Abstract
Objective: Two human papillomavirus (HPV) vaccines are on the market. Based on expected differences in sustained- and cross-protection between the two vaccines, their long-term economic value is modelled and compared for France, Ireland and Italy.
Methods: A Markov cohort model reproducing the natural history of HPV infections, screening and vaccination, is adapted to country-specific data. Two hypothetical HPV vaccines (VA and VB) are compared. At baseline VA provides lifetime protection against HPV‐16 and 10-year protection against HPV‐18 before waning. VB is the same as VA with a 10-year protection against HPV‐6 and 11. Sustained- and cross-protection is varied over wide ranges in VA to define the levels that could make VA cost-effective or dominant compared with VB.
Results: Under baseline conditions VB dominates VA. VA becomes cost-effective when the difference in cross-protection alone reaches 13–15% (undiscounted), and 22–44% (discounted). A combination of sustained- and cross-protection is required for VA to dominate VB (discounted). The results are dependent upon country, the base-case value and the discount applied.
Conclusion: Realistic additional sustained- and cross-protection in one HPV vaccine may confer benefits that offset the economic value of protection against low-risk HPV in the other. The results are country specific.
Introduction
Cervical cancer (CC) is the third largest cause of cancer mortality in women worldwide, accounting for 274,000 deaths in 20021. In 25 countries of the European Union (EU) approximately 31,000 CC cases and 14,000 specific deaths occur each yearCitation[2]. However, incidence and mortality rates vary considerably between countriesCitation[2]. A variety of different screening programmes have been introduced, resulting in a substantial reduction in the CC burden in many countriesCitation[3],Citation[4]. Nevertheless, CC still remains a public health threat that could be reduced by adding vaccination to screening as an additional disease-prevention toolCitation[2].
The human papillomavirus (HPV) is known to be the predominant cause of CCCitation[3]. Vaccination against HPV infection therefore has the potential to help prevent the development of HPV-associated cancer and pre-cancerous disease conditions. At least 130 different HPV types have been discovered but only a few are associated with CC, of which the most common are HPV types 16, 18, 31 and 453. The most frequent are HPV types 16 and 18, which account for 70% of CC cases worldwide. With the addition of HPV types 31 and 45 the frequency reaches 80%Citation[5]. As well as the oncogenic HPV types, low-risk HPV types also prevail but these do not lead to cancerCitation[6]. They do however play a role in the development of diseases such as genital warts (GW), as well as low-grade pre-cancerous lesionsCitation[6–8].
Two vaccines against CC are currently available, a bivalent vaccine targeting HPV types 16 and 189,Citation[10] and a quadrivalent vaccine targeting HPV types 6, 11, 16 and 1811,Citation[12]. Both vaccines use virus-like particles that are made up of recombinant capsid proteins from the relevant HPV types to generate an immune response. Both vaccines have been demonstrated to be highly effective against HPV types 16 and 18 infections and precancerous lesions in large phase III randomised clinical trialsCitation[11],Citation[13]. Other differences between the two vaccines exist. For instance, the adjuvant used in each vaccine to enhance the immunogenicity reaction is different. The bivalent vaccine is formulated with the proprietary Adjuvant System 04 (AS04), that has been shown to produce an enhanced immune response compared with the same non-adjuvanted vaccineCitation[14]. A recent head-to-head comparison of the immunogenicity profile over time of the two vaccines demonstrated that the bivalent vaccine induced a significantly higher neutralising antibody titre and more circulating memory B-cells specific to HPV types 16 and 18, compared with the quadrivalent vaccineCitation[15]. Even though the clinical significance of the difference in immune response is not known, this enhanced immune response may indicate that the bivalent vaccine provides longer protection than the quadrivalent. Furthermore, although there are no head-to-head efficacy trials of the two vaccines and, therefore, any comparison between the two has some limitationsCitation[16], the results from the respective clinical trials indicate that the bivalent vaccine may provide more cross-protection against other oncogenic HPV types than the quadrivalent vaccineCitation[13],Citation[17],Citation[18]. Published data from the clinical trial results of the bivalent vaccine indicate that its cross-protection vaccine efficacy level is 68% against CIN 2+ lesions relative to the ten most frequent non-vaccine HPV types in CC (31, 45, 33, 52, 58, 35, 39, 51, 56, 59)Citation[19]. The quadrivalent vaccine has reported an efficacy of 33% against the ten HPV types in CIN 2+Citation[17].
Previous modelling studies highlighted the effect of cross- and sustained-protection on the cost-effectiveness results of HPVCitation[20–24]. Information on the potential economic value of the factors that may differentiate the two vaccines would therefore be valuable for estimating and comparing the potential advantages and/or disadvantages of both vaccines under different scenarios. Healthcare decision-makers will indeed need to analyze both vaccines’ data and determine, for their population, how the potential efficacy due to the cross-protection and more sustained-protection observed with the bivalent vaccine compares with the prevention of the two additional low-risk HPV types offered by the quadrivalent vaccine against GW.
The impact of different levels of cross- and sustained-protection against CC on the economic value of both vaccines is analysed here below as a sensitivity analysis, given the remaining uncertainty around the specific characteristics (e.g., sustained-protection) of each vaccine. Hypothetical vaccines are therefore compared. If cross- and sustained-protection were equivalent between both vaccines, the additional protection against GW would be a net gain for the vaccine that protects against it. Any additional level of cross- or sustained-protection the bivalent vaccine may have against CC may offset the protection against GW and change the economic results. The authors investigated the amount of additional cross- and/or sustained-protection that would be needed for the bivalent vaccine to become cost-effective (incremental cost-effectiveness ratio below the cost-effectiveness threshold or dominant yields less costs and more QALY gained) compared with the quadrivalent vaccine from an economic perspective.
