Abstract
Objective: To evaluate the actual dosing of etanercept in US commercially insured patients with psoriatic disease, to estimate treatment cost.
Methods: This study evaluated medical and pharmacy claims in a US commercial claims database from November 1, 2003 through June 30, 2008. Biologic-naïve patients diagnosed with psoriasis, psoriatic arthritis, or both, who filled prescriptions for etanercept and were enrolled for ≥6 months before the first claim and 1 year after, were included. Patients with rheumatoid arthritis, ankylosing spondylitis, Crohn's disease, or ulcerative colitis during the study period were excluded.
Results: Among 2,359 patients, diagnoses included psoriasis (n = 1,810), psoriatic arthritis (n = 212), and both diseases (n = 337). Psoriasis patients used a mean 98% (standard deviation [SD] 28%) and psoriatic arthritis patients used 107% (SD 31%) of label-recommended etanercept doses.
Conclusions: Overall, patients with psoriatic disease used 98–104% of the expected etanercept dose, depending on whether the expected dose for patients with both diagnoses was the psoriasis or psoriatic arthritis dose. This study does not evaluate effectiveness or clinicians’ intent and may not be representative of other populations. Nevertheless, average usage was as expected for all cohorts, providing a basis for cost estimation.
Introduction
Psoriasis is a chronic autoimmune skin disease that affects approximately 2% of the United States (US) populationCitation[1],Citation[2]. The most common form of the disease, psoriasis vulgaris (plaque psoriasis), which affects about 85–90% of patients with psoriasis, is characterized by red or pink raised plaques with silvery scales resulting from hyperproliferation of the epidermisCitation[2]. Plaques often form on the elbows, knees, scalp, back, and abdomen, but they may be found anywhere on the bodyCitation[1]. About half of patients with psoriasis also have nail changes including pitting, separation, discoloration, or dystrophyCitation[1].
Psoriatic arthritis is a systemic inflammatory disease, distinct from other forms of arthritis, that is estimated to affect an estimated 0.3–1% of the US population including up to 42% of patients with psoriasisCitation[3]. Characteristics of psoriatic arthritis may include joint and tendon inflammation in the extremities and spine, swelling of the fingers, and abnormal new bone formation at joint marginsCitation[4–6]. Most (but not all) patients with psoriatic arthritis also have psoriatic skin symptoms and nail changesCitation[4–6].
Both psoriasis and psoriatic arthritis cause significant problems for patientsCitation[7],Citation[8]. Patients with psoriasis report difficulties using their hands, walking, sitting or standing for long periods of time, social stigmatization, high stress levels, and depression, which increase in severity as the extent of their disease increasesCitation[8],Citation[9]. Severe psoriasis is also associated with a reduced probability of full-time employment and lower incomeCitation[9]. Because many patients with psoriatic arthritis also have skin symptoms, they may experience the same difficulties as psoriasis patients. Psoriatic arthritis also shares characteristics with rheumatoid arthritis. In one study, disability and quality of life scores of patients with psoriatic arthritis were found to be similar to those of patients with rheumatoid arthritisCitation[10].
Overproduction of the pro-inflammatory cytokine tumor necrosis factor (TNF) is central in the development of both psoriasis and psoriatic arthritisCitation[1],Citation[5]. Therapies that inhibit TNF, including etanercept, adalimumab, and infliximab, are used to treat both psoriasis and psoriatic arthritisCitation[2],Citation[6]. Etanercept was approved in the US for the treatment of psoriatic arthritis on January 15, 200211 and for moderate-to-severe plaque psoriasis on April 30, 200412. Although these agents are effective for many patientsCitation[2],Citation[6], the cost may be of concern to payers.
Before a model can be developed to predict the cost of treating psoriatic disease, the factors that drive cost need to be identified and quantified. One of the drivers of cost is the actual dose used, but published evidence concerning actual usage of TNF inhibitors in psoriatic disease is limited. The study reported by Wu and colleagues evaluated etanercept utilization in psoriasis, but did not include patients with psoriatic arthritis and did not compare total dose over time with the label-recommended dose for the diagnosisCitation[13]. Information about the starting and maintenance doses actually used by patients with psoriasis or psoriatic arthritis is important for determining adherence to the label and thereby predicting therapy costs.
