Abstract
Objective: To critically evaluate published cost-effectiveness studies of novel drug products requiring less-frequent medication dosing compared to conventional formulations of the same drug substance.
Methods: A search was conducted in the Medline and Embase databases for cost-effectiveness studies published before May 2009 that compared two or more drug delivery technologies formulated with the same active drug substance. The Quality of Health Economic Studies (QHES) grading criteria for cost-effectiveness studies was applied to the selected publications.
Results: The literature search identified approximately 907 articles of which six cost-effectiveness studies met the inclusion criteria. The studies spanned four chronic conditions, were conducted from various international perspectives and used decision-analytic models to project economic outcomes. The base-case results of all six studies indicated that the drug product with sustained therapeutic efficacy was either more effective and less costly (‘dominant’) or more cost effective than the conventional formulation of the same drug substance. Quality scores ranging from 70 to 84 (scale 0 to 100) were assigned to the studies, with a mean of 78.
Limitations: This review likely did not capture all relevant drug delivery technologies and drug products. Only one reviewer critically evaluated the cost-effectiveness studies and independently assigned quality scores using the QHES grading criteria, which may be limited in its ability to identify poorly analyzed studies.
Conclusion: Evaluation of the published literature suggests that drug products with less-frequent medication dosing can be cost effective when compared to conventional formulations, but assessments are challenging because of complex relationships among therapeutic drug levels, dosing frequency, medication adherence, and health outcomes. Additional product-specific, comparative, pragmatic studies in this area are needed.
Introduction
Development of pharmaceutical products with improved pharmacokinetic and pharmacodynamic properties has helped to reduce the frequency of medication dosing for treatment of a variety of chronic diseases. Many of these products use drug delivery technologies that decrease or increase the release rate of the drug, or sustain the presence of active drug substance over timeCitation[1], and may be described by terms such as timed-release, controlled-release (CR), sustained-release (SR), delayed-release, extended-release (ER), prolonged-release, or long-acting (LA). Pegylation is also an example of a delivery technology that changes the physiological characteristics of a drug substance and prolongs its clinical responseCitation[2]. Enhanced drug delivery technologies may lead to improved health outcomes because of more consistent exposure to optimal therapeutic drug levels, as well as improved medication adherence because of decreased dosing frequencyCitation[1],Citation[3].
Many different drug delivery technologies may be applied to reduce dosing frequencyCitation[1]. Osmotically controlled oral drug delivery systems are commonly used, in which a soluble drug substance is encased in a polymeric, semi-permeable, rate-controlling membrane. As the drug product passes through the aqueous digestive tract, water causes the polymeric osmotic membranes to swell pushing active drug substance out of the delivery system through an optimally sized orifice. In addition to oral administration, implantable osmotic drug delivery systems have also been developed. Microsphere technology encapsulates active drug in polymeric microspheres. Upon administration of the drug via injection, the microspheres absorb water, leading them to swell and gradually release drug substanceCitation[4]. Pegylation involves the modification of a drug molecule to improve its pharmacokinetic and pharmacodynamic properties. The pegylation process entails attachment of polyethylene glycol units to a therapeutic molecule to create a molecular entity with extended activity compared to its unmodified formCitation[2]. These are only a few examples of drug delivery technologies that may reduce medication dosing frequency; this description is by no means comprehensive.
Although the safety and efficacy of these novel drug products have been documented in the published literature, their potential economic value has not been systematically studied. It is possible that sustained bioavailability and less-frequent medication dosing may be associated with improved clinical outcomes and healthcare cost savings. However, the relationships among therapeutic drug levels, dosing frequency, medication adherence, and clinical and economic outcomes can be complex. The overall objective of this study was to identify and critically evaluate cost-effectiveness studies in the published literature of drug products that require less-frequent medication dosing compared to alternate, more conventional formulations of the same drug substance. The specific goals of this study were to evaluate the economic impact of reduced dosing frequency, to assess the quality of the economic evidence, and to identify challenges in estimating the value of drug product re-formulation.