It is likely that the size of the difference in cross- and sustained-protection required for the two vaccines to provide equivalent benefits will vary between countries, because of country-specific factors such as disease epidemiology, disease management, costs and health economic guidelinesCitation[25]. To explore such country-specific differences the model was applied to three European countries (France, Italy and Ireland). These countries differ in cumulative rate of CC mortality (0.28% in France and 0.29% in Ireland compared with 0.19% in Italy)Citation[2], disease management, associated costs and guidelinesCitation[25]. So the selected countries should provide a reasonable overview of the likely differences across European countries that already have in place a screening programme against CC.
To the authors’ knowledge, no one has yet studied the effects of differences in these particular vaccine attributes over a wide range of values, investigating the effect of discounting, and assessing whether analyses should be country-specific. The present study analysis has been set out to address all these questions.
Methods and material
Model structure
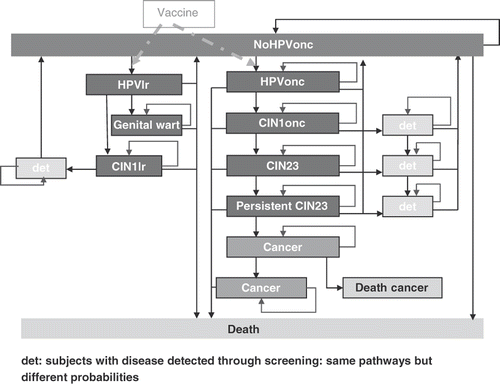
This analysis was conducted using a previously developed lifetime Markov cohort model with a 1‐year cycle reproducing the natural history of oncogenic HPV in cervical cancer, the effect of screening and of vaccinationCitation[24],Citation[26–28]. The initial model has been extended by adding a component covering the low-risk HPV infections (including HPV types 6 & 11) that might evolve to cervical intraepithelial neoplasia (CIN) 1 disease status and/or to GW only. The structure of the new model is shown in .
Figure 1. Model structure. CIN, cervical intraepithelial neoplasia; det, detected; HPVlr, human papillomavirus low-risk types; HPVonc, human papillomavirus oncogenic types.
For each country, an age cohort of girls aged 12 years was entered into the model. This age selection corresponds to the general recommendation for starting vaccination against HPV-infection in most of the European countries. The cohort moves between the different health-states in the model each year according to transition probabilities based on the natural history of the disease and its treatment. The model runs in 1-year cycles for 95 years, thus covering the total lifetime of the cohort. Each health state is associated with a utility score and a cost. Both costs and utility scores are combined with the time spent in each health state to estimate the total accrued cost and number of quality-adjusted life-years (QALYs). The model is run from the healthcare payer perspective and both costs and utility scores are adjusted using country-specific discount ratesCitation[25].
Vaccine profiles
Two baseline vaccine profiles were constructed:
Vaccine A has lifetime protection against HPV types 16, time-limited protection against HPV types 18 that begins to wane 10 years after vaccine initiation, and no cross-protection against non-vaccine oncogenic HPV types under baseline conditions;
Vaccine B has the same initial profile as vaccine A, with additional protection against the low-risk HPV types 6 and 11 causing GW that begins to wane after 10 years.
These baseline profiles permit the potential impact of a large range of values for additional cross- and sustained-protection in vaccine A to be evaluated.
Input data
The model was adapted to each country separately using country-specific epidemiological data, information based on literature reviews and local expert opinion. lists the main input data for the base-case analysis in each of the three countries selected. Published information was used whenever available, but in the absence of data expert opinion was sought (one in Ireland, three in France and three in Italy). In addition to providing assessment for missing input data, the experts also reviewed the model outcome in order to evaluate its applicability to the local situation. The size of the vaccinated cohort was based on national demographic data. It was assumed that there was 100% vaccine coverage rate to obtain maximum effect on the lesions. Also, as no implementation cost was included and the model is static, vaccination coverage rates do not impact on the incremental cost-effectiveness ratio (ICER)Citation[24].
Table 1. Key model input data by country.
The HPV type-specific vaccine efficacy (i.e., against HPV types 16 and 18 for vaccine A and against HPV types 16, 18, 6 and 11 for vaccine B) were identical for both vaccines and across the three countries. This efficacy was conservatively set at 95% based on reported data for the existing bivalent and quadrivalent vaccinesCitation[9],Citation[11],Citation[29],Citation[30].
The vaccine efficacy applied to the infection rate of HPV is that expected on the ‘end lesion’, i.e. cervical cancer for high risk HPV and genital warts for low risk HPV.
Adjustment is thereafter made to account for a different distribution of the HPV types in the precancerous and cervical cancer. With cross-protection, additional vaccine efficacy was applied to all non-vaccine oncogenic HPV types. The overall lesion-specific efficacy applied in the model was calculated by multiplying the percentage of HPV types in each specific lesion (CIN 1, CIN 2/3, CC, GW) by the vaccine efficacy estimated against each HPV type and summing the resultCitation[24].
Sustained-protection is maintained until the vaccine efficacy starts waning. The model then applies a linear reduction in vaccine efficacy over a 5-year period against all HPV types except HPV type 16. No waning against HPV type 16 is assumed because of the reported and observed long-term high immune response from both vaccinesCitation[31],Citation[32]. It was further assumed that a booster dose administered when waning starts confers lifelong protection against the vaccine HPV types and against the cross-protected HPV types. Compliance with the booster was limited to 40%, based on an expected low attendance rate. It was further envisioned that a booster dose would only be administered up to the age of 55 years because after that age no further booster benefit is expected.
