Abstract
Objective: To compare healthcare utilization and costs for major depressive disorder (MDD) patients treated with escitalopram and who were switched to another SSRI for non-medical reasons versus those who did not switch.
Research design and methods: Patients were identified in the Ingenix Impact Database (2003–2006). The analysis group included patients who remained on escitalopram for ≥90 days and switched to another SSRI within 45 days of end of supply days for non-medical reasons; the control group included matched individuals who did not switch within 45 days. Switching for medical reasons was defined as switching within 7 days after having a hospitalization, an emergency room (ER) visit, or a psychotherapy visit. Outcomes (all-cause and MDD-related) were analyzed over 3 months and included use of hospital, ER, outpatient visits and professional services, and healthcare costs. Outcomes were compared between the two groups using descriptive statistics and regression analyses controlling for differences in baseline characteristics. Costs were inflation adjusted to 2006 US dollars.
Results: The study included 2,805 matched pairs. Compared to controls, switchers had higher rates of all-cause and MDD-related hospitalizations (relative risk [RR] = 1.4 and 2.0, respectively) and all-cause and MDD-related ER visits (RR = 1.2 and 1.6, respectively, all p ≤ 0.05). Results from multivariate analyses show that switchers had higher medical costs (+$138), drug costs (+$149) and total healthcare costs (+$322) compared to patients in the control group (all p < 0.0001).
Limitations: This study's limitations include its short observational period and definition of switching for non-medical reasons.
Conclusion: Patients who were switched to another SSRI for non-medical reasons after being stabilized on escitalopram used more resources and had higher healthcare costs within 3 months of switching than patients who did not switch.
Introduction
Major depressive disorder (MDD) is a serious, persistent mental illness with a lifetime prevalence in the US of 17% in 2001, with as many as 10% of the population suffering from the disease in any given yearCitation[1–3]. MDD presents a substantial economic burden to the individual and to societyCitation[2], which was estimated at $83.1 billion in 20004. Approximately 60% of those affected with MDD never seek treatment, whereas those who do seek help are likely undertreatedCitation[5]. Inadequate treatment of MDD results in higher direct and indirect costs due to increased morbidity, absenteeism, and decreased productivityCitation[6],Citation[7].
The main treatment modalities for MDD include antidepressant medication, psychotherapy, and electroconvulsive treatment. Selective serotonin reuptake inhibitors (SSRIs) are a highly effective class of antidepressants with a superior adverse effect and safety profile relative to traditional agents such as tricyclic antidepressantsCitation[8]. SSRIs are also more cost effective in long-term MDD treatment than older antidepressantsCitation[8–11].
Escitalopram is a highly selective SSRI with strong binding to the serotonin reuptake receptor and little affinity to other tested receptorsCitation[12]. Multiple randomized, controlled, double-blind studies have shown high efficacy of escitalopram in acute MDD treatmentCitation[13],Citation[14] and maintenance therapyCitation[15–17]. Escitalopram is well tolerated and has a lower discontinuation rate and a faster onset compared to other SSRIs (citalopram, fluoxetine, paroxetine, fluvoxamine, and sertraline)Citation[13],Citation[18–24], and compared to the serotonin norepinephrine reuptake inhibitor (SNRI) venlafaxineCitation[19],Citation[25],Citation[26]. Recently, Kilts et al. found that escitalopram had superior efficacy in the treatment of more severe depression compared with other antidepressantsCitation[27].
SSRIs are frequently used as a first antidepressant for MDD but have response rates of only 50–60% in daily practiceCitation[28], with up to 44% of MDD patients discontinuing treatment within the first 3 monthsCitation[29]. Adverse effects and lack of response are the most frequent reasons given for discontinuing or switching SSRIsCitation[6],Citation[30]. No consensus exists on the optimal clinical management of MDD patients who do not respond well to a first-line antidepressantCitation[31]. Various authors recommend dose escalation, switching, or polypharmacyCitation[32]. Physicians, however, tend to switch patients from one SSRI to another or to an SNRI to manage side-effects or lack of efficacyCitation[33–35]. While switching to a different SSRI or an SNRI is often effective in MDD patients who do not respond to a first SSRICitation[31],Citation[36–41], switching to a different SSRI for non-medical reasons after a patient has already stabilized on the first SSRI can be associated with a significant discontinuation rate due to adverse side-effects or lack of responseCitation[42]. Potential non-medical reasons for switching antidepressants include financial reasons, such as switching to a generic drug for cost-containment purposes.