Methods
An initial broad systematic literature review was conducted to identify studies that assessed the value of reduced medication dosing frequency. For this preliminary review, only the Medline database was used to search for studies published in the English language between 1998 and 2008 that evaluated the impact of a change in dosing frequency on patient medication adherence. Diverse search terms (‘adherence,’ ‘compliance,’ or ‘persistence’ combined with ‘dose,’ ‘dosing,’ ‘dose frequency,’ or ‘dose administration schedule’ combined with ‘cost,’ ‘cost effectiveness,’ ‘burden,’ ‘health economics,’ or ‘drug cost’ combined with ‘quality of life,’ ‘patient preference,’ or ‘patient satisfaction’ in any combination) were used in an attempt to capture the largest number of potential studies. This initial broad literature search yielded a total of 168 hits of which 21 articles appeared to meet the study objective and were reviewed; 18 articles were original studies that spanned different chronic disease areas such as osteoporosis and hepatitis C, and three articles were systematic reviews. Based on the findings of the preliminary broad literature search, the search terms and inclusion criteria were modified to narrow the focus on original cost-effectiveness analyses of drug products with improved dosing, as described below. Drummond and colleagues describe cost-effectiveness analysis as a form of economic evaluation where both the costs (measured in monetary units) and consequences (measured in natural units) of treatments are examinedCitation[5]. Additionally, cost-effectiveness analysis was defined in this study using the definition established by the Panel on Cost-Effectiveness in Health and Medicine: ‘An analytic tool in which costs and effects of a program and at least one alternative are calculated and presented in a ratio of incremental cost to incremental effect’Citation[6].
A refined literature search was conducted in the Medline and Embase databases for cost-effectiveness studies published before May 2009 that compared two or more drug delivery technologies formulated with the same active drug substance. The search term ‘cost effective’ was combined with each of the following: ‘timed release,’ ‘controlled release,’ ‘sustained release,’ ‘delayed release,’ ‘extended release,’ ‘prolonged release,’ ‘long acting,’ and ‘pegylated.’ A separate search for each set of terms was performed in order to generate a manageable number of hits that could be more feasibly and accurately evaluated. Additional keyword searches were also explored, such as pairing ‘economic’ with each of the previous terms or with ‘once daily,’ ‘dose frequency,’ or ‘formulation change’ in an attempt to capture as many applicable studies as possible. The titles of all articles were reviewed and the abstracts of those likely to meet the inclusion criteria were evaluated. The inclusion criteria specified the following:
Studies must be published in the English language
Studies must be original cost-effectiveness analysis comparing two or more drug products formulated with the same active drug substance
The drug products being compared must use different delivery technologies requiring different dosing frequencies
Drug products must be indicated to treat a chronic disease or condition
The entire article must be available for review
The Quality of Health Economic Studies (QHES) grading system is a validated quantitative instrument developed by health economists to measure the perceived quality of health economic studiesCitation[7]. The grading system consists of 16 criteria which are described in . Each criterion is associated with a weighted score and the scores are summed for a maximum of 100 possible points for each study. One author (MMC) used the QHES grading system to evaluate the quality of cost-effectiveness studies that met the inclusion criteria. For each study, weighted scores were assigned in their entirety for each criterion that was perceived by the author to be satisfactory. Zero points were assigned to each criterion that was perceived to be unsatisfactory. Uncertainties in scoring were resolved through discussions with two of the other authors (DLV and LPG).
Table 1. QHES grading system for cost-effectiveness studies (Chiou et al., 2003)Citation[7].
Results
Literature search and selection
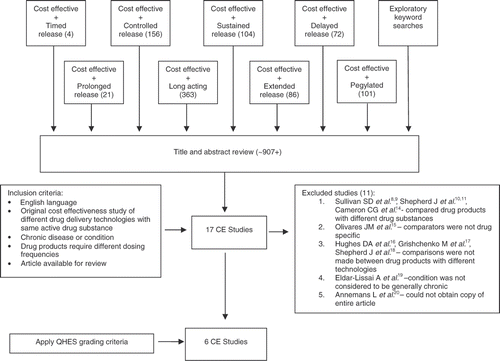
Approximately 907 studies were identified using the refined search strategy. provides a brief summary of results, presenting the number of hits from each keyword search in parentheses. After the titles and relevant abstracts from the search results were reviewed, 17 studies spanning six different chronic conditions were initially determined to meet the inclusion criteria.
