Abstract
Objective: Prostate cancer is a leading cause of cancer death in men in the US. Castration-resistant prostate cancer (CRPC) is an advanced form of the disease and has a poor prognosis and limited treatment options. The objective of this study was to identify patients with CRPC from a medical claims database, and determine the prostate cancer-related economic burden and healthcare utilization of these patients.
Methods: This was a retrospective study using claims and enrollment information from a large US database linkable to laboratory data. Male patients aged 40 or older who were diagnosed with prostate cancer and received surgical or medical castration between July 1, 2001 and December 1, 2007 were considered for study inclusion. Patients with CRPC were initially identified based on at least two increases in prostrate-specific antigen (PSA) values. Due to the small number of patients with available PSA results data, logistic regression modeling using characteristics of patients with known CRPC was used to identify a larger set of patients with likely CRPC. Per-patient per-month healthcare utilization and costs were determined using medical and pharmacy claims data.
Results: The final sample of patients with likely CRPC as determined by regression modeling included 349 patients with known CRPC identified from the database on the basis of PSA results and an additional 2391 with likely CRPC. Within this final sample of 2740 CRPC patients, there was a per-patient per-month average of 1.43 prostate cancer-related ambulatory visits, 0.04 prostate cancer-related inpatient stays, and 0.01 prostate cancer-related ER visits. Average per-patient per-month prostate cancer-related costs were $1152 (SD = $2073) for ambulatory visits, $559 (SD = $2383) for inpatient stays, $72 (SD = $229) for pharmacy costs, and $1 (SD = $14) for ER visits. Total per-patient per-month prostate cancer-related costs were on average $1799 (SD = $3505), and these costs comprised about half of the all-cause healthcare costs for these patients.
Conclusions: CRPC is a costly disease, with ambulatory visits and inpatient care accounting for a substantial proportion of the economic burden. Limitations related to the use of retrospective claims data should be considered when interpreting these results.
Introduction
Prostate cancer is the most frequently diagnosed cancer and a leading cause of cancer death in men in the US. The American Cancer Society (ACS) estimated that in 2008, 186,320 new cases of prostate cancer occurred in the US, and 28,660 men died from prostate cancerCitation[1]. Prostate cancer progresses slowly, and many patients live for a considerable time following diagnosis. The ACS estimated that the 5-year relative survival rate for all prostate cancer stages is near 100%, and the 10-year survival rate is 91%Citation[2]. Castration-resistant prostate cancer (CRPC) is an advanced form of the disease that does not respond well to conventional hormone therapy. Patients with CRPC have a poorer prognosis and frequently have metastatic disease, particularly to bone, which can be a source of severe pain and discomfort. A recent study of men with metastatic CRPC found a median survival time of about 18 monthsCitation[3].
CRPC may be treated with a variety of options, including radiation therapy, radiotherapeutics, steroids, bisphosphonates, and the chemotherapeutic agents estramustine, mitoxantrone, and docetaxelCitation[4–8]. However, most existing treatments for CRPC are palliative and do not have a significant impact on survival. Chemotherapy with docetaxel is considered the current standard of care for patients with CRPC. However, in clinical trials, docetaxel was only found to increase median survival by 2.4 months, and it is not considered a cure for the diseaseCitation[9]. Due to the limited efficacy of current treatment regimens, a number of new therapies for first- or second-line treatment of CRPC are currently in clinical development. Some of these include the RANKL inhibitor denosumab, the CYP 17 inhibitor abiraterone, the VEGF inhibitor bevacizumab, the specific endothelin–A receptor antagonist zibotentan, the epothilone analog ixabepilone, and several immunotherapeutic approachesCitation[10–13].
To date, limited information exists regarding the costs incurred by CRPC patients in the US. CRPC is an advanced state of the disease, and treatments differ from those used in prostate cancer patients who still respond to conventional hormone therapy. Therefore, the costs and healthcare utilization among patients with CRPC may also differ from that observed among patients with pre-CRPC status. A better understanding of costs associated with advanced-stage prostate cancer may help inform treatment decisions or identify areas where new treatment options could reduce the economic burden on the healthcare system. The objective of the present study was to determine the economic burden and healthcare utilization associated with the treatment of CRPC in an insured population of US patients. A challenge associated with performing this study in an observational setting is that there is no standard method for identifying patients with CRPC from health plan databases. A definition of CRPC used in a clinical trial was applied in this study to identify an initial CRPC sampleCitation[11]. To increase sample size, a novel exploratory algorithm was developed to assist in the identification of additional CRPC patients.
