Abstract
Objectives: the study aims to estimate the clinical-impact and cost-effectiveness value of adding human papillomavirus 16/18 vaccination against cervical cancer among women currently undergoing organised screening in Finland.
Methods: A Markov cohort model evaluating high-risk HPV infections and cervical cancer (CC) cases combined with screening has been customised to the Finnish setting. The model outcome for a cohort of 30,000 girls aged 10 years was calibrated to age-specific annual number of Pap smears, CC incidence and mortality.
Results: The observed age-specific incidence and mortality rates of CC closely match the data replicated by the model. The model predicts that with a 90% vaccine coverage rate, CC cases and mortality would be reduced by 70%. In the base-case analysis with a discount rate of 3% the incremental cost per quality-adjusted life-years (QALY) gained, from a healthcare perspective, was €17,294. Without discounting this value is €2,591/QALY gained.
Conclusions: The analysis suggests that implementing prophylactic CC vaccination within the current screening system would substantially reduce CC cases and deaths, as well as the overall disease burden expressed in pre-cancer lesions averted. Vaccination could be a cost-effective intervention in Finland despite the fact that the number of CC cases and deaths are currently relatively low. Conservative estimates of the cost effectiveness of the vaccination were provided since it was not possible to assess herd protection induced by vaccination using this Markov model.
Introduction
It is well-documented that cervical cancer (CC) is a major burden worldwideCitation[1]. The highest level of disease burden is however observed in the less-developed countries that do not have any screening programmes in place.
In countries with an organised screening programme the incidence of the disease has been dramatically reducedCitation[2]; however screening programmes in general focuses on secondary prevention which includes treatment interventions following detection of early stages of disease development. Finland has been one of the most successful countries in the world for implementing a population-based organised CC screening system which has led to a steep decrease in CC burden over time. Since its implementation in the 1960s, both CC incidence and mortality rates have been reduced by 80% to a current annual low level of 150–170 CC cases and 50–60 CC deaths for an eligible target population of 1.2 million womenCitation[3]. Despite recent evidence of an increase in CC incidence, the CC rates in Finland are still strikingly low (4 per 100,000 women per year (adjusted rate to the world standard population structure)Citation[3].
It has now been well-established that CC is caused by the human papillomavirus (HPV)Citation[4]. However, the necessity of having an oncogenic (high-risk) HPV infection for acquiring cancer now provides the opportunity of reducing the disease burden by means of primary prevention or prophylactic vaccination in addition to secondary prophylaxisCitation[5],Citation[6]. Two HPV types, HPV 16 and 18, account for at least 70% of the CC globallyCitation[7]. The first prophylactic vaccine (Gardasil) against these HPV types was introduced into the market in 2006. It is a quadrivalent vaccine, active against HPV types 6, 11, 16 and 18). The second vaccine (Cervarix) which appeared 1 year later in 2007 is a bivalent vaccine against HPV types 16 and 18).
Some public health authorities (e.g., USA, UK, Australia, Canada, Switzerland, Denmark, Italy) have immediately recommended the vaccination of girls and women for the prevention of HPV-related diseases, but among these some policymakers and payers requested more information on the economics of implementing HPV vaccination such as Canada, Australia and UK. Finland is also a country where health authorities require a more in-depth economic assessment of each new healthcare intervention (i.e., vaccine) brought into their market before a decision for universal mass vaccination is given.
In the present study a Markov-based cohort model was used to investigate the clinical and economic consequences of adding prophylactic vaccination against CC to the existing screening programme in Finland. The analysis was designed to answer two questions: (1) What is the clinical impact of introducing a prophylactic HPV 16/18 vaccine into the CC disease management assuming no change in the current screening approach? (2) What is the incremental cost-effectiveness value when implementing prophylactic HPV vaccination in Finland within the currently organised screening programme that results in relatively few remaining CC cases and deaths?