After further evaluation, 11 of the 17 studies were excluded. Five studies compared products with different active drug substances, one study used a comparison group that was not drug specific, three studies did not conduct analysis between drug products that used different technologies, and one article could not be obtained for review. In detail, two studies by Sullivan et al.Citation[8],Citation[9] and two studies by Shepherd et al.Citation[10],Citation[11] that evaluated the clinical and cost effectiveness of pegylated interferon (PegIFN)-α with ribavirin compared to interferon (IFN)-α with ribavirin for treatment of hepatitis C virus (HCV) infection were excluded. The Sullivan et al.Citation[8],Citation[9] studies compared PegIFN–α 2a with IFN–α 2b and the Shepherd et al.Citation[10],Citation[11] studies used pooled data from systematic literature reviews in which clinical results from IFN–α 2a and 2b or PegIFN–α 2a and 2b were combined. Although there is evidence in the published literature that suggests PegIFN–α 2a and 2b regimens have similar efficacy and tolerability among patients with the same disease genotypeCitation[12], the drug molecules have different structural forms which leaves the potential for different pharmacokinetic and pharmacodynamic properties (i.e., the same active drug substance was not compared)Citation[13]. A study by Cameron et al.Citation[14] that evaluated the cost effectiveness of insulin analogues for diabetes mellitus was also excluded. Although insulin analogues can be used as substitutes for conventional insulins in the treatment of type 1 and type 2 diabetes, they are not the same active drug entities. A study by Olivares et al.Citation[15] which evaluated the cost effectiveness of long-acting injectable (LAI) risperidone compared to other antipsychotic formulations for the treatment of schizophrenia was excluded because the comparison group (‘previous antipsychotics’) was not drug specific. The individual drug substance formulations that were being compared to LAI risperidone could not be identified. A publication by Hughes et al.Citation[16] of extended-release (ER) oxybutynin and tolterodine was excluded because the ER formulations were compared against each other, whereas immediate-release (IR) oxybutynin was analyzed against no therapy. Similarly, a study by Grishchenko et al.Citation[17] that evaluated the cost effectiveness of PegIFN–α with ribavirin and a third economic evaluation by Shepherd et al.Citation[18] of PegIFN and IFN were excluded because both studies evaluated treatment strategies (e.g., treatment versus no treatment, or watchful waiting versus early treatment) for HCV infection and did not directly compare PegIFN and IFN treatments with each other. A study by Eldar–Lissai et al.Citation[19] that evaluated the cost effectiveness of using pegylated filgrastim compared to non-peyglated filgrastim for treatment of neutropenia due to cancer chemotherapy was excluded because this medical condition is considered to be generally acute. Additionally, an abstract by Annemans et al.Citation[20] on the cost effectiveness of PegIFN compared to standard IFN therapy for treatment of HCV infection was identified and reviewed. However, an electronic copy of this article could not be obtained from the journal and a copy of the original manuscript was unavailable from the corresponding author; this study was thus excluded from the analysis.
The final articles in this review included six cost-effectiveness studies and are described in . These studies were conducted from various international perspectives and spanned four chronic conditions: overactive bladder/urge incontinence, chronic hepatitis C, attention deficit hyperactivity disorder (ADHD), and schizophrenia. Although pegylation is a chemical modification and a variety of drugs could potentially be included in this general category, studies of pegylated interferon were included in this review because the drug substance modification was specifically intended to alter dosing frequency and multiple economic evaluations have been conducted on this topic. All of the studies included in this review used decision-analytic models to project economic and health outcomes over time. The base-case results of all six studies indicated that the drug product with sustained therapeutic efficacy was either dominant or more cost effective than the conventional formulation of the same drug substance. In cost-effectiveness analysis, an intervention is described as being dominant if it has greater effectiveness and lower costs than its comparatorCitation[5]. provides a summary of each cost-effectiveness analysis and describes study perspectives, data sources, primary outcome measures, and base-case incremental cost-effectiveness ratios (ICERs).