Patients and methods
Study design
This was a retrospective claims data study using medical data, pharmacy data, enrollment information, and laboratory results. Data were obtained from a large managed care claims database. This study included commercially insured and Medicare Advantage patients, and data from July 1, 2001 through December 31, 2007 were used. As of 2007, data for approximately 35.4 million individuals (34.0 million commercially insured and 1.4 million Medicare Advantage patients) with both medical and pharmacy benefit coverage were available. Patients in the database are geographically diverse across the US.
Medical claims data are collected from healthcare sites (inpatient hospital, outpatient hospital, emergency room, physician's office, surgery center, etc.) for specialty, preventive, and office-based treatments. Claims for ambulatory services submitted by individual providers (e.g., physicians) use the HCFA-1500 format. Claims for facility services submitted by institutions (e.g., hospitals) use the UB-82 or UB-92 format. Medical claims include multiple diagnosis codes recorded with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes; procedures recorded with ICD-9-CM procedure codes, Current Procedural Terminology (CPT) codes, or Healthcare Common Procedure Coding System (HCPCS) codes; site of service codes; provider specialty codes; revenue codes (for facilities); and paid amounts.
Claims for pharmacy services are typically submitted electronically by the pharmacy at the time prescriptions are filled. Outpatient laboratory test results from two large laboratory facilities are available from a laboratory results database, and laboratory results data are linked to medical and pharmacy claims data using a patient identification number common to each of the databases. Laboratory results for approximately 30–40% of all outpatient laboratory tests are available in the database; however, results are more frequently available for blood-based tests than for laboratory tests using other methods. Standard Logical Observation Identifiers Names and Codes (LOINC) coding developed by the Regenstrief Institute, Inc to pool laboratory and clinical results was used to identify specific tests and results.
Patient identification
To be considered for inclusion in the study, patients were required to be male, aged 40 or older (defined by index date year), and have at least one medical claim with an ICD-9-CM diagnosis code for prostate cancer (185 [malignant neoplasm of the prostate] or 233.4 [prostate cancer]) during the study period from July 1, 2001 through December 31, 2007. Additionally, evidence of surgical castration or medical castration during the study period was required. Evidence of surgical castration was based on having any of the following procedure or diagnosis codes: CPT codes 54520, 54522, 54530, 54535, 54690; ICD-9 procedure codes 62.3, 62.41, 62.42; or ICD-9 diagnosis code V45.77. Evidence of medical castration was based on having a pharmacy claim for leuprolide, triptorelin, goserelin, or histrelin, or any of the following HCPCS procedural codes: J1950, J9217, J9218, J9219, Q0057, J3315, J9202, J9225, J9226, S0133. NDC codes used to identify pharmacy claims are provided in in the Appendix.
Subjects undergoing medical castration must have had a minimum of 8 weeks of therapy during the study period. Days of therapy were determined from the days supply on pharmacy claims and/or the days supply associated with the dose given in the medical claims. Days supply was calculated for medical claims using the number of billed units on the medical claim, the dose associated with one billed unit, and the days supply associated with the dose. A table of the days supply associated with one billed unit for each HCPCS code is provided in in the Appendix.
Additionally, to be considered for inclusion in the study, patients were required to have at least three laboratory results for the prostate specific antigen (PSA) test between the date of castration and December 1, 2007. Hormone-treated patients with fewer than three PSA laboratory values were considered for inclusion in the study if they received at least one of the following services, which must have occurred at least 2 days following surgical castration or 14 days following the initiation of medical castration: (a) at least one pharmacy claim or medical claim for docetaxel, (b) a medical claim for a bone X–ray or bone scan, (c) a medical claim for at least one PSA test, (d) at least one office visit with an oncologist for prostate cancer, or (e) at least one office visit.