Model validation
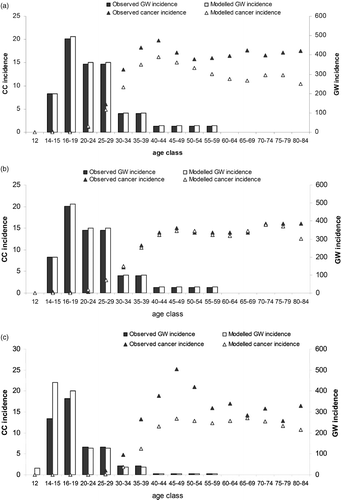
In each country, the incidence of GW and CC cases produced by the model in each age group without vaccination were compared with observed data to validate its predictabilityCitation[33]. For CC prediction, the model should closely replicate the observed data on events seen in the younger age groups (e.g., <45 years of age) as these result from infection, disease progression and treatment that occurred more recently and thus reflect the epidemiological and screening context imputed in the model. In older groups, reported events are likely to occur from infections in the past that differ from the input data in the model. Therefore, appropriate model validation should be considered when the model estimates cases that match observations during younger age-group periodsCitation[34], unless recent changes in disease management have occurred. This is for instance the case in Ireland, where screening has recently been introduced and has been proactively introduced into the modelCitation[35]. As a result, the currently observed CC incidence in Ireland is unlikely to be reproduced by the model over time.
The time needed for a low-risk HPV to evolve to genital warts is less than a yearCitation[36],Citation[37]. The incidence of genital warts is therefore less likely to be influenced by changes in either sexual behaviour or screening. Face validity of the model results was assessed by local experts.
Model outputs
For each vaccine profile in each country the model provides estimates of the number of GW cases, CC cases, CC deaths, QALYs and costs. The main outcome value produced by the model is the ICER between the two vaccines. The ICER is calculated as the difference in costs divided by the difference in QALYs gained between the two vaccines. Two cost-effectiveness threshold levels are evaluated for each country. One is the very cost-effective threshold limit (1 time the gross domestic product (GDP) per capita); the other is the cost-effective threshold limit of 3 times the GDP per capita. Those values refer to the World Health Organization (WHO) threshold limits that determine when a treatment is highly cost-effective or cost-effective only in a given countryCitation[38].
The effect of additional cross- and sustained-protection is measured by comparing the difference in CC cases prevented and the ICER for vaccine A over vaccine B. Multiple scenarios are tested by gradually increasing the level of cross-protection and the level of sustained-protection in the vaccine A profile. Given the current uncertainty in the level of cross- and sustained-protection provided by each vaccine, large ranges are assessed:
For cross-protection the range tested is from 0 to 50%
For sustained-protection the range is from 3 years to lifetime.
Based on the results obtained by changing the modelled levels of cross- and sustained-protection in the profile of vaccine A, three-limit frontier lines were constructed showing the minimal combined levels of cross- and sustained-protection needed to make vaccine A:
Cost-effective compared with vaccine B, i.e. ICER is below the country specific 3 times the GDP per capita which is the cost-effectiveness threshold limit or the cost-effectiveness frontier line
Highly cost-effective compared with vaccine B, i.e. the ICER value is below the country-specific 1 time the GDP per capita which is the very cost-effective threshold limit or the highly cost-effective frontier line
Dominant over vaccine B, i.e. vaccine A yields more QALYs and less costs (dominance frontier line)
All combinations of cross- and sustained-protection levels above the frontier lines represent combined cross- and sustained-protection levels at which vaccine A is economically preferred (cost-effective, highly cost-effective or dominant) than vaccine B. The two cost-effectiveness and dominance frontier lines are reported with and without discounting following local guidelines on health-economic assessment and to show the separate effect of discounting.
Sensitivity analysis
Sensitivity analysis is performed on the dominance and the 3 times GDP cost-effectiveness frontier lines for Italy as an example, varying the key input data. The purpose of this analysis is to show how the outcome of the model is affected by varying the value of the data directly related to the incidence and the cost of GW and CC as well as vaccine characteristics other than cross- and sustained-protection – drivers of the economic differences between the two vaccines. The selected data are for the CC and genital warts management costs and incidence-related parameters: the low- and high-risk HPV incidence (applying ±50% variation simultaneously on all the base-case age-dependent incidence rates that will impact the incidence of GW and CC), screening coverage, cancer, GW costs, For the vaccine characteristics related parameters the input data are: the vaccine efficacy against vaccine HPV types (90% and 100%), the booster sustained-protection conferred by a booster administration after 10, 30 and 50 years instead of lifetime protection used in the base case and the base-case sustained-protection for vaccine B (20, 30 and 50 years). The analysis was also performed on undiscounted values to assess the direct effect of each variable, as the authors were particularly interested in identifying which of the variables had the biggest impact on the ICER result.
Results
Model validation
The model outputs per country without vaccination reasonably mirror the observed data on age-dependent incidence rates of GW and CC in the three countries tested for the younger age range (up to the age of 45 years) (). The differences between observed and modelled data could be explained by changes over time in screening and/or sexual behaviour that are difficult to capture in a cohort model. The numbers produced by the model are a little below the observed values, and thus the model might provide conservative estimates of lesion incidence rates and consequently of vaccine effects.