Whereas some cost comparisons among SSRIs have reported no significant differences in total direct costs of treatmentCitation[43], other pharmacoeconomic analyses have found escitalopram to be more cost-effective than citalopram, fluoxetine, sertraline, paroxetine, and the SNRI venlafaxineCitation[44–53]. There is limited evidence for a modest healthcare cost advantage of switching to a different antidepressant class compared to switching to another SSRICitation[31],Citation[54]. No studies, however, have compared healthcare utilization and costs of MDD patients switching to another SSRI after being stabilized on the first SSRI with those of patients who do not switch. The present analysis retrospectively compares healthcare resource utilization and costs between MDD patients who switched, for non-medical reasons, from escitalopram to another SSRI after being treated with escitalopram for at least 3 months and those who continued to use escitalopram.
Methods
Data source
This study used data from the Ingenix Impact Database, which included complete medical and pharmacy claims for more than 25 million managed-care lives from over 35 health plans, covering all census regions of the US. The data contain the enrollment records, medical claims (type of service, ICD-9 diagnosis code, CPT procedure code, standardized payments) and prescription drug claims (NDC code, days of supply, dose strength, standardized payments) for enrollees from January 1, 2003 through December 31, 2006. The starting date of the data sample is January 1, 2003 because escitalopram received FDA approval for the treatment of MDD in August 2002. The dataset included information on mental health carve-out eligibility, which allowed limitation of the study sample to patients with complete medical service records (i.e., patients with no mental carve-out service) to avoid underestimating the true economic burden of depression.
Definitions
A switcher is defined as a patient who receives a prescription for another SSRI without a refill of escitalopram within 45 days of the end date of the last escitalopram prescription. The 45-day duration was chosen to infer a discontinuation of escitalopram rather than possible add-on treatment of another SSRICitation[55–57]. Patients were considered to have switched antidepressants for a medical reason if they switched within 7 days after a hospitalization, having an ER visit, or a psychotherapy visit. Although the actual reason for switching cannot be determined from the claims data, the authors hypothesize that patients who had a treatment change due to medical reasons, such as hospitalization, ER visit, or psychotherapy visit due to adverse events or relapse of depression, were likely to do so very soon after the event, and thus should not be included in this analysis.
Patients who did not switch to another SSRI during the 45 days of the end date of the last escitalopram prescription constitute the control sample. For switchers, the index date is the date of switching, i.e., the date of the first prescription for another SSRI without a refill of escitalopram within 45 days of the end of the escitalopram prescription. For the control sample, the index date is a randomized escitalopram prescription date after the pre-index period. For both groups, the baseline period was defined as the 3-month pre-index date and the study period as the 3-month period following the index date. The index therapy is any SSRI available during the period analyzed (citalopram, fluoxetine, paroxetine, fluvoxamine, or sertraline).
Sample selection
Adult MDD patients were identified in the database and were grouped into: (1) switchers, for patients who remained on escitalopram for ≥90 days and switched to another SSRI for non-medical reasons in the next 45 days without a refill of escitalopram or (2) a matched control group for patients who remained on escitalopram for at least 90 days and did not switch to another SSRI within the next 45 days. The duration of 90 days was selected to capture patients who had been stabilized on escitalopram. Thus, a switch to another SSRI would be unlikely due to medical reasons. Each patient from the control sample was matched 1 : 1 to the switcher sample if they had the same age (year of birth), gender, and index year. In the event that there were multiple matches of control sample to a switched sample, one matched control was randomly selected. Both groups were required to have: (1) at least two claims with MDD diagnosis (ICD-9 = 296.2x, 296.3x) on different dates, with at least one claim on or after January 1, 2003, or at least one inpatient claim with MDD diagnosis on or after January 1, 2003; (2) continuous escitalopram use for at least 3 months (without a gap of more than 45 days) between the end of one prescription and the beginning of the next prescription); and (3) continuous enrollment in the health plan during the baseline and study periods to ensure continuous insurance eligibility. Exclusion criteria were: (1) being less than 18 years old during the baseline period; (2) using an antipsychotic, SSRI other than escitalopram, or SNRI in the pre-index period; or (3) having serious MDD-related events such as depression-related hospitalization, ER visits, or psychotherapy visits within 7 days before the index date. The last criterion applied to switchers only to ensure that the treatment was switched for non-medical reasons. Psychotherapy visits were identified using CPT codes.