An index date was assumed to be a proxy measure for the time patients became castration-resistant. For patients with at least three PSA results, the index date was defined as the date of the second PSA increase. For patients who did not have two PSA increases, inclusion was based on one of the other services described above. Identification using these services was hierarchical; patients with docetaxel use had an index date based on the first claim for docetaxel. If patients did not receive docetaxel, the index date was based on the first claim for a bone X–ray or bone scan. If patients did not have claims for bone X–ray or bone scan, the index date was based on the date of the third PSA test (in patients with three or more tests), second PSA test (in patients with two tests), or first PSA test (in patients with one test). In patients without claims for PSA tests, the index date was based on the first oncology office visit for prostate cancer. In patients not meeting any of these criteria, the index date was defined as the date of the first office visit. For all patients, the index date must have occurred between January 1, 2002 and December 1, 2007.
Finally, continuous enrollment in the health plan for 180 days prior to the index date (pre-index period), and for a minimum of 30 days following the index date (post-index period), was required for study inclusion. Patients were followed until the earlier of December 31, 2007 or the end of continuous enrollment.
CRPC patient cohort assignment
A definition of CRPC based on PSA increases and developed by oncologists for clinical trials was used hereCitation[11]. Patients were defined as having known CRPC if they had at least two PSA increases following surgical or medical castration (PSA value 1 < PSA value 2 < PSA value 3). If there were more than three PSA values, the increases were not required to be consecutive. For patients receiving surgical castration, the first PSA value considered for the CRPC definition must have occurred at least 2 days following surgical castration. For patients receiving medical castration, the first PSA value considered for the CRPC definition must have occurred at least 14 days following the initiation of medical castration. For all patients, the third PSA value must have been at least 50% (or 10 units) higher than the first PSA value, and each of the three PSA values must have been at least 14 days apart. Patients meeting these criteria were considered to have met the study definition of CRPC and were defined as having known CRPC. Patients who had three or more laboratory values for PSA but did not have at least two increases following castration were defined as not having CRPC. All other patients (who met the inclusion criteria described above but did not have three or more PSA results in the laboratory data) were defined as having unknown CRPC status.
Patients defined as having known CRPC or as not having CRPC based on PSA results were selected and used to model CRPC status, in order to determine which patients initially assigned an unknown CRPC status had likely CRPC. Patients with known CRPC status based on three or more PSA results in the laboratory data were assigned a score of ‘1’ if they met the study definition of CRPC and were defined as having known CRPC, and patients were assigned a score of ‘0’ if they were defined as not having CRPC. Logistic regression was used to model CRPC status as a function of age, pre-index comorbidity, pre-index prostate cancer-related costs, length of post-index enrollment, time from castration to index date, docetaxel use, evidence of bone X–ray or bone scan, number of PSA tests identified in the medical claims, and evidence of oncology or urology office visits following castration. The results of this logistic model were used to identify patients with unknown CRPC status who appeared similar to those with known CRPC. The estimated coefficients from this logistic model were applied to the entire sample of patients with known or unknown CRPC status, and the predicted probability (propensity score) was obtained from the model for each patient. The distribution of the propensity scores for patients known to have CRPC, patients not having CRPC based on PSA results, and patients with unknown CRPC status were examined in combination with the ROC curve from the model to determine the optimal propensity score threshold value to assign patients to a cohort of likely CRPC patients.
Study measures
All-cause healthcare resource utilization was identified based on medical claims for ambulatory visits, emergency department visits, and inpatient admissions during the pre-index and post-index periods. Ambulatory visits included physician office visits and visits at an outpatient facility (including outpatient procedures, outpatient services, and outpatient laboratory and radiology). Inpatient visits were identified based on American Medical Association (AMA) place of service codes in combination with revenue and provider specialty codes. Emergency department, physician office visits, and outpatient visits were identified based on AMA place of service codes. The monthly incidence of each type of visit was calculated, and the count of each type of visit during the post-index period was calculated per-patient per-month (PPPM). All-cause healthcare costs were computed as the combined health plan- and patient-paid amounts identified from medical and pharmacy claims in the post-index period. Costs were calculated for overall costs, medical costs (costs from medical claims), and pharmacy costs (costs from outpatient pharmacy claims). In addition, medical costs were broken down into several sub-categories: costs during ambulatory visits, costs during emergency department visits, costs during inpatient hospitalizations, and costs for other services.