Methods
Model structure
A Markov process cohort model was selected as a modelling tool to simulate over lifetime the development of CC disease using Microsoft Excel softwareCitation[8]. The cycle length of evaluation in the model was 1 year and the total evaluation period was from the age of 10 to 106 years, therefore including 96 cycles. The model was adapted to the Finnish setting by applying Finland-specific epidemiological, screening and treatment practice data. Cost information was assessed through literature review and expert opinion.
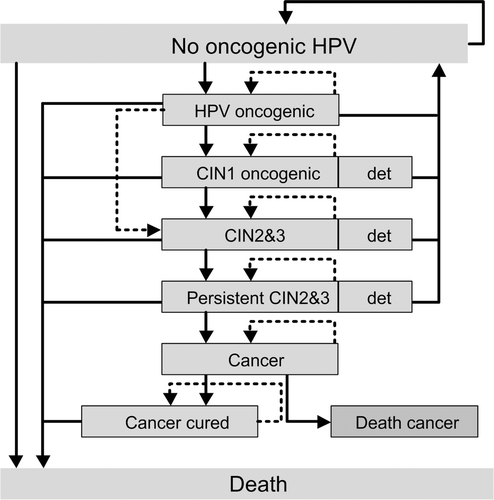
The model () was built up in three integrated parts: the natural history of HPV-infection leading to pre-cancer lesion, cancer cases and specific death; screening that allows the detection and treatment of pre-cancer lesions; and vaccination that reduces type-specific HPV-infection over time. Screening in the model can be either organised (i.e., over specific intervals and age-ranges), or opportunistic (i.e., at a specific age), or a combination of both.
Natural history of HPV infection
Most oncogenic HPV infections regress spontaneously but some will become persistent and go on to develop cervical cancer. However no complete data on the natural history of HPV infection in Finland exists and therefore the authors had to use a proxi data from the literature which were then discussed with local experts. Transition probabilities between the different disease stages were used in the model based on a literature review which extended selection criteria to studies from all countries ().
Table 1. Transition probabilities.
CC screening in Finland
The Finnish CC screening programme is organised nationwide targeting women aged 30–60 years. The estimated total target population is 1.2 million (in some municipalities the age range is from 25 to 65 years). The municipalities are responsible for the costs and the practical implementing of the screening programme. The normal screening interval is every 5 years, but it can be intensified to every year for at-risk groups (i.e., benign findings or suspected mild HPV infection) and screening-positive women or women with symptomsCitation[23]. Women of the target age range are identified by the national population registry and are invited by letter to attend a cervical screening programme free of charge. Overall, around 70% of the invited women will attend the screening, but in the 25–35-year age group the participation rate is lower: 50–60% (). Every year around 170,000 Papanicolaou (Pap) smears are taken during this mass screening programmeCitation[24].
Table 2. Organised cervical cancer mass screening programme in Finland 2005Citation[24].
Screening is based on traditional Pap screening but HPV tests are now carried out but not used in normal screening patterns. Around one in every 100 women screened is sent for further evaluation and 4–9% will have abnormalities that need a follow-up. The screening programme therefore detects around 600 precancerous lesions annually which is between three and five cases in every 1000 women screened. One CC case is detected in every 10,000 screened women.
Only one-third (170,000/520,000) of all the annual Pap smears taken in Finland are from the organised mass screening programmeCitation[25]. The remaining two-thirds (350,000/520,000) are opportunistic/spontaneous screening Paps that potentially affect the effectiveness results of the mass screening programme. However, the data from the opportunistic screening are included into the model as they induce an important additional cost even though there is some evidence that the substantial decrease in CC incidence and mortality in Finland is mainly caused by the organised mass screening programmeCitation[26]. Pap smears taken from this opportunistic screening are not registered. Therefore the age-specific distribution of the organised screening programme is used to extrapolate the age distributions of the opportunistic/spontaneous screening. This widens the total targeted population for screening from 1.2 to 2.1 million eligible women per year (women aged 17–65 years) ().
Table 3. Model input data.