Table 2. Description of cost-effectiveness studies included in the review.
Table 3. Summary of methods and base-case results of studies included in the review.
Quality assessment
The results of the quality assessment are presented in . The weighted potential score for each grading criterion is shown in parentheses in the far left column. Certain criteria such as subgroup analysis was not applicable for all studies. In these circumstances, scores were assigned liberally and the entire value of the criterion was assigned to the study; the total scores represent the sum of values assigned with the liberal scoring method. Total scores were also calculated using a conservative scoring method where zero points were assigned to each criterion that was not applicable to the study. The conservative scores are presented in parentheses next to the total scores. The studies received scores ranging from 70 to 84, with a mean score of 78.
Table 4. Results of quality assessment using QHES grading systemCitation[6].
Overall, the quality of data inputs was satisfactory. All of the studies attempted to obtain the majority of clinical estimates and cost data from the published literature, including head-to-head randomized trials of the interventions, systematic reviews, and patient databases. However, two studies relied on expert opinion (clinician panels) for some clinical inputs such as probabilities of changing treatment (Guest et al.Citation[21]), proportions of non-responders, mean daily dosages and transition probabilities (Faber et al.Citation[25]), as well as resource utilization estimates (Guest et al.Citation[21], Faber et al.Citation[25]). In general, the quality assessment scores indicate that the studies were adequately analyzed and well-reported; however, several important shortcomings were recognized. Three studies did not satisfactorily present the structure of the decision-analytic model or did not describe the main assumptions of the model (Siebert et al.Citation[22], Buti et al.Citation[23], Simon et al.Citation[24]). One study did not present primary study results in ratios of incremental costs to incremental effects (ICERs) (Edwards et al.Citation[26]). Nearly all studies failed to discuss potential sources of bias in the data and data sources, and how bias could impact results (Guest et al.Citation[21], Buti et al.Citation[23], Simon et al.Citation[24], Faber et al.Citation[25], Edwards et al.Citation[26]). Additional reporting issues that reduced the quality scores included:
The authors did not address uncertainty in the decision-analytic model (Simon et al.Citation[24]).
The authors described the study perspective to be that of payers, but also estimated direct costs borne by patients (e.g., travel expenses) and indirect costs to society arising from lost productivity. A societal perspective would have been more appropriate given the additional costs and the authors’ description of the ‘substantial economic burden’ which overactive bladder places on society. (Guest et al.Citation[21]).
The study presented an overall conclusion implying more significant differences between treatment outcomes than what the results appeared to suggest. (Siebert et al.Citation[22]).
The source of funding was not stated (Buti et al.Citation[23]).
Discussion
A systematic literature review assessing the economic value of drug delivery technologies that reduce dosing frequency was conducted. Although several published studies evaluated the impact of drug delivery and dosing frequency on medication adherence, there was a relative lack of published economic analyses on this topic, especially cost-effectiveness studies comparing drug products with the same active drug substance. Among the six cost-effectiveness studies that were identified and reviewed, the base-case results indicated that the drug product with sustained therapeutic efficacy was either dominant or more cost effective than the conventional formulation of the same drug substance.