The goal of this study was to evaluate the actual dosing of etanercept in US commercially insured patients with psoriasis and/or psoriatic arthritis. The recommended dosing of etanercept according to the US label for patients with moderate-to-severe plaque psoriasis starts at 100 mg/week for the first 12 weeks (administered as two 50-mg doses, 3 or 4 days apart), followed by a step down to 50 mg/weekCitation[14]. For patients with psoriatic arthritis, the recommended dosage of etanercept is 50 mg/week throughout the entire treatmentCitation[14]. This study evaluates actual usage in comparison to the US label-recommended dosing in a large commercial database.
For patients diagnosed with both psoriasis and psoriatic arthritis, it is not possible to know the primary intent of treatment and thus the relevant expected dose because the treatment may be either for psoriasis (recommended starting dose, 100 mg/week) or psoriatic arthritis (recommended starting dose, 50 mg/week). It was hypothesized that the intent of treatment and thus the starting dose might be predicted based on characteristics of either the patient or the prescriber.
Patients and methods
Study population
This retrospective study examined pharmacy claims for etanercept for patients with a diagnosis of psoriasis, psoriatic arthritis, or both, using the Ingenix administrative claims database from a large, geographically diverse US managed-care plan. At the time the study was conducted, the administrative claims database included data for approximately 14 million health-plan enrollees with both medical and pharmacy benefits. The health plan comprises discounted fee-for-service independent practice association plans spanning the US, primarily in the southern and midwestern regions. The providers of pharmacy and medical services submit their claims for payment directly to the health plan. All study data were de-identified and accessed using methods compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. Institutional review board approval was not necessary because there was no patient intervention and the limited data were utilized in accordance with appropriate data use agreements with the covered entities as defined in HIPAA.
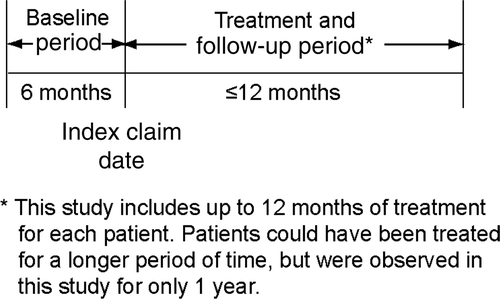
The study examined patient claims from November 1, 2003 through June 30, 2008. For inclusion in the study, patients were required to have at least one claim for etanercept based on National Drug Code (NDC) numbers between May 1, 2004 and June 30, 2007 (identification period). The process for qualifying patients to be included in the study is summarized in . Inclusion criteria required that patients were age 18 or older, continuously enrolled in the health plan for a 6-month baseline period before the date of the first claim for etanercept (the index date) and a 12-month follow-up period (). Patients were required to have at least one medical claim with a diagnosis of psoriasis (ICD-9-CM 696.1), psoriatic arthritis (ICD-9-CM 696.0), or both during the 6 months before through 1 month after the first claim for etanercept (the index claim). The intent of allowing diagnoses recorded through 1 month after the index claim was to ensure that all diagnoses were captured. For each individual patient, the time from the start of the 6-month baseline period through the 12-month follow-up period could have occurred at any time during the 4.5-year study period ().
Figure 1. Among 23,367, 511 health plan enrollees, 2,359 patients met selection criteria for this analysis.
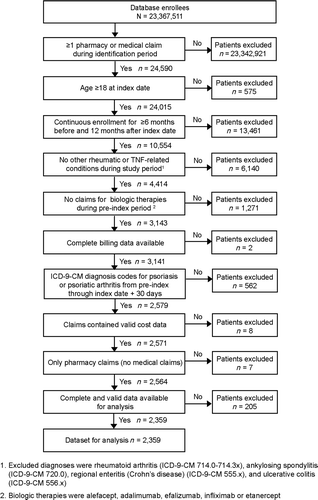
Figure 2. The record for each patient was examined over a 6-month baseline period and a follow-up period of up to 12 months after the index claim for etanercept.