Statistical analysis
All-cause and MDD-related outcomes were analyzed over 3 months post-index date and included healthcare resource utilization, in particular hospitalizations, emergency room (ER) visits, urgent care (measured as either a hospitalization or an ER visit), and non-urgent care (outpatient visits and professional services). MDD-related outcomes were identified by claims with ICD-9 codes for MDD while all-cause outcomes included all claims irrespective of the ICD-9 classification. Outcomes also included healthcare costs, broken down into medical and drugs costs. All medical costs are presented in 2006 inflation-adjusted US dollars.
Outcomes were compared descriptively between the two groups using the paired t-test for continuous variables and the McNemar test for categorical variables. Outcomes were further analyzed using regression analyses adjusting for differences in Deyo-Charlson comorbidities, selected baseline comorbidities (such as presence of anxiety or substance abuse), and healthcare resource use at baseline. Multivariate logistic regression was used to assess the difference in healthcare utilization between switchers and controls. The total medical, total drug, and overall total healthcare costs were estimated using a generalized linear model (GLM) model with a log-link function and gamma error distributionCitation[55]. This GLM model is commonly used when the data are highly skewed and the assumption of normality is challenged — a characteristic typical of medical expense data. The gamma distribution is suggested by prior studies as a closer approximation to distribution of costs dataCitation[58–60]. The expected cost for each group was calculated by estimated cost generated in the GLM model. The incremental cost of switchers was then calculated by subtracting the average costs of controls from the average annual costs of switchers.
Results
Patients
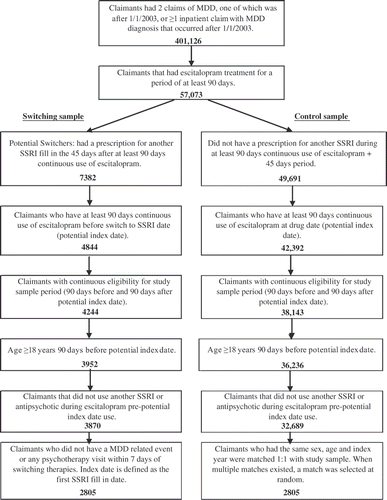
A total of 57,073 patients was identified with an MDD diagnosis and escitalopram use for a minimum of 90 days. Among these, 2,805 patients met the inclusion criteria for the switcher sample. A total of 32,689 patients met the inclusion criteria for the control sample, among which 2,805 were matched 1 : 1 to the switcher sample. reports the sample counts by selection criteria.
Figure 1. Sample counts by selection criteria. MDD, major depressive disorder; SSRI, selective serotonin reuptake inhibitor.
The baseline characteristics, resource utilization and healthcare costs of each group are summarized in . By construction, the two groups have the same age (mean 45.9 years) and gender distribution (74.2% female). They also had the same Deyo-Charlson comorbidities index, but in general switchers were more likely to have comorbidities, in particular schizophrenia (0.6 vs. 0.1%; p = 0.0010). They were also more likely to use healthcare services at baseline and to have higher prescription drug costs, medical services costs, and total costs (all p < 0.01).
Table 1. Demographics, comorbidities, healthcare utilization and healthcare costs — 3-month baseline period switching from escitalopram to another SSRI.
Healthcare resource utilization
Results of the unadjusted analysis indicated that in the 3-month treatment period, compared to patients in the control group, patients who switched to another SSRI had significantly higher urgent care utilization (any cause, relative risk [RR] = 1.2; MDD-related, RR = 1.7; all p < 0.05) (). Compared to patients in the control group, switchers had higher rates of outpatient visits (any cause, RR = 1.1; MDD-related, RR = 1.5; both p < 0.01) and higher rates of professional service utilization (any cause, RR = 1.0; MDD-related, RR = 1.1; both p ≤ 0.01).
Table 2. 3-month healthcare utilization of MDD claimants by index therapy — switching from escitalopram to another SSRI.