The average quality score among the six cost-effectiveness studies identified in the review was 78 on a scale of 0 to 100. Although this may appear to generally reflect good quality, important shortcomings were identified. Specifically, it is recommended that authors refrain from using expert opinion (e.g., clinician panels) for model inputs, particularly effectiveness estimates, and rely on data from well-designed clinical trials, observational studies, and large existing healthcare administrative databases whenever possible. This recommendation is in line with current cost-effectiveness analysis guidelinesCitation[6]. The results of this study suggest that drug products with less-frequent medication dosing can be cost effective when compared to conventional formulations, but the evidence base is relatively limited. Additional product-specific, head-to-head comparative studies are necessary to better understand the value of drug delivery technologies that reduce dosing frequency for the treatment of chronic conditions. It is recommended that these studies use a pragmatic design in order to generate real-world safety and effectiveness data that are more generalizable to healthcare settings and could be used to populate economic models. Pragmatic studies may better evaluate relationships between dosing frequency and medication adherence due to their emphasis on maximizing external validity and are more likely to measure health outcomes that are important to patients, such as improvements in quality of life.
Previous studies have addressed similar issues related to the value of reduced dosing frequency. Wertheimer and colleagues published a review describing novel drug delivery systems in different disease areas. Their review stated: ‘Although advanced formulations may be more expensive than conventional dosage forms, they often have a more favorable pharmacologic profile and can be cost-effective.’Citation[27] Shi et al.Citation[28] and Richter et al.Citation[29] conducted systematic literature reviews to evaluate the impact of dosing frequency on medication compliance and health outcomes. Both studies suggested that reductions in dose frequency through use of novel dosage forms may improve health outcomes and provide economic valueCitation[28],Citation[29]. Although these studies evaluated the impact of formulation changes with reduced dosing frequency, they did not specifically focus on economic assessments. HaeuslerCitation[30] performed a systematic literature review that evaluated how drug product formulation changes can potentially offer value to patients and society. In that study, only one example was described of a formulation change for an antidepressant (venlafaxine) from capsules to controlled-release tablets. Although this review had a similar objective to Haeusler's study, the evaluations differed in focus. Haeusler specifically evaluated how formulation changes impact pill burden, drug costs, and medication adherence while this study focused on the cost effectiveness of novel drug delivery technologies, including injectables, to reduce dosing frequency. Haeusler concluded that less frequently dosed formulations of established therapies could potentially offer clinical and pharmacoeconomic benefits to patients and society, and similar to this study, noted that additional research to generate evidence is necessaryCitation[30].
It is challenging to estimate the potential value of novel drug delivery technologies due to differences between chronic diseases and the complex relationships among therapeutic drug levels, dosing frequency, medication adherence, and clinical and economic outcomes. The level and quality of evidence demonstrated in this study indicate that drug delivery technologies with less-frequent dosing can potentially provide valuable benefits in the treatment of chronic diseases and may be cost-effective. However, it is also important to recognize the potential safety concerns associated with sustained pharmacological activity such as different incidences of adverse events, or increased risk of liver injury due to accumulation of active metabolites or prolonged drug exposureCitation[31]. There is also concern that the consequences of a missed or late dose of a long-acting drug will be magnified due to the extended amount of time (longer interval) between dosage administrationsCitation[32]. The impact of these risks on health outcomes will vary for different drug formulations and for different chronic conditions.
The opportunities and challenges of assessing the economic value of drug delivery technologies can be exemplified by closer examination of two cases: schizophrenia and chronic hepatitis C. Schizophrenia is a debilitating psychiatric disorder generally associated with poor treatment outcomes and high relapse rates attributed to frequent therapy non-adherenceCitation[33],Citation[34]. The conventional immediate-release (IR) oral risperidone formulation requires twice or three times daily administration. The long-acting injectable (LAI) formulation (available in 2003) requires administration once every 2 weeks. Two double-blind, randomized phase III trials evaluated the safety and efficacy of LAI risperidoneCitation[33]. In these studies, the therapeutic efficacy of LAI risperidone was not inferior compared to IR risperidone, measured by the Positive and Negative Syndrome Scale (PANSS). Both formulations were well-tolerated, and had similar safety profiles and discontinuation ratesCitation[35],Citation[36]. Despite these similarities in clinical efficacy, the published literature indicates that LAI risperidone is associated with fewer relapses (duration and frequency), reduced hospitalizations, and lower total per patient medical resource utilization costsCitation[26],Citation[33],Citation[34]. This evidence strongly suggests that long-acting delivery technology adds clinical and economic value to schizophrenia treatment strategies. Although definitive evidence was not identified in the published literature to explain why reduced dosing frequency appears to improve clinical outcomes among schizophrenic patients, it is hypothesized that sustained therapeutic activity reduces the number of ‘opportunities’ for a patient to miss or delay medication dosing. It is likely that there is no actual change in patient medication adherence defined as the extent to which a patient becomes more or less compliant with a prescribed medication regimen. Instead, LAI risperidone may be associated with better clinical outcomes due to its ability to prolong the interval between each dose, thereby reducing the number of potential relapses within a patient population challenged by frequent medication non-adherenceCitation[33],Citation[34].