Patients with medical claims with diagnoses of other diseases for which some TNF inhibitors are approved, including rheumatoid arthritis (ICD-9-CM 714.0–714.3x, 714.9, 714.89); ankylosing spondylitis (ICD-9-CM 720.0); regional enteritis (Crohn's disease) (ICD-9-CM 555.x); or ulcerative colitis (ICD-9-CM 556.x) during the study period (182 days before to 365 days after the date of the index claim) were excluded. Patients were also excluded if they had any claims for biologic therapies (alefacept, adalimumab, efalizumab, infliximab or etanercept) during the baseline period. Use of methotrexate, cyclosporine, phototherapy (UVA/UVB), and topical therapies any time during the study period was allowed. Therapies used were identified in the records by NDC or, if applicable, HCPCS codes. If a patient switched to another biologic therapy during the follow-up period, only the period of etanercept use was analyzed.
Patients were considered to have discontinued their course of etanercept therapy if they had a gap of 28 days or more after the end of the days’ supply of the previous etanercept prescription. If those patients restarted etanercept therapy, only the first period of therapy was included in the analysis. Restarts were not included because the etanercept label does not specify the recommended dose when resuming therapy after discontinuation, and therefore the expected dose could not be determined.
In addition to the inclusion and exclusion criteria listed above, patients were excluded for potential data validity problems in their records, such as administration of etanercept under a batch billing arrangement (multiple service dates billed together) or a value in the claim that suggested errors in adjudication (including a negative value for cost or quantity dispensed, a cost greater than three standard deviations above or below the mean for that dose of etanercept, or a claim with a weekly dose >200 mg).
Three cohorts were defined based on diagnosis in the claims, during the baseline period through 1 month after the index date: patients with psoriasis only; patients with psoriatic arthritis only; and patients with both psoriasis and psoriatic arthritis. Patients who had claims for psoriasis and psoriatic arthritis within the baseline period through 1 month after the index date were included in the ‘both psoriasis and psoriatic arthritis’ cohort.
Outcome measures
Expected doses were based on recommended doses in US labeling. For psoriasis, the recommended dosage of etanercept is 100 mg/week for the first 12 weeks, followed by 50 mg/week for the duration of therapy; for psoriatic arthritis, the recommended dosage is 50 mg/week for the duration of therapy. No expected duration of therapy is specified because psoriasis and psoriatic arthritis are chronic conditions, for which the label does not include a recommended treatment duration.
The study compared the actual etanercept doses received to the expected doses for psoriasis or psoriatic arthritis according to US labeling, and reported actual doses as percentages of the expected dose for psoriasis or psoriatic arthritis. For example, a patient who received 2,000 mg of etanercept during the follow-up period when the label-recommended dose was 2,200 mg for his diagnosis during that period would have received 91% of the recommended dose. A patient who received 2,300 mg when the expected dose was 2,200 mg would have received 104% of the expected dose. Average treatment duration and the proportion of patients with two or more claims (indicating prescription refills) were also observed during the follow-up period.
Etanercept is supplied as 25 mg or 50 mg syringes, four to a carton; drug quantities were standardized to the number of syringes. A number of common inconsistencies were identified during the data cleaning process. For example, the days of supply were often recorded as 30 or 31 days; however, because etanercept is dosed weekly, days of supply were corrected to represent a 28-day month or a 4-week supply. For each claim, the daily dose in milligrams was determined by multiplying the number of syringes dispensed on a prescription by the drug strength (25 mg or 50 mg) and dividing by days of supply. The daily dose was multiplied by seven to get the weekly dose. The total dose for each patient was the sum of the weekly doses for all of his or her claims. For example, if a patient's claims during the follow-up period totaled 44 weeks of etanercept at 50 mg/week, the total dose in the follow-up period would be 2,200 mg. The total dose included the last prescription claim and the duration of that claim based on the days of supply.
Statistical analysis
All descriptive statistics were calculated as mean and standard deviation (SD) and median with 25th and 75th percentiles. Statistics were calculated for the diagnosis-based cohorts (psoriasis, psoriatic arthritis, and both psoriasis and psoriatic arthritis), and for patients starting at 100 mg/week, 50 mg/week, and other dosages (either 25 mg or 75 mg/week). Statistical significance was calculated using a two-tailed comparison, with α = 0.05.