After controlling for differences in baseline healthcare utilization and comorbidities, switchers continued to be more likely to use healthcare resources. In particular, they were more likely to use MDD-related urgent care compared to patients in the control group (odds ratio [OR] = 1.49; p < 0.05) (). This included higher likelihood of hospitalization (any cause, OR = 1.24; MDD-related, OR = 1.70; both p < 0.05), although the likelihood of using the ER was not significantly different between the two groups (any cause, OR = 1.10; MDD-related, OR = 1.38; both p > 0.05). Switchers also had significantly greater numbers of all-cause and MDD-related hospitalization days compared to patients in the control group (all p < 0.05). Switching was also associated with greater utilization of MDD-related non-urgent care resources. Over the 3-month post-index date, switchers were also more likely to have outpatient visits and to use professional services.
Table 3. Effect of switching to another SSRI relative to continuing escitalopram on 3-month rate of urgent care and non-urgent care of MDD claimants.
Healthcare costs
Unadjusted analysis of healthcare costs showed that, in the 3-month study period, compared to controls, switchers had higher medical costs, although not statistically significant ($108; p = 0.6388), and higher drug costs ($163; p < 0.0001) (). Overall, switchers had $270 higher total costs than patients in the control group, though this difference was not statistically significant (p = 0.505).
Table 4. 3-month healthcare costs of MDD claimants by index therapy switching from escitalopram to another SSRI.
After controlling for differences in patient characteristics, results from multivariate analyses confirm these findings and show that switchers incurred even higher medical costs compared to controls ($138; p = 0.0084) while the difference in drug costs was smaller relative to descriptive results ($149; p < 0.0001). Overall, switchers incurred $322 (p < 0.0001) higher total costs in the 3-month period after switching compared to patients who remained on escitalopram ().
Table 5. Multivariate regression1 for 3-month healthcare costs of MDD claimants switching from escitalopram to another SSRI.
Discussion
Health insurance payers may use formularies and formulary management techniques including step-edits, prior authorizations, drug utilization review and tiered co-payments, to encourage switching patients on brand product to other alternatives, especially once generic drugs become available. In addition, some payers (or their pharmacy benefit management companies) may contact physicians and their patients by either letters or telephone calls to encourage therapeutic substitution to alternative generic treatments. These letters often include estimates of how much patients can save on their co-payments by using a lower-cost generic alternative. However, this policy may not be cost saving due to subsequent negative outcomes associated with switching among patients who were stabilized on the brand product. In this study, the authors investigated the outcomes associated with switching due to non-medical reasons to alternative SSRIs among depression patients who were stabilized on escitalopram.
There are inherent risks associated with switching medications in healthcare in general, and especially in the treatment of depressive disorders, where patients often have idiosyncratic responses to medications in the same class (e.g., SSRIs). These risks include side-effects associated with the initiation of a new drug, adverse effects resulting from the discontinuation of an ongoing medication, and reduced efficacy. These factors are likely to affect the healthcare resource utilization and costs incurred by patients who switched. While most MDD patients who switch from one SSRI to another do so because of adverse effects or lack of efficacyCitation[30],Citation[61], the purpose of switching could also be non-medical (e.g., reducing pharmacy cost). In this analysis, a retrospective claims data analysis was used to compare healthcare resource utilization and costs in MDD patients switching from escitalopram to another SSRI for non-medical reasons versus patients continuing escitalopram therapy.
Compared to patients in the control group, patients who switched to another SSRI had more comorbidities at baseline, were more likely to use healthcare services and had higher healthcare costs. They were also more likely to go to the hospital, to visit the ER, have outpatient visits, and receive professional services (all p < 0.05) in the 3-month period after switching. The MDD-related resource utilization rates in all of these categories were also significantly higher in the switcher group compared to the control group over the same time period (all p < 0.05). These findings were confirmed by multivariate analysis that controlled for differences in comorbidities and baseline healthcare resource utilization between cohorts. These results suggest that SSRI switching for non-medical reasons may have been associated with exacerbation of existing adverse effects, development of new ones, or both, resulting in increased healthcare resource utilization.