Similar opportunities and challenges in assessing the economic value of drug delivery were encountered in the treatment of chronic hepatitis C virus (HCV) infection, which is one of the main causes of chronic liver disease and the most common indication for liver transplantationCitation[37]. A standard interferon (IFN) treatment regimen for HCV involves a subcutaneous injection three times per week over 48 weeks. IFN requires frequent dosing due to its half-life of approximately 8 hours. Pegylation technology was identified as a way to sustain the bioavailability of IFN over a longer period of time. Pegylated interferon (PegIFN) has sustained absorption, a slower rate of clearance, and a longer half-life than its unmodified formCitation[38]. The improved pharmacokinetic and pharmacodynamic properties of PegIFN allow for once-weekly dose administration for the treatment of HCV. Three economic evaluations of treatment regimens for hepatitis C were reviewed to assess the potential economic implications of sustained delivery. All of the studies used efficacy data from randomized controlled trials in Markov modeling frameworks to analyze the cost effectiveness of PegIFN compared with the non-pegylated form (with or without ribavirin)Citation[22–24]. The clinical trial data indicate that a PegIFN treatment regimen has better clinical efficacy than an IFN treatment regimen, measured as the percentage of patients who achieve sustained virologic response (SVR) post-treatment and by biochemical response rates. The trials also showed that both treatment regimens yield similar adverse effects and have similar safety profilesCitation[37],Citation[39],Citation[40]. The economic studies indicated that PegIFN was more cost effective than IFN regimens, due in part to reduced liver complications associated with PegIFNCitation[22],Citation[23]. Although two economic evaluations conducted by Shepherd et al.Citation[10],Citation[11] were excluded from this review due to their use of pooled clinical data for PegIFN–α and IFN 2a and 2b, those results were consistent with the above and showed that PegIFN treatment regimens yield higher SVR rates and are cost effective (£12,123/QALY) when compared to IFN treatment regimensCitation[10],Citation[11]. The improved clinical efficacy (higher SVR rates) of PegIFN may largely be attributed to the sustained biological activity of the interferon molecule (prolonged exposure of HCV to drug), but it is hypothesized that improvements could also potentially be attributed to better medication adherence, although this may be less likely in a randomized controlled trial setting. Studies that specifically evaluated the impact of reduced dosing frequency on therapy adherence in treatment of chronic HCV infection could not be identified in the published literature.
Several important limitations to this literature review were recognized. Given the numerous keywords that could have been used to search for potential studies of many different drug delivery technologies, this review likely did not capture all relevant articles. However, this study started with broad search criteria that eventually became more focused; this methodology allowed screening of as many potential studies as possible. Second, only one reviewer critically evaluated the final list of studies and independently assigned quality scores. Although the reviewer attempted to be consistent in the scoring technique, the grading system entails some subjectivity. Overall, the total scores may indicate higher quality than each study actually presented due to the manner in which Chiou and colleaguesCitation[7] stated certain quality criteria. Rather than evaluate how well the authors fulfilled each criterion, the QHES grading system evaluated many of the domains in a ‘check-list’ manner that identified whether or not a specific criterion was present. As a result of this design, the grading system is significantly limited in its ability to identify poorly analyzed or poorly presented studies. Also, Chiou and colleaguesCitation[7] did not provide benchmarks or thresholds for total scores, which further limit the grading system in its ability to quantitatively categorize and distinguish high-quality studies from low-quality studies. Finally, the results of this evidence assessment cannot be generalized to all studies of novel drug delivery technologies because the potential economic value of reduced dosing frequency is disease and product-specific. Additional data derived from well-conducted studies are necessary in order to further establish the evidence base.