Vaccination
The base-case analysis considers vaccine efficacy against HPV 16/18 infection as well as cross-protection against other oncogenic HPV types (e.g., HPV 31, 45 and 52)Citation[27]. All these HPV types together are responsible for more than 80% of the CC cases globally and presumably this is also the case for FinlandCitation[28]. The vaccine efficacy in preventing HPV infection against 16/18 is assumed to be 95%, and 27% for cross-protection against the other oncogenic HPV types. The overall vaccine efficacy against HPV infection in the model is therefore estimated at 77% and determined by taking the proportion of HPV 16/18 in CC (74%) multiplied by the vaccine efficacy (95%) plus the proportion of other oncogenic types (26%) multiplied by the vaccine efficacy (27%) against these types. The same approach was applied to cervical intraepithelial neoplasia (CIN) 1 and CIN 2/3 ().
Model calibration
Calibration of the model is an essential part of model development. In this case manual adaptation of the transition probabilities was used. The model is calibrated by matching the epidemiological numbers (i.e., CC incidence and mortality) estimated by the model with observed data. The initial model structure is based on the natural history of HPV infection and has been calibrated in other settings as well. Calibration and validation are important here as a proxi data from other countries were used in the absence of any Finland-specific data on the natural history of HPV. Finnish country-specific data (e.g., overall age-specific death rate) were retrieved from Finnish databases and the relevant literature (e.g., Statistics FinlandCitation[36] etc.).
Cost data and utilities
Cost data were collected from the official Finnish unit cost reportCitation[37]. This report presents the average production costs for the year 2006 of different healthcare production sectors such as primary healthcare or specialised healthcare. In addition, the official price list of two university hospitals is usedCitation[38],Citation[39]. Cost data include the cost of screening, diagnosis, treatment and follow-up of HPV-related diseases as well as vaccination costs and utility scores per specific disease stage (CIN 1, CIN 2/3, cancer) selected for the model are presented in . The analysis is performed from the perspective of the healthcare payer system.
Table 4. Cost and utility data.
Analysis
The base-case analysis compares an unvaccinated population undergoing the current cervical cancer screening programme with a population receiving screening and an HPV 16/18 vaccination at the age of 11 years. The vaccination coverage rate was set at 90%, which corresponds to the typical paediatric vaccination coverage in FinlandCitation[44]. Clinical events (i.e., precancerous lesions, CC cases and deaths) were derived from the model for both vaccinated and unvaccinated populations (single cohort of girls n = 30,000) from age 10 years until death. The cohort size was derived from the average annual number of females born over the past 35 years in Finland between 1970 and 200536.
In addition it was assumed that adolescents would receive three doses of the vaccine and would be fully immunised after 1 year, and that vaccine efficacy would not wane over time. Results from the extended phase of a clinical trial indicate that waning is not expected in the short term (e.g., up to 15 years)Citation[45].
The outcome measures of the model included the accumulated number of precancerous lesions, CC cases and deaths avoided and life-years (LY) and quality-adjusted life-years (QALY) gained for one age cohort analysed over a lifetime horizon with the vaccines and screening compared with screening alone. The accumulated total cost difference and the number of LYs and QALYs gained per woman, expressed as the incremental cost-effectiveness ratio (ICER), are also reported. Results are presented with 0% and 3% discount rates according to the latest guidelines in FinlandCitation[46]. The discount rate converts values that will occur in the future to their present values.
Sensitivity analyses
Deterministic one-way sensitivity analyses were performed on the screening parameters (i.e., total annual Pap smears, screening patterns, Pap test sensitivity for detecting CIN 1 and CIN 2–3) and costs. Gamma distribution was used for costs and beta distribution for utility valuesCitation[47]. Probabilistic sensitivity analysis was conducted with the simulation run over a 1000 iterations by using @RISK software (Palisade)Citation[48]. The end-result was an acceptability curve in which the level of cost effectiveness is defined as a function of the cumulative probability rate that varies between zero and one.
