AHEAD): treatment with galantamine in the UK. Int J Geriatr Psychiatry 2003; 18: 740–747
- Caro JJ, Salas M, Ward A, et al. Assessment of health economics in Alzheimer's disease. Economic analysis of galantamine, a cholinesterase inhibitor, in the treatment of patients with mild to moderate Alzheimer's disease in the Netherlands. Dement Geriatr Cogn Disord 2002; 14: 84–89
- Garfield FB, Getsios D, Caro JJ, et al. Assessment of Health Economics in Alzheimer's Disease (AHEAD): treatment with galantamine in Sweden. Pharmacoeconomics 2002; 20: 629–637
- Caro J, Salas M, Ward A, et al. Assessing the health and economic impact of galantamine treatment in patients with Alzheimer's disease in the health care systems of different countries. Drugs Aging 2004; 2: 677–686
- Green C. Modelling disease progression in Alzheimer's disease: a review of modelling methods used for cost-effectiveness analysis. Pharmacoeconomics 2007; 25: 735–750
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984; 141: 1356–1364
- Saxton J, Swihart AA. Neuropsychological assessment of the severely impaired elderly patient. Clin Geriatr Med 1989; 5: 531–543
- Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11(Suppl 2)S33–39
- Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–2314
- Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study – clinical global impression of change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11(Suppl 2)S22–32
- Knopman DS, Knapp MJ, Gracon SI, et al. The Clinician Interview-Based Impression (CIBI): a clinician's global change rating scale in Alzheimer's disease. Neurology 1994; 44: 2315–2321
- Diesfeldt HF. Observations on behavior, psychological study and 1-year survival in psychogeriatric patients. Gerontologie 1980; 11: 205–212
- Beecham J, Knapp MRJ. Costing psychiatric intervention. Measuring Mental Health Needs, GJ Thornicroft, CR Brewin, JK Wing. Gaskell/Royal College of Psychiatrists, London 1992
- Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data, 2nd. Wiley, New York 2002
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370
- Wimo A, Wetterholm AL, Mastey V, . Evaluation of the resource utilization and caregiver time in anti-dementia drug trials: a quantitative battery. Health Economics of Dementia, A Wimo, B Jönsson, G Karlsson, B Winblad, et al. John Wiley & Sons, London 1988; 465–99
- Guideline on medicinal products for the treatment of Alzheimer's disease and other dementias. Committee for Medicinal Products for Human Use (CHMP), 2008. London: doc. ref. cpmp/ewp/553/595 rev. 551.
- Vellas B, Coley N, Andrieu S. Disease modifying trials in Alzheimer's disease: perspectives for the future. J Alzheimer's Dis 2008; 15: 289–301
- Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol 2005; 62: 1601–1608
- Scarmeas N, Brandt J, Blacker D, et al. Disruptive behaviour as a predictor in Alzheimer's disease. Arch Neurol 2007; 64: 1755–1761
- Habermann S, Cooper C, Katona C, et al. Predictors of entering 24-h care for people with Alzheimer's disease: results from the LASER–AD study. Int J Geriatr Psychiatry 2009; 24: 1291–1298