Prostate cancer-related healthcare utilization was calculated for ambulatory visits, emergency department visits, and inpatient admissions during the pre-index and post-index periods. Any ambulatory or emergency department visit with a primary diagnosis of prostate cancer, primary diagnosis or procedure code for surgical or medical castration, primary diagnosis or procedure code for PSA tests (CPT codes 84152, 84153, or 84154; HCPCS G0103 or G9080; or ICD-9 diagnosis code 790.93), procedure code for radical prostatectomy (CPT codes 55810–55815, 55840–55845, 55866, or ICD-9 procedure code 60.5), or procedure code for docetaxel infusion (J9170) was considered prostate cancer-related. Any inpatient stay with a primary diagnosis of prostate cancer was considered prostate cancer-related. The monthly incidence of each type of visit was calculated, and the count of each type of visit during the post-index period was calculated as a count PPPM. Prostate cancer-related healthcare costs were calculated for overall costs, medical costs, and pharmacy costs; medical costs were broken down into ambulatory costs, emergency costs, inpatient costs, and other costs. Pharmacy costs were calculated from pharmacy claims for leuprolide, triptorelin, goserelin, histrelin, or docetaxel. In addition, all pharmacy claims with a provider specialty of oncology or urology were included as prostate cancer-related pharmacy costs.
All pre-index and post-index costs were calculated as costs PPPM, and were adjusted for inflation to 2007 dollars using the medical component of the consumer price indexCitation[14]. All study measures are presented for the overall sample and stratified by whether patients have known CRPC or likely CRPC. Means and standard deviations are presented for continuous measures of patient demographics, patient characteristics, healthcare utilization, and costs. Numbers and percents are presented for dichotomous measures of patient demographics and patient characteristics. Per-patient per-month incidences are presented for dichotomous utilization measures. Medians and interquartile ranges are also presented for per-patient per-month healthcare costs. To assess similarities and differences in the two study samples, patient demographics, patient characteristics, and utilization were compared between known and likely CRPC patients using chi-squared tests for discrete data and t-tests for continuous data. Due to non-normality of data, cost results were compared using Wilcoxon rank sum tests. All analyses were conducted using SAS, version 9.1 [SAS Institute, Inc., Cary, NC, USA].
Results
Of 140,190 patients identified from the database with a prostate cancer claim, 20,188 met the surgical or medical castration criteria (). Of these, 15,361 patients met age and enrollment criteria. However, only 974 of the 15,361 patients had at least three PSA results in the laboratory claims data. Of the 974 patients, 349 had two or more PSA increases and were defined as having known CRPC, while 625 patients did not have two or more PSA increases and were classified as not having CRPC.
Figure 1. Patient identification. PSA, prostrate-specific antigen; CRPC, castration-resistant prostate cancer.
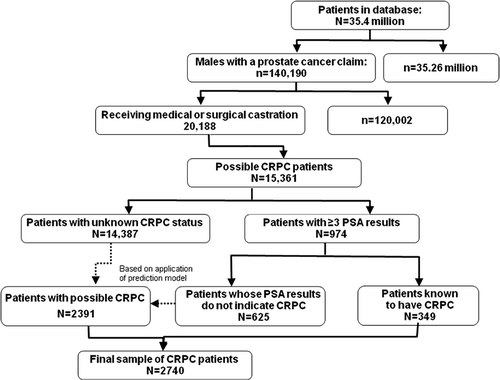
Logistic regression modeling of CRPC status was carried out for the 974 patients with available PSA laboratory data, the estimated coefficients were applied to the entire population of 15,361 patients, and predicted probabilities were calculated. Patients meeting the study definition (the 349 patients with known CRPC) had the highest average predicted probability (0.52), while patients not meeting the study definition (the 625 patients classified as not having CRPC) and patients with unknown CRPC status had much lower average predicted probabilities of 0.27 and 0.22, respectively. Propensity scores near the 25th percentile of the known CRPC sample (0.3270) were chosen to be the threshold value to separate the 14,387 patients initially classified as having unknown CRPC status into groups of likely CRPC patients and likely non-CRPC patients. At this threshold, the sensitivity was 0.75 and the specificity was 0.73. This is similar to the propensity score value that optimizes the sensitivity and specificity for this model (0.32); at this value, the sensitivity of this model is 0.76 and the specificity is 0.73. The final sample of CRPC patients used for analysis consisted of all 349 known CRPC patients and an additional 2391 patients identified as having likely CRPC based on the propensity score model, for a total of 2740 patients. The c-statistic for the logistic regression model was 0.8066.