Results
Model validation
The observed age group-specific CC incidence and death rates (averages from 1991 to 2005) closely match the model output values (, ). Overall, predicted and observed CC incidence and mortality rate (adjusted for world standard population age-structure per 100,000 women) coincide well: model predicted CC incidence of 3.84 vs. observed 3.87; model predicted mortality rate of 1.37 vs. observed 1.22, respectively). Averages and adjusted numbers are used as actual numbers – these were quite low and there was deviation between years. Slight deviations between age groups were also observed. True numbers in each age group were low, and total cancer death rate and age-specific correlations were in line with the observed data. It would have been possible to get the model to generate exact numbers (e.g., CC incidence) for the time period in question, but reported CC incidence and mortality are an average over 15 years and the environment (i.e., HPV types, CC incidence and prevalence) is changing constantly, and as the primary target of this modelling is to predict future changes, the difference between predicted and observed numbers was thought to be acceptable.
Figure 2. (a) Comparison between model-predicted and observed data for age-specific cervical cancer incidence and (b) cervical cancer mortality.
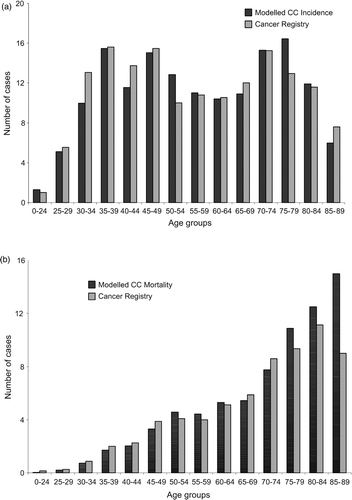
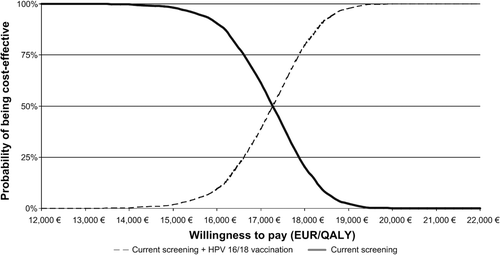
Figure 3. Results of probabilistic sensitivity analysis expressed as cost-acceptability curves, for screening plus vaccination and screening alone.
The model predicts lifetime numbers of pre-cancerous lesions for the total cohort at 15,496 (CIN 1) and 2,088 (CIN 2–3). These numbers cannot be compared with observed data as pre-cancerous lesions are not registered (except carcinoma in situ). Moreover, presumably many CIN 1 lesions go undiscovered because of their propensity towards relatively rapid regressionCitation[49],Citation[50].
Health outcomes of vaccination
Vaccinating one birth cohort of girls with a coverage rate of 90% at the age of 11 years is expected to reduce the predicted lifetime number of CC cases and deaths by 71 and 73%, respectively, compared with screening alone. In addition, the model also predicts that vaccination would result in a substantial reduction in high-grade lesions (i.e., CIN 2/3) across all ages ().
Table 5. Effect of vaccination on clinical endpoints.
Cost effectiveness of vaccination
The base-case analysis with cross-protection shows that the incremental cost per QALY gained would be €2,592 (undiscounted) and €17,294 for a 3% discount rate of costs and effect ().
Table 6. Cost-effectiveness results (cohort of 30,000 girls; lifetime horizon).
Sensitivity analysis
Deterministic one-way sensitivity analysis performed on the screening parameters showed that the sensitivity level of detecting CIN 2/3 has the greatest effect on the screening results (i.e., number of CIN and CC). The total number of annual Pap smears taken was also analysed but was not included in the sensitivity analysis as the base-case assumption precisely duplicated the observed incidence and mortality rates. The second deterministic analysis (including cost parameters) showed that changes in discount rates and vaccine costs had by far the greatest impact on the cost-effectiveness ratio.
The overall results from probabilistic sensitivity analysis are expressed as cost-effectiveness acceptability curves ().
Discussion
Results of the cost-effectiveness model developed for Finland indicate that adding HPV vaccination to the current screening programme would result in a significant reduction in CC cases and deaths to 71 and 73%, respectively. This management strategy is moreover very cost-effective compared with screening alone despite the very low number of cervical cancer cases and deaths observed (ICER = €17,294/QALY gained (annual discount rate of 3%).