Conclusion
Evaluation of the relatively limited published literature suggests that drug products with less-frequent medication dosing may be cost effective when compared to alternate conventional formulations. However, assessing the economic benefits of drug products with improved dosing is challenging because the relationships among therapeutic drug levels, dosing frequency, medication adherence, and health outcomes are complex and vary among different diseases. Additional product-specific, comparative, pragmatic studies are necessary in order to identify the clinical and economic impact of drug re-formulation and to estimate the value of drug delivery technologies that reduce dosing frequency in treatment of chronic conditions.
Transparency
Declaration of funding: This study was funded by Amylin Pharmaceuticals, Inc.
Declaration of financial/other relationships: MC, RA, LG, and DV have disclosed that they have served as consultants to Amylin Pharmaceuticals, Inc. JB has disclosed that she is an employee of Amylin Pharmaceuticals. DB has disclosed that he is an employee of Eli Lilly and Company.
References
- Rathbone MJ, Hadgraft J, Roberts MS, et al. Modified-Release Drug Delivery Technology, 2nd. Informa Healthcare USA, New York 2008; 2
- Bailon P, Won CY. PEG-modified biopharmaceuticals. Expert Opin Drug Deliv 2009; 6: 1–16
- Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care 2009; 15: e22–33
- “Fact Sheet Medisorb® Microspheres Technology” Accessed June 30, 2009: http://www.alkermes.com/media/42408/general%20medisorb%20techonology%20fact%20sheet%20jan%202009%20-%20011409%20.pdf
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes, 3rd. Oxford University Press, New York 2005
- Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in Health and Medicine. Oxford University Press, New York 1996
- Chiou CF, Hay JW, Wallace JF, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care 2003; 41: 32–44
- Sullivan SD, Craxi A, Alberti A, et al. Cost effectiveness of peginterferon alpha-2a plus ribavirin versus interferon alpha-2b plus ribavirin as initial therapy for treatment-naïve chronic hepatitis C. Pharmacoeconomics 2004; 22: 257–265
- Sullivan SD, Jensen DM, Bernstein DE, et al. Cost-effectiveness of combination peginterferon alpha–2a and ribavirin compared with interferon alpha–2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99: 1490–1496
- Shepherd J, Brodin H, Cave C, et al. Pegylated interferon alpha–2a and 2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2004; 8: 1–125, iii–iv
- Shepherd J, Brodin HF, Cave CB, et al. Clinical and cost-effectiveness of pegylated interferon alfa in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Int J Technol Assess Health Care 2005; 21: 47–54
- McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361: 580–593
- Pedder SCJ. Pegylation of interferon alfa: structural and pharmacokinetic properties. Semin Liver Dis 2003; 23(Suppl 1)19–22
- Cameron CG, Bennett HA. Cost-effectiveness of insulin analogues for diabetes mellitus. CMAJ 2009; 180: 400–407
- Olivares JM, Rodriguez-Martinez A, Burón JA, et al. Cost-effectiveness analysis of switching antipsychotic medication to long-acting injectable risperidone in patients with schizophrenia: a 12 and 24-month follow-up from the e-STAR database in Spain. Appl Health Econ Health Policy 2008; 6: 41–53
- Hughes DA, Dubois D. Cost-effectiveness analysis of extended-release formulations of oxybutynin and tolterodine for the management of urge incontinence. Pharmacoeconomics 2004; 22: 1047–1059
- Grishchenko M, Grieve RD, Sweeting MJ, et al. Cost-effectiveness of pegylated interferon and ribavirin for patients with chronic hepatitis C treated in routine clinical practice. Int J Technol Assess Health Care 2009; 25: 171–180