The average age of patients in the final sample was 72.9 years (), and patients in the known CRPC group were younger than the patients in the likely CRPC group (68.2 vs. 73.6 years). Slightly more patients were enrolled in a commercial plan than a Medicare Advantage plan (55 vs. 45%), and a higher proportion of likely CRPC patients than known CRPC patients were enrolled in a Medicare Advantage plan (46 vs. 39%). Most patients were from either the South or the Midwest (42.7 and 40.7%, respectively), while relatively few patients were from the Northeast or the West (9.8 and 6.8%, respectively). A variety of comorbid conditions were identified in the patient population, including hypertension, diseases of the heart, diseases of the urinary system, disorders of lipid metabolism, secondary malignancies, eye disorders, lower respiratory disease, anemia, diabetes mellitus, non-traumatic joint disorders, and connective tissue disease. Overall, patients had an average pre-index Charlson comorbidity index of 4.4 (data not shown).
Table 1. Patient demographics.
Within the final patient sample, a much higher proportion of patients underwent medical castration as compared to surgical castration (95.8 vs. 4.2%) (). Of the other clinical characteristics examined, 92.9% (known CRPC: 84.0%; likely CRPC: 94.2%) of patients received a bone scan at any time following castration, 24.7% (known CRPC: 24.9%; likely CRPC: 24.6%) received docetaxel at any time following castration, 88.8% (known CRPC: 88.3%; likely CRPC: 88.8%) had a urology office visit at any time following castration, and 50.9% (known CRPC: 56.2%; likely CRPC: 50.1%) had an oncology office visit at any time following castration. The average length of time from castration to index date (a proxy date for the time until a patient became castration-resistant) was 719.7 days, and the average length of the post-index follow-up period was 406.5 days. Known CRPC patients had a shorter time from castration to the index date compared to likely CRPC patients (664.5 vs. 727.8 days), and known CRPC patients had a longer post-index follow-up period compared to likely CRPC patients (593.2 vs. 379.2 days).
Table 2. Patient clinical characteristics.
All-cause and prostate cancer-related healthcare utilization were measured during the post-index period (). Overall, patients had a PPPM average of 0.10 inpatient stays, 0.16 ER visits, and 3.34 ambulatory visits per month. The monthly incidences of all-cause inpatient, ER, and ambulatory visits were 0.05, 0.06, and 4.66, respectively. All-cause healthcare utilization was higher among likely CRPC patients compared to known CRPC patients (). When considering only prostate cancer-related healthcare utilization, patients had an average of 0.04 inpatient stays, 0.01 ER visits, and 1.43 ambulatory visits per month (). The monthly incidences of prostate cancer-related inpatient, ER, and ambulatory visits were 0.02, 0.003, and 0.60, respectively. Prostate cancer-related healthcare utilization was similar between known CRPC patients and likely CRPC patients ().
Table 3. Post-index prostate cancer-related and all-cause healthcare utilization.
Next, all-cause and prostate cancer-related healthcare costs were determined for patients in the post-index period (). The all-cause mean (median) total healthcare costs were $3507 ($1560) PPPM; $3212 ($1242) of this total cost were for medical costs, and the remaining $295 ($168) were for pharmacy costs. When medical costs were broken down further, ambulatory costs were found to be the most expensive subset (mean = $1851, median = $694), followed by inpatient costs (mean = $1208, median = $0) and ER costs (mean = $57, median = $1). The distribution of all-cause costs was similar between the known CRPC and likely CRPC groups, with the exception of ambulatory and other medical costs, which had slightly higher median costs in the known CRPC group (). The mean (median) costs for prostate cancer-related healthcare services were $1799 ($474) PPPM. Of this total, $1728 ($417) were for medical costs and $72 ($0) were for pharmacy-related costs. Of the medical costs, an average of $1152 ($328) were for ambulatory costs, $559 ($0) were for inpatient costs, and $1 ($0) was for ER costs. The distribution of prostate cancer-related costs was significantly different in the known CRPC group compared to the likely CRPC group, with higher median costs in known CRPC patients.