Despite a higher acquisition cost of escitalopram compared to other SSRIs, the present study found no evidence for a cost advantage of switching to other SSRIs in the first 3 months after the index date. In the unadjusted analyses, the switching group incurred higher MDD-related medical costs compared to the control group over the same period. Notably, the total drug costs were $163 higher in the switcher group, which could be an indication that the cost of medications required to manage the adverse events associated with non-medical antidepressant switching outweighed the decrease in SSRI acquisition costs in the first 3 months after the index date. In addition, the higher drug cost in the switcher group may be in part related to the need for dosage adjustment after initiating a new treatment. Although the extent to which this factor affected the cost difference was not analyzed in this study, it is one of several factors that should be considered before a patient's treatment is altered for non-medical reasons. Multivariate regression analyses showed that the total healthcare costs were $322 higher in the 3-month period after switching for patients who switched compared to the control cohort ($2,808 vs. $2,486; p < 0.0001).
The present study has some limitations. First, because adverse effects are normally more pronounced in the first weeks after the start of SSRI treatment, and possibly after switching to a new SSRI, 3 months may not be a long enough period to estimate conclusively the long-term cost-effectiveness of switching SSRIs. The downward trend of diminishing side-effects with time may, however, be counterbalanced or even exceeded by an upward trend due to the greater baseline levels of adverse effects of the SSRIs switched to, because escitalopram has a more favorable adverse effect profile than the other SSRIs. A 3-month period was chosen for this study in order to maximize the sample size of the switching group. Second, this study suffers from the usual limitations associated with claims data, including the absence of clinical data (such as smoking status, pregnancy, weight) and markers of disease severity as well as ICD-9 miscoding, which could contribute to physicians’ decision to switch antidepressants. Therefore, this study would not be able to identify all switchings due to non-medical reasons. Last, the study findings may be sensitive to the definition of switching for non-medical reasons. Although one cannot infer from claims data the real reason behind a switch in medications, a plausible algorithm was developed to identify patients who switch medications for non-medical reasons. However, the absence of hospitalization, ER visits or psychotherapy visits in the 7 days prior to the switch date does not rule out the possibility that a patient did not experience a lack of efficacy or suffer from serious side-effects and, consequently, that the decision to switch was not directed by medical considerations. The increase in healthcare costs after the index date in the switching group may therefore be partly due to the inclusion of patients who had not been responding adequately to the initial therapy or who had continued to have significant adverse side-effects. This trend is likely to be offset by patients from the control group who had also failed to respond adequately to escitalopram treatment or continued to experience adverse reactions without using urgent care resources or having a psychotherapy visit within 7 days prior to the index date.
Conclusion
This large claims data analysis has provided evidence that MDD patients who were switched to another SSRI for non-medical reasons after being stabilized on escitalopram for at least 3 months required greater healthcare resource utilization and healthcare costs in the 3 months after switching compared with MDD patients who remained on escitalopram. Given the clinical risks and lack of a cost advantage, the findings of this study suggest that switching from escitalopram to another SSRI appears to be an ineffective cost-saving measure. This implies that policy intending to lower drug costs by encouraging switching to generic products may not be cost saving from a payer perspective.
Transparency
Declaration of interest: This study was funded by Forest Laboratories, Inc. and was presented at the American Psychiatric Association annual meeting, Washington, DC, May 3–8, 2008.
Declaration of financial/other relationships: E.Q.W., R.B.H., A.P.Y. and J.T. have disclosed that they are employees of Analysis Group, Inc., Boston, MA, which received funding for this study. M.H.E. and A.B. have disclosed that they are employees of Forest Research Institute Inc., Jersey City, NJ.