- Shepherd J, Jones J, Hartwell D, et al. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2007; 11: 1–205, iii
- Eldar-Lissai A, Cosler LE, Culakova E, et al. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health 2008; 11: 172–179
- Annemans L, Warie H, Nechelput M, et al. A health economic model to assess the long term effects and cost-effectiveness of PEG IFN alpha-2a in hepatitis C virus infected patients. Acta Gastroenterol Belg 2004; 67: 1–8
- Guest JF, Abegunde D, Ruiz FJ. Cost effectiveness of controlled-release oxybutynin compared with immediate-release oxybutynin and tolterodine in the treatment of overactive bladder in the UK, France and Austria. Clin Drug Invest 2004; 24: 305–321
- Siebert U, Sroczynski G, Rossol S, et al. Cost effectiveness of peginterferon α–2b plus ribavirin versus interferon α–2b plus ribavirin for initial treatment of chronic hepatitis C. Gut 2003; 52: 425–432
- Buti M, Medina M, Casado MA, et al. A cost-effectiveness analysis of peginterferon alfa–2b plus ribavirin for the treatment of naïve patients with chronic hepatitis C. Aliment Pharmacol Ther 2003; 17: 687–694
- Simon K, Gladysz A, Rotter K, et al. Cost effectiveness of replacing recombinated interferon α–2b with its pegylated form in combination with ribavirin for the therapy of chronic HCV infection in Poland. Adv Clin Exp Med 2006; 15: 453–462
- Faber A, van Agthoven M, Kalverdijk LJ, et al. Long-acting methylphenidate – OROS in youths with attention-deficit hyperactivity disorder suboptimally controlled with immediate-release methylphenidate: a study of cost effectiveness in The Netherlands. CNS Drugs 2008; 22: 157–170
- Edwards NC, Locklear JC, Rupnow MFT, et al. Cost effectiveness of long-acting risperidone injection versus alternative antipsychotic agents in patients with schizophrenia in the USA. Pharmacoeconomics 2005; 23(Suppl 1)75–89
- Wertheimer AI, Santella TM, Finestone AJ, et al. Drug delivery systems improve pharmaceutical profile and facilitate medication adherence. Adv Ther 2005; 22: 559–577
- Shi L, Hodges M, Yurgin N, et al. Impact of dose frequency on compliance and health outcomes: a literature review (1966-2006). Expert Rev Pharmacoecon Outcomes Res 2007; 7: 1–16
- Richter A, Anton SF, Koch P, et al. The impact of reducing dose frequency on health outcomes. Clin Ther 2003; 25: 2307–2335
- Haeusler JMC. Change in formulation and its potential clinical and pharmacoeconomic value: example of extended release venlafaxine. Curr Med Res Opin 2009; 25: 1089–1094
- Lemke TL, Williams DA, Roche VF, et al. Foye's Principles of Medicinal Chemistry, 6th. Lippincott, Williams & Wilkins, Baltimore 2007
- Hughes D. Less is more: medicines that require less frequent administration improve adherence, but are they better?. Pharmacoeconomics 2006; 24: 211–213
- Möller HJ. Long-acting injectable risperidone for the treatment of schizophrenia. Drugs 2007; 67: 1541–1566
- Taylor M, Currie A, Lloyd K, et al. Impact of risperidone long acting injection on resource utilization in psychiatric secondary care. J Psychopharmacol 2008; 22: 128–131
- Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003; 160: 1125–1132
- Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005; 15: 111–117
- Manns MP, McHutchison JG, Gordon SF, et al. Peginterferon alfa–2b plus ribavirin compared with interferon alfa–2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet 2001; 358: 958–965
- Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa–2a in patients with chronic hepatitis C. N Engl J Med 2000; 343: 1666–1672
- Lindsay KL, Trepo C, Heintges T, et al. A randomized, double-blind trial comparing pegylated interferon alfa–2b to interferon alfa–2b as initial treatment for chronic hepatitis C. Hepatology 2001; 34: 395–403
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa–2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–982