Cost-effectiveness of vaccine A and B
The model-generated lifetime numbers of CC cases, GW cases, and total costs per birth- and per 100,000 cohort are reported for each country in without any vaccination. presents the cost, QALYs and incremental cost-effectiveness ratio (ICER) results of the baseline profiles of vaccine A compared with vaccine B in each country, both discounted and undiscounted. As expected, vaccine B always dominates vaccine A.
Table 2. Lifetime modelled number of CC, GW and total costs (€) per 100,000 and per actual 12-year-old age cohort in France, Ireland and Italy pre-vaccination.
Table 3. Incremental lifetime cost per QALY of vaccine A compared with B in each country (base case).
Changing cross- and sustained-protection level in vaccine A
shows for each country the 3 GDP per capita cost-effectiveness, the 1 GDP per capita cost-effectiveness and the dominance frontier line (compared with vaccine B). All combinations above the frontier lines represent combined cross- and sustained-protection levels at which vaccine A is economically acceptable (cost-effective, highly cost-effective) or superior (dominant) to vaccine B. The lines are presented both without (left part of ) and with discounting (right part of ).
Figure 3. Cost-effectiveness and dominance frontier for vaccine A compared with vaccine B by cross- and sustained-protection level with and without discounting in France, Ireland and Italy. VacA, vaccine A; X-protection, cross-protection (The dominance and cost effectiveness frontier lines determine the combined level of cross (x axe) and sustained (y axe) protection for vaccine A to dominate vaccine B (dominance frontier) and be cost effective compared with vaccine B (cost effectiveness frontier).
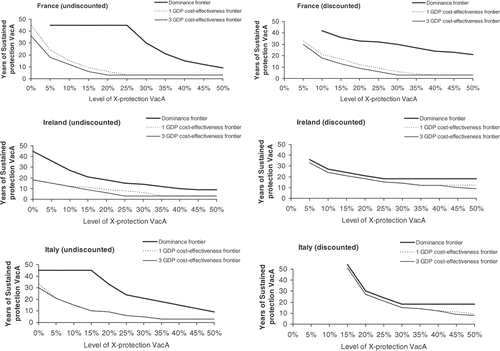
Without discounting, for every country, a cost-effective result can be reached by combining both cross- and sustained-protection within the range tested, including cross- or sustained-protection alone. The difference between the cost-effective and highly cost-effective frontier lines is marginal. The dominance frontier line shifts to the right and upwards relative to the cost-effectiveness frontier lines (see , left-hand graphs). It shows that vaccine A needs higher levels of cross-protection as well as longer sustained-protection to become dominant over vaccine B, relative to the conditions needed to become cost-effective compared with vaccine B. Dominance can also be reached with either sustained or cross-protection alone in all countries except France, where sustained-protection alone is not enough to reach the dominance condition. The area between the dominance and cost-effectiveness frontier lines is country-specific, with Ireland displaying the smallest shift between the two curves.
Discounting has a substantial effect on the results (right-hand graphs in ). For low additional cross-protection levels the area between the dominant and the cost-effectiveness frontier lines collapses for countries where the discount rates for cost and effect are 3% or above (i.e. Ireland and Italy). Also, the sustained-protection levels needed in combination with the higher cross-protection levels remain low. This indicates that under-discounted conditions a small additional sustained-protection level has a large effect in reaching dominance for vaccine A.
Compared with the undiscounted results, the cost-effectiveness frontier lines shift to the right and upwards. The dominance frontier lines, however, move in the opposite direction, downwards and to the left. Thus, to reach the cost-effectiveness frontier lines under discounted conditions the difference in sustained and cross-protection between the vaccines needs to be larger, while for the dominance frontier line it needs to be lower. Also, the magnitude of the difference between discounted and undiscounted frontier lines is country-specific depending on the discount rates applied in a country. France for instance, which uses the smallest values, has the smallest difference between the two graphs.
The minimum additional levels of cross- or sustained-protection alone needed to reach the cost-effectiveness frontier lines are presented in for each country with and without discounting.
Table 4. Minimum level of cross- and sustained-protection required alone for vaccine A to be cost-effective compared with vaccine B in each country without and with discounting.
Sensitivity analyses
Results of the sensitivity analysis on CC and GW related parameters are presented in and on vaccine characteristics in with and without discounting for both the dominance and the cost-effectiveness lines. The frontier lines obtained take on many different shapes.
Figure 4. Sensitivity analysis on Italian undiscounted and discounted dominance and cost-effectiveness frontiers. Effect of uncertainty around cervical cancer and genital wart management cost and incidence related parameters under a + and − 50% of base-case value for HPV low-risk and HPVonc oncogenic types incidence, CC costs and GW cost. CC, cervical cancer; GW, genital warts; HPV‐LR, human papillomavirus low risk; HPVonc, human papillomavirus; VacA, vaccine A; X-protection, cross-protection.
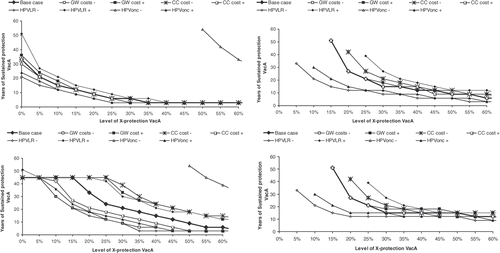
Figure 5. Sensitivity analysis on Italian undiscounted and discounted dominance and cost-effectiveness frontiers. Effect of uncertainty on vaccines characteristics related parameters: vaccine type efficacy (VE) of 90% and 100%, booster duration of 10, 30 and 50 years and base-case duration of protection (BC) for vaccine B of 20, 30 and 50 years.