References
- Bloom BS. Prevalence and economic effects of depression. Manag Care 2004; 13: 9–16
- Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289: 3095–3105
- Kessler RC, Nelson CB, McGonagle KA, et al. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry Suppl 1996; 17–30
- Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000?. J Clin Psychiatry 2003; 64: 1465–1475
- Davidson JR, Meltzer-Brody SE. The underrecognition and undertreatment of depression: what is the breadth and depth of the problem?. J Clin Psychiatry 1999; 60(Suppl 7)4–9, discussion 10-11
- Bradvik L, Mattisson C, Bogren M, et al. Long-term suicide risk of depression in the Lundby cohort 1947-1997 – severity and gender. Acta Psychiatr Scand 2008; 117: 185–191
- Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA 1997; 277: 333–340
- Panzarino PJ, Jr, Nash DB. Cost-effective treatment of depression with selective serotonin reuptake inhibitors. Am J Manag Care 2001; 7: 173–184
- Frank L, Revicki DA, Sorensen SV, et al. The economics of selective serotonin reuptake inhibitors in depression: a critical review. CNS Drugs 2001; 15: 59–83
- Peveler R, Kendrick T, Buxton M, et al. A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine. Health Technol Assess 2005; 9: 1–134, iii
- Revicki DA, Simon GE, Chan K, et al. Depression, health-related quality of life, and medical cost outcomes of receiving recommended levels of antidepressant treatment. J Fam Pract 1998; 47: 446–452
- Burke WJ. Escitalopram. Expert Opin Investig Drugs 2002; 11: 1477–1486
- Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry 2002; 63: 331–336
- Murdoch D, Keam SJ. Spotlight on escitalopram in the management of major depressive disorder. CNS Drugs 2006; 20: 167–170
- Gorwood P, Weiller E, Lemming O, et al. Escitalopram prevents relapse in older patients with major depressive disorder. Am J Geriatr Psychiatry 2007; 15: 581–593
- Kornstein SG, Bose A, Li D, et al. Escitalopram maintenance treatment for prevention of recurrent depression: a randomized, placebo-controlled trial. J Clin Psychiatry 2006; 67: 1767–1775
- Rapaport MH, Bose A, Zheng H. Escitalopram continuation treatment prevents relapse of depressive episodes. J Clin Psychiatry 2004; 65: 44–49
- Baldwin DS. Escitalopram: efficacy and tolerability in the treatment of depression. Hosp Med 2002; 63: 668–671
- Baldwin DS, Reines EH, Guiton C, et al. Escitalopram therapy for major depression and anxiety disorders. Ann Pharmacother 2007; 41: 1583–1592
- Gorman JM, Korotzer A, Su G. Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: pooled analysis of placebo-controlled trials. CNS Spectr 2002; 7: 40–44
- Lam RW, Andersen HF. The influence of baseline severity on efficacy of escitalopram and citalopram in the treatment of major depressive disorder: an extended analysis. Pharmacopsychiatry 2006; 39: 180–184
- Lepola U, Wade A, Andersen HF. Do equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo-controlled studies in major depressive disorder. Int Clin Psychopharmacol 2004; 19: 149–155
- Lepola UM, Loft H, Reines EH. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol 2003; 18: 211–217
- Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. Int Clin Psychopharmacol 2005; 20: 131–137
- Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry 2004; 65: 1190–1196
- Llorca PM, Fernandez JL. Escitalopram in the treatment of major depressive disorder: clinical efficacy, tolerability and cost-effectiveness vs. venlafaxine extended-release formulation. Int J Clin Pract 2007; 61: 702–710
- Kilts CD, Wade AG, Andersen HF, et al. Baseline severity of depression predicts antidepressant drug response relative to escitalopram. Expert Opin Pharmacother 2009; 10: 927–936
- Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA 2001; 286: 2947–2955
- Melfi CA, Chawla AJ, Croghan TW, et al. The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Arch Gen Psychiatry 1998; 55: 1128–1132
- Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care 1995; 33: 67–74
- Papakostas GI, Fava M, Thase ME. Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry 2008; 63: 699–704
- Nelson JC. Managing treatment-resistant major depression. J Clin Psychiatry 2003; 64(Suppl 1)5–12
- Dording CM, Mischoulon D, Petersen TJ, et al. The pharmacologic management of SSRI-induced side effects: a survey of psychiatrists. Ann Clin Psychiatry 2002; 14: 143–147
- Fava M. Management of nonresponse and intolerance: switching strategies. J Clin Psychiatry 2000; 61(Suppl 2)10–12
- Thase ME. Therapeutic alternatives for difficult-to-treat depression: a narrative review of the state of the evidence. CNS Spectr 2004; 9: 808–816, 818-821