To perform this economic analysis the model must first be calibrated against Finnish HPV-related prospective screening data, CC incidence and mortality data. The estimate for total annual Pap smears taken may be more precise than in other studies, but a more accurate estimate is deemed necessary because of the low disease burden of CC cases and deaths in Finland. Combining data from the Finnish CC mass screening programme with estimates from the opportunistic Pap smears result in an annual number of treated moderate and high-risk precancerous lesions (CIN 2/3) of around 2000–2500 cases in FinlandCitation[23]. The number of treated cervical pre-cancer lesions (CIN 2/3) predicted by the model is 2,088 which is within the range of the observed real-life situation. The model predicts that adding vaccination to screening would result in a 58% reduction in CIN 2/3 cases compared with screening alone.
In recent years a number of mathematical models have been published for predicting the clinical and economic impact of prophylactic HPV vaccination. The authors selected the cohort model as the analysis tool as it was considered to answer the question about cost effectiveness and clinical impact of vaccination against cervical cancer wellCitation[51]. Cohort models often require fewer data and assumptions compared to more sophisticated models such as dynamic models, and are also easier to describe and present to decision-makers may have limited health economic and/or modelling experience.
With a static Markov cohort model it was not possible to take into account the dynamic process of viral transmission within the population. Therefore it was not feasible to assess the herd protection factor induced by vaccinationCitation[52–54]. Without considering herd protection the cost effectiveness of a vaccination programme is likely to be underestimatedCitation[55]. Markov models therefore provide conservative estimates of the cost-effectiveness results of vaccination programmes. Furthermore, cohort models cannot explore the potential benefit incurred by vaccinating boys which would necessitate the use of a dynamic model.
Reduced transmission of the HPV virus is expected to benefit boys as well, albeit to a lesser extent than girls. Reduction in HPV 16/18 infection would limit the incidence of other cancer types (e.g., anal, penile, head and neck cancer), however this benefit has not been taken into account in current study model. Finally, the base-case analysis used conservative assumptions regarding screening by not allowing for any change in the future.
Cross-protection on non-vaccine HPV types was included in the base case in order to follow the latest assessments on HPV vaccine efficacyCitation[27],Citation[56].
Costs for the administration of HPV vaccination when they occur at the age of 11 years in schools would be low and were therefore not taken into account in this analysis. Costs of adverse events were also not included in the analysis, as clinical trials have shown that both HPV vaccines are generally safe and well-toleratedCitation[27],Citation[57]. Total costs calculated in the current study evaluation also lack costs resulting from treatment and follow-up of other cervical abnormalities detected with screening. These will be evaluated in the future with a cost of illness study focusing on the cost of managing CC prevention.
The reduction in CC cases and mortality predicted by the model is in line with previously published estimates from Goldie et al.Citation[58], Taira et al.Citation[59], Kohli et al.Citation[60], and Suarez et al.Citation[8]. (62%, 66%, 76% and 67–74% reduction, respectively). Barnabas et al.Citation[61], using a dynamic model, reported a 91% reduction in HPV 16-related CC incidence in Finland with 90% vaccine coverage, but unfortunately they did not include HPV 18 in their study.
Most published cost-effectiveness results have focussed their analysis on North America and the UKCitation[58],Citation[60],Citation[62–66]. Making a direct comparison with those studies is difficult due to differences in screening pattern, cost structures, and epidemiology of the disease in those different areas. Nevertheless, the current study results are within the range reported by other evaluationsCitation[8]. This may be surprising as Finland has an extensive organised screening programme combined with an even more extensive opportunistic screening process.
The study results therefore indicate that even in a country with a low disease burden such as Finland, the addition of HPV 16/18 vaccination of girls aged 11 years to a screening programme can still provide significant benefits and be cost-effective compared with screening alone. Comparing the current analysis with other new vaccine evaluations conducted by Finnish authoritiesCitation[67],Citation[68] (National Public Health Institution's analyses on rotavirus and pneumococcal conjugate vaccine), the study results appear to be within the acceptable cost-effectiveness range from a healthcare payer perspective.