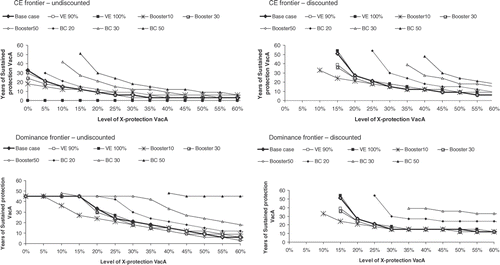
For the CC and GW related parameters. In the undiscounted analysis a decrease in GW incidence (low-risk HPV, HPV‐LR–) or costs as well as an increase in CC (oncongenic HPV, HPVonc+) incidence or costs leads to a dominance frontier line lower than the reference (base-case) line. This means that the levels of sustained and cross-protection needed for vaccine A to dominate vaccine B will both be lower than in the reference condition. The reverse is true for either a higher incidence in GW and/or cost or a lower incidence in CC and/or costs. The difference between the scenarios is however more pronounced with the dominance frontier than with the cost-effectiveness frontier.
The lowest frontier is obtained with a 50% reduction in HPV low-risk incidence rate. Under that scenario cross-protection alone can be enough to offset the benefit of GW prevention. On the other hand, the highest frontier line is obtained with a 50% reduction in oncogenic HPV. Under that scenario no combination of sustained- and cross-protection can be found in order for vaccine A to dominate or be cost-effective compared with vaccine B if the cross-protection is below 50%.
For the vaccine related parameters, a more sustained-protection for vaccine B at base line will limit the possibility vaccine A to dominate vaccine B. The effect of different vaccine efficacy or booster duration is less marked. The dominance frontier is lowered only when a 10-year booster duration is assumed and low cross-protection reached.
Discounting completely modifies the shape of the frontier lines for every scenario tested. With discounting, at low level of cross-protection, a lower level of sustained-protection is needed when the GW incidence is reduced or the CC incidence is increased. This is however not observed with sensitivity on cost inputs nor for an increase in GW nor for a decrease in CC incidence rate. The difference between the cost-effectiveness frontier line and the dominance frontier line is however less pronounced with discounting than without (see ). With a 50-year based case sustained-protection for vaccine B, no combination of sustained and cross-protection will allow vaccine A to dominate vaccine B under discounted value (no line therefore appears on the graph).
Discussion
Two HPV vaccines are available on the market. A recent clinical study has highlighted the different immunogenicity profile of both vaccines over time with the bivalent vaccine inducing a significantly higher neutralizing antibody titre and more circulating memory B-cells specific to HPV types 16 and 18, compared with the quadrivalent vaccineCitation[15]. Also the respective clinical trials of both vaccines indicate that the bivalent vaccine may provide more cross-protection against other oncogenic HPV types than the quadrivalent vaccineCitation[13],Citation[17],Citation[18]. This modelling exercise is performed to assess the potential economic value of having additional cross- and sustained-protection levels in an hypothetical bivalent HPV vaccine compared with an hypothetical quadrivalent HPV vaccine in three European countries displaying a reasonable variation in the number of CC cases and GW to be managed each year.
The study analysis shows that with the introduction of vaccination to improve the management of HPV-associated disease, including CC, the level of cross- and sustained-protection against oncogenic HPV types can modify the economic value of vaccination. This has implications for the comparison between the two vaccines.
If there were no difference in cross- or sustained-protection between the two vaccines, a quadrivalent vaccine could be dominant over a bivalent vaccine for obvious reasons. However, a difference in cross- and sustained-protection, separately or combined, in favour of the bivalent vaccine may reverse the above statement. The quantification of that modification is country-specific. The results of the study model show that in some countries cross-protection will play a more prominent role while in others sustained-protection is the critical factor.
For example, the effect of additional cross-protection alone seems to be greater in France, where under discounted conditions, a cross-protection against non-vaccine oncogenic HPV types of 22% is sufficient to be cost-effective compared with vaccine B, while levels of 41% and 44% are needed in Italy and Ireland, respectively. Without discounting, the level of additional cross-protection required in each country is 15%, 15% and 13%, respectively.
The impact of sustained-protection alone is more important in Ireland, where 16 years of sustained-protection without any cross-protection is sufficient for the bivalent vaccine to be cost-effective when compared with the quadrivalent vaccine with 10 years of sustained-protection. In Italy 29 years of sustained-protection is needed, and in France sustained-protection alone is insufficient. Under discounted conditions a combination of both sustained and cross-protection was needed to reach cost-effectiveness at the selected threshold in the three countries. It is interesting to note that the difference between the level of cross- and sustained-protection needed to reach the high cost-effectiveness limit (1 time the GDP per capita) versus the cost-effectiveness limit (3 times the GDP per capita) is very marginal if any difference exists. This is due to the much larger level of elasticity in costs than in QALYs. As a result of the elasticity difference between both variables, the incremental cost-effectiveness ratio which is a ratio of cost difference over QALY difference, grows exponentially and therefore jumps immediately from the 1 time GDP to the 3 times GDP threshold lines with small changes in sustained- and/or cross-protection levels.
When the benefits of both cross- and sustained-protection are combined, the bivalent vaccine becomes dominant in certain scenarios.