For patients diagnosed with both psoriasis and psoriatic arthritis, a multivariate analysis was conducted using logistic regression to examine the factors associated with the initiation of etanercept treatment at either 50 mg or 100 mg/week. Factors that were considered included demographic characteristics; pre-index clinical characteristics such as medication use, body mass index, Quan–Charlson comorbidity score, and healthcare costs and utilization; and provider specialty on the index date. The results of the multivariate analysis are presented as odds ratios associated with each independent variable.
Results
Demographic and clinical characteristics
The study population included 24,590 patients who were identified in the health plan with one or more medical or pharmacy claims for etanercept from May 1, 2004 through June 30, 2007. Of these patients, 10,554 were aged 18 or greater and continuously enrolled in the health plan for 6 months before the index date and 12 months after it. Based on the inclusion and exclusion criteria described above, 2,359 patients were eligible for analysis (). The most common reasons for excluding patients were diagnoses other than psoriasis or psoriatic arthritis (n = 6,140) and claims for a biologic during the pre-index period (n = 1,271). The analysis included 1,810 patients (77% of the total population) with psoriasis, 212 patients (9%) with psoriatic arthritis, and 337 patients (14%) with diagnoses of both psoriasis and psoriatic arthritis. shows the distribution of dosages prescribed for each cohort.
Table 1. Number and percent of patients included in each analysis, by initial diagnosis and starting dosage*.
Key demographic characteristics of the study population are summarized in . The psoriasis, psoriatic arthritis, and psoriasis/psoriatic arthritis cohorts included similar proportions of men (57–60%). The mean age among all cohorts was approximately 44 years and was similar in all cohorts. Among patients with psoriasis, approximately 71% used topical therapies in the baseline period (); of the psoriatic arthritis patients, approximately 31% used topical therapies and 37% used methotrexate in the baseline period. Among patients with both psoriasis and psoriatic arthritis, topical therapies were the most common treatments in the baseline period, used by 64% of patients. Mean baseline Quan–Charlson comorbidity indexCitation[15] scores among the groups ranged from 0.26 to 0.30 (median for all groups, 0), indicating low levels of comorbidity (). Approximately 79% of patients with psoriasis were treated by dermatologists, as were 45% of patients with both psoriasis and psoriatic arthritis. Most patients with psoriatic arthritis (77%) were treated by rheumatologists (). Before the start of therapy with etanercept, patients with both psoriatic arthritis and psoriasis incurred the greatest mean monthly treatment costs ($582, including both medical and pharmacy costs); mean costs were $520 for patients with psoriasis and $491 for patients with psoriatic arthritis ().
Table 2. Patient demographics and clinical characteristics.
Treatment patterns
The mean (SD) actual total dose of etanercept used during the follow-up period was 1,901 (1,290) mg for the psoriasis cohort, 1,599 (1,083) mg for the psoriatic arthritis cohort, and 1,964 (1,234) mg for the cohort diagnosed with both psoriasis and psoriatic arthritis (). The mean (SD) actual dose was similar to the expected dose based on product label recommendations for both the psoriasis (98% [28%]) and psoriatic arthritis (107% [31%]) cohorts. For the cohort diagnosed with both psoriasis and psoriatic arthritis, the mean (SD) actual dose was 89% (30%) of the expected label-recommended dose for psoriasis and 132% (45%) of label dosing for psoriatic arthritis. For the overall population, the weighted average actual dose was between 98% (29%) and 104% (33%) of the expected dose, depending on whether usage of patients with both psoriasis and psoriatic arthritis was compared with the expected dose for psoriasis or for psoriatic arthritis ().
Figure 3. The weighted average actual dose was between 98% and 104% of the expected dose, depending on whether usage of patients with both psoriasis and psoriatic arthritis was compared with the expected dose for psoriasis (lower bound) or for psoriatic arthritis (upper bound).