Table 4. Post-index prostate cancer-related and all-cause care costs (per-patient per-month).
Prostate cancer-related healthcare utilization was lower in the pre-index period than in the post-index period (data not shown), and this was reflected by lower average healthcare costs during the pre-index period. Mean (median) prostate cancer-related healthcare costs were $750 ($352) PPPM in the pre-index period, compared to $1799 ($474) PPPM in the post index period (). Additionally, expenditure as a percent of total costs increased for inpatient services and decreased for ambulatory services from the pre-index period to the post-index period. In the pre-index period, average inpatient services made up $102 of the $750 total for prostate cancer-related healthcare spending (13.6%), but in the post-index period average inpatient costs made up 31.1% of the total prostate cancer-related healthcare spending. However, ambulatory services made up $567 of the $750 total for prostate cancer-related healthcare spending (75.6%) in the pre-index period, but only accounted for 64.0% of the total post-index prostate cancer-related healthcare spending.
Table 5. Pre-index prostate cancer-related healthcare costs (per-patient per-month).
Discussion
The goal of this study was to identify patients with CRPC from an administrative claims database and determine the healthcare utilization and economic burden associated with CRPC. A final sample of 2740 CRPC patients was used for determination of CRPC-related healthcare utilization and costs following determination of CRPC status. Ambulatory visits were the most frequently observed category of prostate cancer-related healthcare during the post-index period (on average patients had 1.43 prostate cancer-related ambulatory visits per month), followed by inpatient stays (a monthly average of 0.04 per patient) and ER visits (a monthly average of 0.01 per patient). Patients had average monthly costs of $1799 relating to prostate cancer healthcare utilization in the post-index period. Reflecting levels of utilization, ambulatory costs ($1152) made up the largest portion of prostate cancer-related costs, followed by inpatient costs ($559), pharmacy costs ($72), and ER costs ($1). Prostate cancer-specific costs were a large proportion of overall healthcare costs for patients in this study, making up about 50% of the average monthly $3507 all-cause costs. In this study, the average PPPM prostate cancer-related costs more than doubled from the pre-index period to the post-index period, suggesting that the economic burden increases significantly as disease progresses to a castration-resistant phase. This may be due to increased morbidity in CRPC patients, requiring more frequent and expensive medical care. Further research is warranted to more specifically determine the cost drivers of CRPC.
To date there is little available information on the costs and healthcare utilization associated with CRPC in a general patient population. Some previous studies examining costs associated with treating CRPC have been conducted in a clinical setting, and have evaluated patients receiving a specific treatment. For example, in a small study of 29 patients receiving strontium-89 and/or vinblastine/estramustine for androgen-independent prostate cancer, the mean total cost per patient over 6 months of treatment was found to be $12,647Citation[15]. Additionally, several previous studies have reported on the economic burden of prostate cancer in a general population of US patients, although the studies were not necessarily specific to CRPC patients. A retrospective study of about 3000 men with prostate cancer receiving androgen deprivation therapy found that the mean total cost of healthcare over a 36-month period was $48,350 per patient. When compared to controls, costs associated with androgen deprivation therapy and to a lesser extent bone fractures appeared to drive the prostate cancer-related burdenCitation[16]. In a study of 4553 prostate cancer patients identified using a national disease registry, the mean annual prostate cancer-related costs were $7740 per patient; however, average cost per patient varied substantially by treatment type, with patients receiving some treatments such as radiation therapy incurring higher annual costs than patients receiving other types of therapyCitation[17]. Another retrospective study of prostate cancer patients in Canada examined how total healthcare costs varied with disease progression, and found that costs were high around the time of diagnosis ($3289 per 100 days) and 18 to 6 months before death ($5629 per 100 days)Citation[19]. Also, in a retrospective study of 2056 patients with prostate cancer treated between 1995 and 2000, patients identified as having metastatic prostate cancer had average annual costs of $30,626, and patients identified as having PSA progression had average annual costs of $18,948Citation[19]. Although the current study method of patient selection differed from that used in other studies, the economic burden reported here is similar to that from previous studies examining the costs associated with advanced prostate cancer.