It is also important to note that prophylactic HPV 16/18 vaccination may provide additional benefits not captured by the present model such as the specific alleviation of the disease burden placed on the healthcare system and to their individuals (patients and carers) such as anxiety, stress and sufferingCitation[69],Citation[70].
The analysis was performed from a healthcare payer perspective and therefore the cost-effectiveness results may underestimate the total economic benefit of vaccination to society. Around half of the new CC cases in Finland are diagnosed in women under 60 years of age and indirect costs from loss of income due to illness may heavily influence the economic results. The current study model does not capture this potential additional cost benefit. The purpose of the current study analysis was to show to healthcare payers (i.e., government) the potential costs and benefits that can be expected from HPV 16/18 vaccination.
In Finland, there is no official threshold for the acceptance or rejection of a cost-effectiveness evaluation. Some unofficial estimates are reported to be between €30,000 and 50,000 per QALY gained, or even higher with interventions with high social value (i.e., cancer drugs for small targeted population). These estimates are usually in the context of pharmaceutical treatment of illnesses. However, little debate has been noted on preventive interventions in Finland. Long-term preventive healthcare interventions such as vaccination are disproportionably disadvantaged by the discount rate used for outcomes and costs. The base-case results are shown here with 0% and 3% discount rates according the latest Finnish guidelineCitation[46], and indicate the strong effect of even moderate discount values.
Conclusion
In summary, the authors have calibrated and validated a mathematical cohort model replicating the natural history of HPV infection and disease in a setting where screening of pre-cancerous lesions is well implemented and certified as of good quality. The analysis suggests that it is still possible to substantially reduce CC cases and CC deaths by additionally introducing vaccination and consequently reducing the burden placed on the healthcare system, even in a country with a relatively low CC burden. From a healthcare payer perspective the addition of HPV 16/18 vaccination to the current screening programme is likely to be very cost effective in the Finnish context.
Transparency
Declaration of funding: GlaxoSmithKline Biologicals was the funding source of this study.
Declaration of financial/other relationships: S.T. and N.D. have disclosed that they are employees of GlaxoSmithKline; P.N. has disclosed that he is a member of the External End Point Committee of the GSK phase III HPV vaccine trial; J.P. and M.L. have disclosed that they have received research grants from Merck & Co and GlaxoSmithKline; J.H. has disclosed that he is a former employee of GlaxoSmithKline.
Acknowledgments: The authors would like to thank Dr Seija Grenman and Dr Päivi Pakarinen from Turku University hospital and Helsinki University hospital for their valuable insights, and Dr Risto Sankila from Cancerregistry Finland. Finally the authors thank Elhem Sbaa (Keyrus Biopharma for GlaxoSmithKline Biologicals, Belgium) for publication coordination.
Notes
*Gardasil is a registered trade mark of Merck & Co Inc, New Jersey, USA.