Discounting also impacts heavily on the end result. The application of discounting shifts the results to either lower or higher levels of cross- and sustained-protection. This is because discounting has two opposing effects. The discount rate on cost has a large impact on a booster vaccination, which will have a smaller influence on cost if its administration is delayed. Better sustained-protection will therefore reduce the total cost of HPV disease management. On the other hand, discount rates reduce the benefits of cross-protection as most of the gains (especially the CC-related ones) occur many years after vaccination. This explains the larger effect of additional sustained-protection in the discounted versus the undiscounted analysis, where the additional effect of cross-protection is also reduced. Consequently, undiscounted sustained-protection has a lower impact, while cross-protection has a larger impact (because all long-term benefits are taken into account).
As well as the effect of discounting, the end results are also influenced by a number of key factors such as the spread of the different HPV types in cancer and pre-cancerous lesions in a country, the local screening practices, the incidence and management of GW, the local treatment patterns and the associated costs.
Specific sensitivity analysis performed on the dominance frontier line of Italy by varying the main variables related to GW and CC incidence and costs highlights the effect of each parameter separately. Also, by varying the parameters included in the analysis we obtain the range of dominance frontiers observed among the different countries included in present study. Any reduction in GW incidence or cost as well as any increase in CC incidence or cost leads to lower cross- or sustained-protection levels required for the bivalent vaccine to dominate the quadrivalent vaccine. Consequently a different model calibration will also lead to different results. The relative value of these parameters in a country is important to determine the relative value of each vaccine. The effect of changes in CC and GW incidence is more important under discounted conditions than the effect of changes in costs. So, countries with a low incidence of GW may benefit more from the cross-protection difference between the vaccines, but that phenomenon could be counter-balanced if the cost of treatment of GW is high. The same can be said about the proportion of non-vaccine oncogenic HPV types in cancer in a particular country. If that proportion is high, the bivalent vaccine will have a better health economic profile. But if the proportion of HPV types 16 and 18 is high, as for example in Ireland, cross-protection may not contribute much to the benefit of vaccination with vaccine A because there is little scope for cross-protection against non-vaccine HPV types to generate extra benefits compared with vaccine B. Hence, in Ireland, a higher cross-protection level is needed to offset the protection against low-risk HPV. On the other hand different assumptions regarding the vaccine specific HPV types efficacy does not significantly modify the results. Also the duration of booster protection has a limited effect except when low cross-protection is attained and thus a high level of sustained-protection is needed for vaccine B to dominate vaccine A. The base-case duration of sustained-protection for vaccine B does however change the results. With 50 years sustained-protection and under discounted conditions, vaccine A cannot dominate vaccine B, even with the highest level of sustained- and cross-protection.
Even though placebo controlled trials have been conducted for each vaccine separately, no head-to-head clinical, efficacy trial has been initiated yet, so that only indirect comparison can be made with the limitations that this method hasCitation[16]. Published data from the clinical trial results of the bivalent vaccine have indicated that its cross-protection vaccine efficacy is 68% against CIN 2+ lesions related to the ten most frequent non-vaccine HPV types in CC (31, 45, 33, 52, 58, 35, 39, 51, 56, 59)Citation[19]. These HPV types are responsible for 22% of the CC cases worldwide (WHO data)Citation[39]. In the study model, all non-16/18 HPV types are responsible for 30% of CC cases. A vaccine efficacy of 68% against ten HPV types results in an absolute efficacy of 15% (= 68% × 22%). This equals an efficacy of 50% against all non-16/18 HPV types (50% × 30% = 15%). This is also consistent with the vaccine efficacy reported for the bivalent vaccine on non-HPV 16/18 CIN 2+ lesions, which is 54%Citation[13]. On the other hand, the quadrivalent vaccine has reported an efficacy of 33% against the ten HPV types in CIN 2+Citation[17]. This corresponds to an absolute efficacy of 7% (= 33% × 22%). Against all non-vaccine HPV types the efficacy would be 23% (23% × 30% = 7%). So the estimated absolute efficacy difference between the two vaccines is around 27% in cross-protection. This efficacy value is roughly in the middle of the range tested on cross-protection in the study sensitivity analysis.
The strengths of the model presented here include its simplicityCitation[40], transparency and adaptability to reflect country-specific epidemiology and disease management patterns. But the analysis has some limitations as well. The assumptions used in the base-case condition of each vaccine such as the limited 10-year period of sustained-protection after vaccination, have a certain effect on the end-results. The difference in cost and/or QALY gain between the two vaccines will be smaller if the time difference of sustained-protection is delayed from the age at vaccination as most of the HPV infections will have been covered before any difference between the two vaccines is starting to emerge. This does not apply for cross-protection as the effect of additional cross-protection is independent of the base-case condition. Because the model is not dynamic, it is unable to capture indirect protective benefits of vaccination such as herd effects, and thus it may underestimate both the potential benefit of vaccination and the difference between vaccine types. If one vaccine provides higher cross-protection than the other, it is likely that this would also translate into a greater herd protection effect. The conventional Markov process cohort approach was chosen because it is simpler and requires fewer data, and is applicable to countries that do not have access to detailed data on HPV transmission through sexual behaviour. Another limitation of using a cohort model is that it is not possible to estimate the vaccination effect at the population level over time which would report more closely what will be observed in a real world setting.
The analysis and results here presented remain a modelling exercise. Additional data on cross- and sustained-protection are required on both products to permit a more straightforward comparison between the vaccines. The modelled results of the study require confirmation by real-world data from long-term follow-up studies. Such data will not be available for several years. In the meantime, the present model offers a valuable method of estimating the potential value of differences in sustained and cross-protection between the available HPV vaccines.