The findings of this study must be considered within the limitations of the data and study design. Claims data are collected for the purpose of payment and not research. While these data increase understanding of real-world treatment patterns, they are subject to possible coding errors. A diagnosis code may be included as a rule-out criterion rather than to indicate presence of a disease. Additionally, there is an inherent limitation in using PSA levels as an indicator of CRPC, as a small percentage of patients (up to 20%) may experience PSA flares following docetaxel therapyCitation[20], and there is a risk for PSA flares following medical castration due to initiation of hormonal therapy. However, several previous studies support the definition of CRPC based on PSA level increases that was used hereCitation[11],Citation[21–23]. The presence of a prescription claim does not necessarily mean the drug was taken as prescribed, and some patients may have received drugs without the presence of a prescription claim (for example, by receiving drug samples or filling a prescription outside of the healthcare pharmacy system). Outcome measures were calculated PPPM to adjust for varying lengths of follow-up. However, calculating outcomes as monthly measures in high-mortality diseases such as CRPC may bias the results. For example, a patient with a very short follow-up period may have died. In this case, costs during the follow-up period may be very large for end-of-life care, and these large costs may be an overestimation of typical follow-up costs. Because death information was not collected for this population, it is not known if patients left the health plan because of death or for other reasons.
There are also limitations to the generalizability of this study. The data used for this study come from a managed care population. Therefore, results of this analysis are primarily applicable to the burden of illness of CRPC patients in managed care settings. In addition, patients with both Medicare Advantage and commercial plans were included for analysis. There may be differences in the way services are covered under a commercial or a Medicare Advantage plan. In addition, Medicare began offering a new pharmacy benefit, Medicare Part D, starting in January 2006, which may cover drugs differently than in prior plans. This may result in different utilization patterns among commercial patients, Medicare Advantage plans prior to 2006, and Medicare Advantage plans following the 2006 switch to Medicare Part D. However, the plans used for analysis include a wide geographic distribution of patients across the United States, and therefore should be generalizable to managed care populations on a national level.
Finally, initial analysis of the patient cohort yielded few patients with a clear diagnosis of CRPC, as defined by PSA result increases. Because PSA laboratory results information was not available for the entire sample of hormone-treated prostate cancer patients, only a small proportion of patients met the PSA results criteria required to be identified as having known CRPC. Due to the limitations of the available laboratory results data, a second, larger sample was identified based on the results of a prediction model that used logistic regression to relate baseline patient characteristics with known CRPC status. This broader CRPC population (with characteristics similar to patients in the known CRPC sample) was selected for study inclusion, but the true CRPC status of these patients is not known. A limitation of this approach was that non-specific markers of CRPC, including medical claims for bone scans, or for office visits with oncologists, were used to initially select some of the patients in the likely CRPC population. Therefore, the approach we used could potentially have lead to overidentification of patients with CRPC, and it is possible that patients without CRPC were included in the study sample of likely CRPC patients. Alternatively, it is also possible that patients with CRPC were not included in the final sample. Future research should focus on a review of medical charts to determine whether patients identified as part of the likely CRPC sample are true CRPC patients, in order to fully confirm the exploratory algorithm.
Conclusions
In this study a novel algorithm was developed in order to identify patients with CRPC from a health plan database. Using patients identified with this algorithm, a retrospective analysis was performed to better understand the economic burden and healthcare utilization associated with CRPC in a US population. Average prostate cancer-related costs made up about 50% of total all-cause healthcare costs incurred by patients in this study. In addition, average monthly prostate cancer-related costs more than doubled following determination of CRPC status. Costs were primarily driven by frequent ambulatory visits and expensive inpatient stays. Further research into the specific ambulatory services that drive costs is warranted. More effective treatment options are needed to manage this disease.