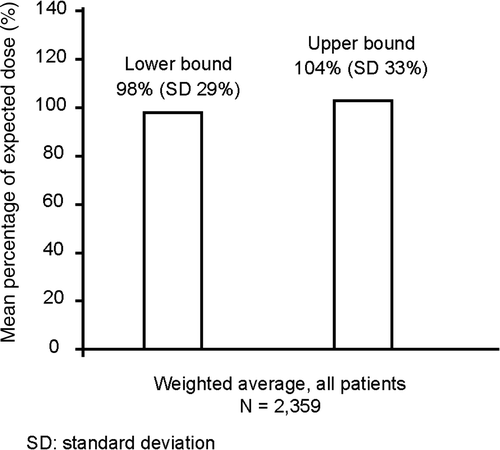
Table 3. Etanercept usage patterns: actual dose as a percentage of expected dose.
On average, patients with diagnoses of both psoriasis and psoriatic arthritis received etanercept treatment for longer than those with a single diagnosis. The mean (SD) duration of treatment was 217 (125) days for patients with both diagnoses, 184 (126) days for patients with psoriasis only, and 213 (137) days for patients with psoriatic arthritis only ().
Among patients with psoriasis who started on etanercept at 100 mg/week and stayed on therapy for at least 12 weeks, 50% stepped down to the 50 mg/week dose. The median time to step-down was 100 days (14 weeks). Among patients with both psoriasis and psoriatic arthritis, 51% stepped down from 100 mg to 50 mg/week, after a median of 106 days. A small number of patients (<1%) stepped down to other doses.
Overall, 17% of patients filled only a single prescription for etanercept, including 18% of psoriasis patients, 22% of patients with psoriatic arthritis, and 12% of patients with both diagnoses; the remaining patients had claims for at least one refill (). These proportions were similar among all dose groups within each diagnosis category.
Results of the logistic regression showed that among patients with both psoriasis and psoriatic arthritis, the strongest predictor of starting at 100 vs. 50 mg/week was provider specialty (dermatologist vs. rheumatologist, OR 30.0; p < 0.0001 and other specialty vs. rheumatologist, OR 10.8; p < 0.0001). Use of topical therapies in the pre-index period was also significantly associated with a 100 mg/week starting dose of etanercept (OR 2.2; p = 0.0060). Pre-index factors that did not predict starting dose included age, sex, body mass index, geographic region, use of methotrexate therapy, use of phototherapies (including light-activated pharmacotherapies), and number of previous ambulatory visits for either psoriasis or psoriatic arthritis treatment.
Discussion
In this study of etanercept usage over a 1-year follow-up period, etanercept usage was stable, predictable, and close to expected dosing based on product labeling in cohorts of patients with psoriasis, psoriatic arthritis, and both diagnoses. While individual patient dosing patterns may have differed from expected dosing, average usage patterns were as expected for all cohorts, regardless of diagnosis or starting dose. This suggests that etanercept usage patterns can be predicted reliably in a patient population using etanercept for psoriasis, psoriatic arthritis, or both. From a cost perspective, the 50% of patients who did not step down to 50 mg are balanced by patients who started at lower-than-average doses or who stepped down before 12 weeks.
Additionally, factors that suggest the intent to treat the patient's psoriasis symptoms (treatment by a dermatologist or other non-rheumatologist and previous use of topical therapies) provide insight into the likelihood of starting with a 100 mg/week dose for a patient with both psoriasis and psoriatic arthritis. Some studies have suggested that patients with a higher body mass index might be more likely to require higher doses of etanercept to experience a treatment effectCitation[16],Citation[17], but body mass index was not correlated with dose in this study.
Published evidence is limited regarding the actual utilization of etanercept for psoriasis and psoriatic arthritis outside of clinical trials. Wu and colleagues analyzed etanercept usage in patients with psoriasis, but the Wu study did not include patients with psoriatic arthritis, did not compare total dose over time with the label-recommended dose for the diagnosis, and may have provided a misleading interpretation of etanercept usage patterns (e.g., defining the absence of a dose decrease as a dose increase)Citation[13]. Other economic studies of etanercept therapy in psoriatic disease included an overall review of the purchase price of psoriasis therapiesCitation[18], an analysis of the projected cost-effectiveness of multiple psoriasis therapies based on data from clinical trialsCitation[19], and health technology assessments that used clinical data and economic models to evaluate the cost effectiveness of etanercept therapyCitation[20],Citation[21]. Except for Wu et alCitation[13], no studies based on actual utilization etanercept for psoriatic diseases in clinical practice were identified. This study was designed to address this unmet need.