Conclusion
Results of the present modelling exercise indicate that the extent of additional cross- and sustained-protection the two currently available vaccines against CC would have, play a key role in defining the cost-effectiveness value of each vaccine. Better cross- and longer sustained-protection against oncogenic HPV types in one vaccine could completely offset the clinical and financial benefits of protection against GW by the other vaccine. The level of cross- and sustained-protection at which one vaccine becomes dominant over the other is however country-specific, and needs to be assessed specifically in each country to provide relevant estimates.
Transparency
Declaration of interest: This study was supported by GlaxoSmithKline Biologicals.
Declaration of financial/other relationships: N.D. and B.S. have disclosed that they are employees of GlaxoSmithKline Biologicals, Rixensart, Belgium.
Acknowledgments: The authors thank the local experts who have helped with the data selection and model validation process per country: Prof Capri in Italy, Bruno Detournay in France and Sandra Redmond in Ireland. The authors also thank Laure Delbecque and Carole Nadin who provided medical writing services.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108
- Arbyn M, Raifu AO, Autier P, et al. Burden of cervical cancer in Europe: estimates for 2004. Ann Oncol 2007; 18: 1708–1715
- Al-Daraji WI, Smith JH. Infection and cervical neoplasia: facts and fiction. Int J Clin Exp Pathol 2009; 2: 48–64
- Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 2005; 14: 677–686
- Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121: 621–632
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens – Part B: biological agents. Lancet Oncol 2009; 10: 321–322
- Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol 1995; 33: 2058–2063
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. Volume 90, 2007. Lyon: World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.
- Keam SJ, Harper DM. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix trade mark]. Drugs 2008; 68: 359–372
- Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil®). Drugs 2006; 66: 1263–1271
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95: 1459–1466
- Munoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102: 325–339
- Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374: 301–314
- Giannini SL, Hanon E, Moris P, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24: 5937–5949
- Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix(TM) and Gardasil(R) human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin 2009; 5: 702–716
- Song F, Loke YK, Walsh T, et al. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009; 338: b1147
- Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; Types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 2009; 199: 926–935
- Jenkins D. A review of cross-protection against oncogenic HPV by an HPV‐16/18 AS04-adjuvanted cervical cancer vaccine: importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gynecologic Oncology 2008; 110: S18–25
- Skinner R, Apter D, Chow SN, et al. Cross-protection efficacy of Cervarix(tm) against oncogenic HPV types beyond HPV-16/18. (Abstract n O-29.01 presented at the 25th International Papillomavirus Conference, May 8–14, Malmö, Sweden), 2009.
- Cuzick J, Castanon A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102: 933–939
- Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 2008; 337: a769
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359: 821–832
- Liu PH, Hu FC, Lee PI, et al. Cost-effectiveness of human papillomavirus vaccination for prevention of cervical cancer in Taiwan. BMC Health Serv Res 2010; 10: 11
- Suárez E, Smith JS, Bosch FX, et al. Cost-effectiveness of vaccination against cervical cancer: a multi-regional analysis assessing the impact of vaccine characteristics and alternative vaccination scenarios. Vaccine 2008; 26: F29–45
- Pharmacoeconomic guidelines around the world. http://www.ispor.org/PEguidelines/index.asp. 2008. Accessed: 11–2008.
- Anonychuk AM, Bauch CT, Fikre Merid M, et al. A cost-utility analysis of cervical cancer vaccination in preadolescent Canadian females. BMC Public Health 2009;9:401-doi:10.1186/1471-2458-9-401.
- Colantonio L, Gómez JA, Demarteau N, et al. Cost-effectiveness analysis of a cervical cancer vaccine in five Latin American countries. Vaccine 2009; 27: 5519–5529
- Debicki D, Ferko N, Demarteau N, et al. Comparison of detailed and succinct cohort modelling approaches in a multi-regional evaluation of cervical cancer vaccination. Vaccine 2008; 26: F16–28
- Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356: 1928–1943
- Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006; 367: 1247–1255
- David MP, Van Herck K, Hardt K, et al. Long-term persistence of anti-HPV‐16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol 2009; 115: S1–6
- Olsson SE, Villa LL, Costa RL, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007; 25: 4931–4939
- Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health 2003; 6: 9–17
- Elbasha EH, Dasbach EJ, Insinga R. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007; 13: 28–41
- O'Neill W. Cervical cancer screening in Ireland. Eur J Cancer 2000; 36: 2233–2234
- Stone KM. Human papillomavirus infection and genital warts: update on epidemiology and treatment. Clin Infect Dis 1995; 20(Suppl 1): S91–97
- Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis 2005; 191: 731–738
- WHO guide for standardization of economic evaluations of immunization programmes: final version July 2008. Geneva: World Health Organization.
- Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161–2170
- Ferko N, Postma M, Gallivan S, et al. Evolution of the health economics of cervical cancer vaccination. Vaccine 2008; 26: F3–15
- Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001; 285: 2995–3002
- The role of human papillomavirus vaccines in reducing the risk of cervical cancer in Ireland: a Health Technology Assessment, 2008. Cork, Ireland: Health Information and Quality Authority.
- Bory JP, Cucherousset J, Lorenzato M, et al. Recurrent human papillomavirus infection detected with the hybrid capture II assay selects women with normal cervical smears at risk for developing high grade cervical lesions: a longitudinal study of 3,091 women. Int J Cancer 2002; 102: 519–525
- Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7932 women. Br J Cancer 2001; 84: 1616–1623
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362: 1871–1876
- Dalstein V, Riethmuller D, Pretet JL, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer 2003; 106: 396–403
- Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 1999; 180: 1415–1423
- Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338: 423–428
- Molano M, Van den BA, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol 2003; 158: 486–494
- Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, et al. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet 2001; 358: 1782–1783
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286: 3106–3114
- Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003; 12: 485–490
- Monsonego J, Breugelmans JG, Bouee S, et al. [Anogenital warts incidence, medical management and costs in women consulting gynaecologists in France]. Gynecol Obstet Fertil 2007; 35: 107–113
- Expert opinion. 2007.