- Fava M, McGrath PJ, Sheu WP. Switching to reboxetine: an efficacy and safety study in patients with major depressive disorder unresponsive to fluoxetine. J Clin Psychopharmacol 2003; 23: 365–369
- Nurnberg HG, Thompson PM, Hensley PL. Antidepressant medication change in a clinical treatment setting: a comparison of the effectiveness of selective serotonin reuptake inhibitors. J Clin Psychiatry 1999; 60: 574–579
- Perahia DG, Quail D, Desaiah D, et al. Switching to duloxetine from selective serotonin reuptake inhibitor antidepressants: a multicenter trial comparing 2 switching techniques. J Clin Psychiatry 2008; 69: 95–105
- Ruhe HG, Huyser J, Swinkels JA, et al. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry 2006; 67: 1836–1855
- Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials 2004; 25: 119–142
- Wohlreich MM, Mallinckrodt CH, Watkin JG, et al. Immediate switching of antidepressant therapy: results from a clinical trial of duloxetine. Ann Clin Psychiatry 2005; 17: 259–268
- Miner CM, Brown EB, Gonzales JS, et al. Switching patients from daily citalopram, paroxetine, or sertraline to once-weekly fluoxetine in the maintenance of response for depression. J Clin Psychiatry 2002; 63: 232–240
- Russell JM, Berndt ER, Miceli R, et al. Course and cost of treatment for depression with fluoxetine, paroxetine, and sertraline. Am J Manag Care 1999; 5: 597–606
- Armstrong EP, Skrepnek GH, Haim Erder M. Cost-utility comparison of escitalopram and sertraline in the treatment of major depressive disorder. Curr Med Res Opin 2007; 23: 251–258
- Demyttenaere K, Hemels ME, Hudry J, et al. A cost-effectiveness model of escitalopram, citalopram, and venlafaxine as first-line treatment for major depressive disorder in Belgium. Clin Ther 2005; 27: 111–124
- Fantino B, Moore N, Verdoux H, et al. Cost-effectiveness of escitalopram vs. citalopram in major depressive disorder. Int Clin Psychopharmacol 2007; 22: 107–115
- Fernandez JL, Montgomery S, Francois C. Evaluation of the cost effectiveness of escitalopram versus venlafaxine XR in major depressive disorder. Pharmacoeconomics 2005; 23: 155–167
- Francois C, Toumi M, Aakhus AM, et al. A pharmacoeconomic evaluation of escitalopram, a new selective serotonin reuptake inhibitor. Comparison of cost-effectiveness between escitalopram, citalopram, fluoxetine, and venlafaxine for the treatment of depression in Norway. Eur J Health Econ 2003; 4: 12–19
- Hemels ME, Kasper S, Walter E, et al. Cost-effectiveness of escitalopram versus citalopram in the treatment of severe depression. Ann Pharmacother 2004; 38: 954–960
- Kulp W, von der Schulenburg JM, Greiner W. Cost-effectiveness of outpatient treatment in depressive patients with escitalopram in Germany. Eur J Health Econ 2005; 6: 317–321
- Sorensen J, Stage KB, Damsbo N, et al. A Danish cost-effectiveness model of escitalopram in comparison with citalopram and venlafaxine as first-line treatments for major depressive disorder in primary care. Nord J Psychiatry 2007; 61: 100–108
- Sullivan PW, Valuck R, Saseen J, et al. A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS Drugs 2004; 18: 911–932
- Wade AG, Toumi I, Hemels ME. A pharmacoeconomic evaluation of escitalopram versus citalopram in the treatment of severe depression in the United Kingdom. Clin Ther 2005; 27: 486–496
- Khandker RK, Kruzikas DT, McLaughlin TP. Pharmacy and medical costs associated with switching between venlafaxine and SSRI antidepressant therapy for the treatment of major depressive disorder. J Manag Care Pharm 2008; 14: 426–441
- Wu E, Greenberg PE, Yang E, et al. Comparison of escitalopram versus citalopram for the treatment of major depressive disorder in a geriatric population. Curr Med Res Opin 2008; 24: 2587–2595
- Wu E, Greenberg P, Yang E, et al. Comparison of treatment persistence, hospital utilization and costs among major depressive disorder geriatric patients treated with escitalopram versus other SSRI/SNRI antidepressants. Curr Med Res Opin 2008; 24: 2805–2813
- McCombs JS, Luo M, Johnstone BM, et al. The use of conventional antipsychotic medications for patients with schizophrenia in a Medicaid population: therapeutic and cost outcomes over 2 years. Value Health 2000; 3: 222–231
- Blough DK, Madden CW, Hornbrook MC. Modeling risk using generalized linear models. J Health Econ 1999; 18: 153–171
- Diehr P, Yanez D, Ash A, et al. Methods for analyzing health care utilization and costs. Annu Rev Public Health 1999; 20: 125–144
- Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004; 161: 692–699
- Bull SA, Hunkeler EM, Lee JY, et al. Discontinuing or switching selective serotonin-reuptake inhibitors. Ann Pharmacother 2002; 36: 578–584