Limitations of this study included the fact that the use of any claims database does not provide information about severity of disease at diagnosis, the effectiveness of therapy over time, or the intent of the treating clinician (e.g., to treat psoriatic arthritis or psoriasis), and, as any database, may be subject to coding errors. While this database is largely representative of the US managed-care population, it may not represent usage patterns in other populations (e.g., Medicaid or Medicare). In addition, because approximately half of the patients in this study were in the southern US, it is possible that differences may exist in other populations with different geographic distributions. However, the actual usage is likely to be between the high and low bounds of expected usage data. For payers covering the large population of commercially insured patients being treated with etanercept, this study provides valuable information about the actual dosing of etanercept for these diseases.
In this study of patients diagnosed with psoriasis, psoriatic arthritis, or both in a large US managed-care population, the actual usage of the TNF inhibitor etanercept in the overall population was very close to expected dosing based on product labeling. Based on the information in this study, payers can reliably predict the overall usage of etanercept therapy in the treatment of psoriasis and psoriatic arthritis.
Transparency
Declaration of funding: This study was funded by Amgen Inc.
Declaration of financial/other relationships: ST and RS have disclosed that they are employed by i3 Innovus, which receives research funding from Amgen Inc. C.W., D.G., and D.J.H. have disclosed that they are employees of Amgen Inc. and own Amgen stock/stock options.
Acknowledgements: The authors would like to acknowledge the writing assistance of Sue Hudson of Medical Writing Associates and the programming assistance of Randall Gerdes of i3 Innovus; their services were funded by Amgen Inc.
References
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263–271
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496–509
- Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005; 64(Suppl 2)ii14–17
- Mease PJ. Psoriatic arthritis assessment and treatment update. Curr Opin Rheumatol 2009; 21: 348–355
- Ritchlin C. Psoriatic disease – from skin to bone. Nat Clin Pract Rheumatol 2007; 3: 698–706
- Ritchlin CT, Kavanaugh A, Gladman DD, et al. Treatment recommendations for psoriatic arthritis. Ann Rheum Dis 2009; 68: 1387–1394
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005; 53: 573
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc 2004; 9: 136–139
- Horn EJ, Fox KM, Patel V, et al. Association of patient-reported psoriasis severity with income and employment. J Am Acad Dermatol 2007; 57: 963–971
- Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol 2001; 28: 1842–1846
- United States Department of Health and Human Services – Food and Drug Administration. Product Approval Information – Licensing Action, 2002.
- United States Department of Health and Human Services – Food and Drug Administration. Product Approval Information – Licensing Action. 2004.
- Wu EQ, Feldman SR, Chen L, et al. Utilization pattern of etanercept and its cost implications in moderate to severe psoriasis in a managed care population. Curr Med Res Opin 2008; 24: 3493–34501
- Amgen Inc., Wyeth. Enbrel (etanercept) full prescribing information. 2008.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol 2008; 58: 443–446
- Driessen RJ, Boezeman JB, van de Kerkhof PC, et al. Three-year registry data on biological treatment for psoriasis: the influence of patient characteristics on treatment outcome. Br J Dermatol 2009; 160: 670–675
- Pearce DJ, Thomas CG, Fleischer AB, Jr, et al. The cost of psoriasis therapies: considerations for therapy selection. Dermatol Nurs 2004; 16: 241–428, 432
- Sizto S, Bansback N, Feldman SR, et al. Economic evaluation of systemic therapies for moderate to severe psoriasis. Br J Dermatol 2009; 160: 1264–1272
- Woolacott N, Bravo Vergel Y, Hawkins N, et al. Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 2006; 10: iii–iv, , xiii–xvi, 1–239
- Woolacott N, Hawkins N, Mason A, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess 2006; 10: 1–233, i–iv