- Sexually transmitted infections 2006: Annual summary report, 2008. Dublin: The Health Protection Surveillance Centre.
- Giorgi Rossi P, Ricciardi A, Cohet C, et al. Epidemiology and costs of cervical cancer screening and cervical dysplasia in Italy. BMC Public Health 2009; 9: 71
- Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003; 9: 37–48
- Van De Velde N, Brisson M, Boily MC. Modeling human papillomavirus vaccine effectiveness: quantifying the impact of parameter uncertainty. Am J Epidemiol 2007; 165: 762–775
- Melnikow J, Nuovo J, Willan AR, et al. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998; 92: 727–735
- Holowaty P, Miller AB, Rohan T, et al. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst 1999; 91: 252–258
- Schlecht NF, Platt RW, Duarte-Franco E, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst 2003; 95: 1336–13343
- Capri S, Bamfi F, Marocco A, et al. Capitolo 6: Impatto clinico ed economico della vaccinazione anti-HPV. Ital J Public Health 2007; 4: 55–81
- Bergeron C, Breugelmans JG, Bouee S, et al. Coût du depistage et de la prise en charge des lesions precancereuses du col uterin en France. Gynecol Obstet Fertil 2006; 34: 1036–1042
- Antilla A, Aoki D, Arbyn M, et al. Cervix cancer screening. IARC Handbooks of Cancer Prevention 10. IARC Press, Lyon 2005
- Coleman MP, Gatta G, Verdecchia A, et al. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol 2003; 14(Suppl 5): v128–149
- Exbrayat C. Col de l'utérus. In: Remontet L, Buemi A, Velten M, et al., eds. Evolution de L'incidence et de la Mortalité par Cancer en France de 1978 à 2000. Saint-Maurice Cédex: Institut de Veille Sanitaire - Departement Maladies Chroniques et Traumatismes, 2002:107–112.
- Ferlay J, Bray F, Pisani P, et al. Globocan 2002: Cancer incidence, mortality and prevalence worldwide. IARC [IARC CancerBase No 5 v.2.0]. 2004. IARC Press. Accessed: 21-8-2008.
- Donnelly DW, Gavin AT, Comber H. A report of cancer incidence, mortality, treatment and survival in the North and South of Ireland: 1994–2004. Cork, Ireland: National Cancer Registry Ireland/Northern Ireland Cancer Registry, 2009.
- Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol 1995; 141: 680–689
- Bergeron C, Cartier I, Guldner L, et al. Lésions précancéreuses et cancers du col de l'utérus diagnostiqués par le frottis cervical, Ile-de-France, enquête Crisap, 2002. Bulletin Epidémiologique Hebdomadaire, 2005; 5–8
- Fender M, Schott J, Baldauf JJ, et al. [EVE, a regional campaign for the screening of cervical cancer. Organization, 7-years results and perspectives]. Presse Med 2003; 32: 1545–1551
- Rash B, Martin-Hirsch P, Schneider A, et al. Resource use and cost analysis of managing abnormal Pap smears: a retrospective study in five countries. Eur J Gynaecol Oncol 2008; 19: 225–232
- Dee A, Howell F, O'Connor C, et al. Determining the cost of genital warts: a study from Ireland. Sex Transm Infect 2008; 85: 402–403
- Bamfi F, Marocco A, Capri S, et al. Genital warts in Italy: a cost of illness analysis. (Abstract n° PIN37 presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 11th Annual European Congress, 8–11 November, Athens, Greece). Value Health 2008;11:A440.
- Arveux P, Benard S, Bouee S, et al. [Invasive cervical cancer treatment costs in France]. Bull Cancer 2007; 94: 219–224
- Rash B, Van Kriekinge G, Standaert B. Comparing management patterns and associated costs for women with abnormal cervical cytology in 5 different countries. (Abstract n° PCN37 presented at ISPOR 9th Annual European Congress, Copenhagen (DK), October 28–31). Value in Health 2006;9:A287.
- Appendix 11: Human Papillomavirus. In: Stratton KR, Durch JS, Lawrence S, eds. Vaccines for the 21st Century: a Tool for Decision Making. Washington D.C.: National Academy Press, 2000:213–221.
- Gold MR, Franks P, McCoy KI, et al. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care 1998; 36: 778–792
- Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004; 96: 604–615
- Insinga R, Glass A, Rush B. Health state transitions following an abnormal pap smear: implications for health utility assessment in cost-effectiveness analyses. (Abstract W-02 presented at 22nd International Papillomavirus Conference & Clinical Workshop 2005, April 30 – May 6, Vancouver, BC, Canada), 2005.
- Myers ER, Green S, Lipkus I. Patient preferences for health states related to HPV infection: visual analog scale versus time trade-off elicitation. (Abstract n° 542 presented at the Twenty-First International Papillomavirus Conference, México City, México. February 20–27), 2004.
- WHO/ICO (Institut Català d'Oncologia) information centre on HPV and cervical cancer. http://www.who.int/hpvcentre/statistics/dynamic/ico/DataQuerySelect.cfm. 2009. Accessed: 20-2-2009.
- Aubin F, Pretet JL, Jacquard AC, et al. Human papillomavirus genotype distribution in external acuminata condylomata: a Large French National Study (EDiTH IV). Clin Infect Dis 2008; 47: 610–615