Transparency
Declaration of funding: The study and medical writing support were sponsored and fully funded by AstraZeneca LP (Wilmington, DE).
Declaration of financial/other relationships: B.A., F.N., and D.P. have disclosed that they are employees of AstraZeneca. E.B. and L.B. have disclosed that they are employees of i3 Innovus, a company that received funding from AstraZeneca to conduct this study.
Acknowledgments: The authors would like to thank Jesse Potash at i3 Innovus for help with preparation of this manuscript. In addition, the authors would like to thank Priyanka Koka of i3 Innovus for her programming support for this study.
References
- American Cancer Society. Cancer Facts & Figures 2008. Available at: www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. Accessed November 29, 2009.
- American Cancer Society. What are the key statistics about prostate cancer? Available at: http://www.cancer.org [Last accessed April 8, 2010].
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512
- Fosså SD, Slee PH, Brausi M, et al. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. J Clin Oncol 2001; 19: 62–71
- Quilty PM, Kirk D, Bolger JJ, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 1994; 31: 33–40
- Saad F, Karakiewicz P, Perrotte P. The role of bisphosphonates in hormone-refractory prostate cancer. World J Urol 2005; 2: 14–18
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14: 1756–1764
- James ND, Bloomfield D, Luscombe C. The changing pattern of management for hormone-refractory, metastatic prostate cancer. Prostate Cancer Prostatic Dis 2006; 9: 221–229
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512
- Rosenberg JE, Weinberg VK, Kelly WK, et al. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: randomized phase 2 study of ixabepilone or mitoxantrone and prednisone. Cancer 2007; 110: 556–563
- James ND, Caty A, Borre M, et al. Safety and efficacy of the specific endothelin A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol 2009; 55: 1112–1123
- Di Lorenzo G, Figg WD, Fossa SD, et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol 2008; 54: 1089–1094
- Harzstark AL, Ryan CJ. Therapies in development for castrate-resistant prostate cancer. Expert Rev Anticancer Ther 2008; 8: 259–268
- US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Chained Consumer Price Index for all urban consumers (C–CPI–U) 1999-2008, Medical Care. Series ID: SUUR0000SAM. Available at: http://data.bls.gov/cgi-bin/surveymost?su [Last accessed June 9 2008].
- Sherman EJ, Pfister DG, Ruchlin HS, et al. The Collection of Indirect and Nonmedical Direct Costs (COIN) form: a new tool for collecting the invisible costs of androgen independent prostate carcinoma. Cancer 2001; 91: 841–853
- Krupski TL, Foley KA, Baser O, et al. Healthcare cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol 2007; 178: 1423–1428
- Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer 2007; 109: 518–527
- Krahn MD, Zagorski B, Laporte A, et al. Healthcare costs associated with prostate cancer: estimates from a population-based study. BJU Int 2010; 105: 338–346
- Penson DF, Moul JW, Evans CP, et al. The economic burden of metastatic and prostate specific antigen progression in patients with prostate cancer: findings from a retrospective analysis of health plan data. J Urol 2004; 171: 2250–2254
- Emmenegger U, Ko YJ. PSA-based treatment response criteria in castration-resistant prostate cancer: promises and limitations. Can Urol Assoc J 2009; 3: 375–376
- Dawson N, Payne H, Battersby C, et al. Health-related quality of life in pain-free or mildly symptomatic patients with metastatic hormone-resistant prostate cancer following treatment with the specific endothelin A receptor antagonist zibotentan (ZD4054). J Cancer Res Clin Oncol: published online 14 Apr 2010, doi:10.1007/s00432-010-0864-1.
- Lindqvist O, Rasmussen BH, Widmark A. Experiences of symptoms in men with hormone refractory prostate cancer and skeletal metastases. Eur J Oncol Nurs 2008; 12: 283–290
- Maffezini M. Highlighting unmet needs: real patients, difficult choices. Eur Urol 2008; 9: 4–12
Appendix
Table A1. NDC codes used to identify medical castration in the pharmacy claims.
Table A2. Days supply associated with one billed unit for HCPCS for medical castration.