†Cervarix is a registered trade mark of GlaxoSmithKline Biologicals, Rixensart, Belgium.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108
- Adams M, Jasani B, Fiander A. Human papilloma virus (HPV) prophylactic vaccination: challenges for public health and implications for screening. Vaccine 2007; 25: 3007–3013
- Anttila A, Pukkala E, Soderman B, et al. Effect of organised screening on cervical cancer incidence and mortality in Finland, 1963-1995: recent increase in cervical cancer incidence. Int J Cancer 1999; 83: 59–65
- Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol 2005; 32(Suppl 1)S16–24
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24(Suppl 1)S1–15
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–19
- Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121: 621–632
- Suarez E, Smith J, Bosch F, et al. Cost-effectiveness of vaccination against cervical cancer: a multi-regional analysis assessing the impact of vaccine characteristics and alternative vaccination scenarios. Vaccine 2008; 26S: F29–45
- Beby-Defaux A, Bourgoin A, Ragot S, et al. Human papillomavirus infection of the cervix uteri in women attending a Health Examination Center of the French social security. J Med Virol 2004; 73: 262–268
- Boulanger JC, Sevestre H, Bauville E, et al. [Epidemiology of HPV infection]. Gynecol Obstet Fertil 2004; 32: 218–223
- Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7932 women. Br J Cancer 2001; 84: 1616–1623
- Dalstein V, Riethmuller D, Sautiere JL, et al. Detection of cervical precancer and cancer in a hospital population; benefits of testing for human papillomavirus. Eur J Cancer 2004; 40: 1225–1232
- Riethmuller D, Gay C, Bertrand X, et al. Genital human papillomavirus infection among women recruited for routine cervical cancer screening or for colposcopy determined by Hybrid Capture II and polymerase chain reaction. Diagn Mol Pathol 1999; 8: 157–164
- Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001; 285: 2995–3002
- Holowaty P, Miller AB, Rohan T, et al. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst 1999; 91: 252–258
- Melnikow J, Nuovo J, Willan AR, et al. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998; 92: 727–735
- Schlecht NF, Platt RW, Duarte-Franco E, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst 2003; 95: 1336–1343
- Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 1999; 180: 1415–1423
- Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338: 423–428
- Molano M, Van den BA, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol 2003; 158: 486–494
- Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, et al. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet 2001; 358: 1782–1783
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286: 3106–3114
- lääkäriseura Duodecimin ja Suomen kolposkopiayhdistyksen asettama työryhmä. Suomalaisen. Käypä hoito -suositus: Kohdunkaulan, emättimen ja ulkosynnytinten solumuutokset - diagnostiikka, hoito ja seuranta. Duodecim 2006; 122: 1808–1833
- Finnish Cancer Registry. http://www.cancerregistry.fi. 2007
- Monto K, Nieminen P. Gynekologisten irtosolunäytteiden määrät ja raportointitavat Suomessa. Suom Lääkäril 2005; 18-19/2005: 2093–2097
- Nieminen P, Kallio M, Anttila A, et al. Organised vs. spontaneous Pap-smear screening for cervical cancer: a case-control study. Int J Cancer 1999; 83: 55–58
- Paavonen J, Jenkins D, Bosch F, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161–2170
- Paavonen J. Mitä HPV-rokotteilta voidaan odottaa?. Suomen Lääkärilehti 2009; 11: 1017–1021
- Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol 1995; 141: 680–689
- Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000; 132: 810–819
- Nieminen P, Vuorma S, Viikki M, et al. Comparison of HPV test versus conventional and automation-assisted Pap screening as potential screening tools for preventing cervical cancer. BJOG 2004; 111: 842–848
- Clifford G, Franceschi S, Diaz M, et al. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006; 24(Suppl 3)S3–26/34
- Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005; 366: 991–998
- Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364: 1757–1565
- FIGO. (International Federation of Gynecology and Obstetrics) 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006; 95(Suppl 1)S1–257
- Statistics Finland. www.stat.fi. 2008
- Hujanen T, Kapiainen S, Tuominen U, et al, Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006 (Healthcare unit costs in Finland 2006). 3/2008, 1–107. 2008. Helsinki, Stakes. Työpapereita
- Helsingin ja Uudenmaan airaanhoitopiiri. Hinnasto 2008. (Official price list of Hospital District of Heslinki and Uusimaa). 2008.
- Varsinais-Suomen sairaanhoitopiiri. Palveluhinnasto 2008. (Official price list of Hospital District of Southwest Finland, 2008). http:///www.vsshp.fi. 2008
- Price SLD, [Finnish Pharmaceutical Data Ltd/Suomen Lääkedata Oy; 2008
- Health Outcomes Data Repository. https://crc-limited.co.uk. 2007. Cardiff Research Consortium.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23: 1107–1122
- Myers ER, Green S, Lipkus I, Patient preferences for health states related to HPV infection: visual analog scale versus time trade-off elicitation. (Abstract n° 542 presented at the Twenty-First International Papillomavirus Conference, México City, México. February 20–27). 2004
- Rokotuskattavuus Suomessa. (Vaccination coverage in Finland). http://www.ktl.fi/portal/suomi/terveyden_ammattilaisille/rokottaminen/rokotuskattavuus/. 2010. Terveyden ja Hyvinvoinnin Laitos (National Institute for Health and Welfare). 23-2-2010.
- Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247–1255
- Sosiaali- ja terveysministeriön asetus lääkkeiden hintalautakunnalle tehtävästä hakemuksesta ja hintailmoituksesta. (Guideline for Health Economic Evaluation). 27 Mar. 2009. Sosiaali- ja terveysministeriö (Ministry of Social Affairs and Health). 201/2009. http://www.finlex.fi/fi/laki/alkup/2009/20090201 Accessed 23 Feb. 2010
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000; 17: 479–500
- @RISK for Excel. Version 4.5.5 (US/English) [Palisade Corporation; 2005
- Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008; 100: 513–517
- Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007; 370: 890–907
- Dasbach EJ, Elbasha EH, Insinga RP. Mathematical models for predicting the epidemiologic and economic impact of vaccination against human papillomavirus infection and disease. Epidemiol Rev 2006; 28: 88–100
- Brisson M, Edmunds WJ. Economic evaluation of vaccination programs: the impact of herd-immunity. Med Decis Making 2003; 23: 76–82
- Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev 1993; 15: 265–302
- Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis 2005; 191(Suppl 1)S97–106
- Edmunds WJ, Medley GF, Nokes DJ. Evaluating the cost-effectiveness of vaccination programmes: a dynamic perspective. Stat Med 1999; 18: 3263–3282
- Harper D, Gall S, Naud P, et al, Sustained immunogenicity and high efficacy against HPV-16/18 related cervical neoplasia: long-term follow up through 6.4 years in women vaccinated with Cervarix™ (GSK's HPV 16/18 AS04 candidate vaccine). Late-breaking abstracts presented for the 39th Annual Meeting of the Society of Gynecologic Oncologists, 9–12 March, Tampa, USA). Gynecol Oncol 2008;109:158
- Barr E, Gause CK, Bautista OM, et al. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. Am J Obstet Gynecol 2008; 198: 261.e1–11
- Goldie SJ, Grima D, Kohli M, et al. A comprehensive natural history model of HPV infection and cervical cancer to estimate the clinical impact of a prophylactic HPV-16/18 vaccine. Int J Cancer 2003; 106: 896–904
- Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004; 10: 1915–1923
- Kohli M, Ferko N, Martin A, et al. Estimating the long-term impact of a prophylactic human papillomavirus 16/18 vaccine on the burden of cervical cancer in the UK. Br J Cancer 2007; 96: 143–150
- Barnabas RV, Laukkanen P, Koskela P, et al. Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLoS Med 2006; 3: e138
- Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004; 96: 604–615
- Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology 2002; 13: 631–639
- Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA 2003; 290: 781–789
- Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003; 9: 37–48
- Van De Velde N, Brisson M, Boily MC. Modeling human papillomavirus vaccine effectiveness: quantifying the impact of parameter uncertainty. Am J Epidemiol 2007; 165: 762–775
- Lasten rotavirusrokotustyöryhmä. Kansanterveyslaitoksen asettama lasten rotavirusrokotustyöryhmän selvitys. B28/2007. 26-11-2007. Helsinki, Kansanterveyslaitos (National Public Health Institute, Finland). Kansanterveyslaitoksen julkaisuja
- Lasten pneumokokkirokotustyöryhmä. Kansanterveyslaitoksen asettama lasten pneumokokkirokotustyöryhmän selvitys. B12/2008. 2008. Helsinki, Kansanterveyslaitos (National Public Health Institute, Finland). Kansanterveyslaitoksen julkaisuja
- Bell S, Porter M, Kitchener H, et al. Psychological response to cervical screening. Prev Med 1995; 24: 610–616
- Lerman C, Miller SM, Scarborough R, et al. Adverse psychologic consequences of positive cytologic cervical screening. Am J Obstet Gynecol 1991; 165: 658–662