Abstract
Background:
Acute myeloblastic leukaemia (AML) patients are at high risk of suffering from invasive fungal infections (IFI). Posaconazole demonstrated higher efficacy than standard azole agents (SAA) in the prophylaxis of IFI in this population.
The authors estimated the cost effectiveness of posaconazole versus SAA in France.
Methods:
A decision-tree model was developed to compare posaconazole with SAA with the results of a published clinical trial. Clinical events were modelled with chance nodes reflecting probabilities of IFI, IFI-related death, and death from other causes. Medical resource consumption and costs were obtained from results of the clinical trial and from a dedicated survey on the costs of treating IFI using a retrospective chart review design.
Results:
IFI treatment costs were estimated using medical files from 50 AML patients from six French centres, with a proven and probable IFI, who had been followed-up for 298 days on average. Direct costs directly related to IFI were estimated at €51,033, including extra costs of index hospitalisation, costs of antifungal therapy and additional hospitalisations related to IFI treatment. The model indicated that the healthcare costs for the posaconazole strategy were €5,223 (€2,697 for prophylaxis and €2,526 for IFI management), which was €859 less than the €6,083 in costs with SAA (€469 for prophylaxis and €5614 for IFI management). A sensitivity analysis indicated that there was an 80% probability that prophylaxis using the posaconazole strategy would be superior.
Conclusion:
The findings from this analysis suggest that posaconazole use is a clinically and economically dominant strategy in the prophylaxis of IFI in AML patients, given the usual limits of economic models and the uncertainty of costs estimates.
Introduction
Acute myeloid leukaemia (AML) is a relatively rare disease of the myeloid line of white blood cells, characterised by rapid proliferation of abnormal cells which accumulate in the bone marrow and consequently interfere with the production of normal blood cells. AML is the most common acute leukaemia affecting adults and its incidence increases with age.
In France, the number of new AML cases was recently estimated at 2,600 per year based on epidemiological data. French researchers estimated the 5-year survival rate in individuals with AML to be 19%Citation1.
Invasive fungal infections (IFI) are a major cause of morbidity and mortality in AML patients receiving intensive myelotoxic chemotherapy. Established risk factors are previous fungal infection, neutropenia exceeding 10 days, older age, active cancer, corticosteroid therapy, administration of broad spectrum antibiotics, allogeneic HSCT, central venous catheter and organ dysfunctionCitation2.
Posaconazole has recently been approved in Europe for prophylaxis of invasive fungal infections in patients receiving remission induction chemotherapy for AML or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are at an increased risk of developing invasive fungal infections. This indication was based on the results of a recently published clinical trialCitation3. This trial recruited patients who received cytotoxic chemotherapy as treatment for AML or MDS. It compared posaconazole to fluconazole or itraconazole as the primary prophylaxis of IFI (proven or probable) and followed-up patients for at most 12 weeks (100 days).
A total of 304 patients were treated with posaconazole for 29 days and 298 were treated with fluconazole or itraconazole for 25 days, on average.
Proven or probable fungal infection was diagnosed in 2% of the patients in the posaconazole group as compared with 8% in the control group (p < 0.001) during the treatment phase (7 days after the last dose of study drug administered during the last chemotherapy cycle)and 4.5% and 11%, respectively during the 100-day period (p = 0.003). Aspergillosis was the most common breakthrough infection and occurred less frequently (p = 0.009) in patients receiving posaconazole (1%) compared to control patients (9%). Lastly, as well as the reduction in fungal infections rates, overall survival was significantly longer in the posaconazole group (p = 0.04).
From a medical standpoint, the use of posaconazole prophylaxis should be recommended in these patientsCitation4,Citation5. However, there are some concerns about the cost effectiveness of this strategy in France as the costs of prophylaxis therapy are included in the tariff of the diagnosis-related group (DRG) for AML. Cost-effectiveness studies should compare the extra costs of posaconazole to the economic benefit of avoiding IFIs.
Very little is known about the costs of IFI in France, although Chapuis et al.Citation6 published some results based on costs in 2000, but these applied to resources consumed between 1993 and 1996 and before the availability of the new antifungal agents. At the time of this study, one-third of inpatients with IFI died during their first hospital stay and less than one-quarter survived an entire year.
From the international literature, the authors retrieved both a USCitation7 and a German study on the costs of IFICitation8. US researchers estimated that the extra costs during the first stay of an IFI patient over a non-infected patient were $47,915. The German group applied standard costs to consumed resources collected during a 3-month clinical trial. They calculated the average cost of IFI treatment to be €23,000 for this period. Other studies were performed but some focused on the costs associated with a specific antifungal agent or compared two treatment strategiesCitation9–13. None of these studies, however, included the long-term costs of IFI, in particular, those involving cure, maintenance treatment, and an eventual secondary prophylaxis for patients who now survive longer with the new antifungal therapies.
Given the situation described above, it is important to estimate the cost effectiveness of posaconazole because of the burden of IFI treatment on the French healthcare system. Another important point to consider is that the current French formulation policy does not favour the use of posaconazole in current practice.
Methods
The main perspective of this economic study was the one of the French healthcare system combining the French sickness funds and the one of hospitals. The study used a two-step design. The first step, a cost study, consisted of a retrospective chart review of patients with proven or probable IFICitation14 to estimate the costs of the condition from IFI occurrence until death or date of last report, lost to follow-up or date of data collection. This study proved to be necessary because available IFI cost estimates for France were too oldCitation6 to be considered relevant for the study’s purpose. These new cost estimates were introduced in a medico-economic model aimed at estimating the cost effectiveness of posaconazole in the prophylaxis of IFI for patients who received myelotoxic chemotherapy.
The observational cost study was conducted according to French regulations to ensure privacy and patients’ rights and therefore was carried out anonymously. The design was the following:
Adult patients with AML and presenting with a probable or proven IFI were retrospectively selected in six French haematological wards, starting backward from December 2006 in order to include recent patients and their management during a sufficient period.
From the start of the IFI, resources consumed for the treatment and secondary prophylaxis were collected in the medical files.
Most of the patients presented their first occurrence of IFI during hospitalisation for the treatment of AML.
Resources consumed were the estimated additional length of stay (LOS) as compared with complication-free stays for AML treatment (national estimate) for the index hospitalisation.
Other resources included the antifungal agents consumed during hospitalisation and in the outpatient setting, and additional hospitalisations due to the treatment of IFI.
Table 1. DRG of acute leukaemia hospitalizations with and without complication: tariffs and daily tariff of extra LOSCitation15.
Additional LOS was then costed using the tariff of extra LOS over the upper limit of the DRG plus the potential daily tariff in cases of hospitalisation in a positive pressure roomCitation15. Antifungal agents were then costed using the official tariffs, the duration of consumption and the location of their consumption. Expensive agents consumed in hospitals are charged on top of the DRGs by hospitals to the sickness funds (expensive and innovative medications registered on drug list off DRG). The less-expensive medications are included in the DRGs and their costs were only allocated to the hospitals. Agents consumed by outpatients were cost on the basis of official tariffs and are charged to the French sickness funds.
Table 2. Unit costs (taxes included) of antifungal agentsCitation15.
Table 3. Parameters of the model, distribution laws, baseline value, standard deviations used in the sensitivity analyses and sources.
Additional hospitalisations were costed according to the tariff of the DRG as the main reason for hospitalisation. Only hospitalisations due to IFI management were included in this analysis.
Costs not considered in this analysis were examinations that were not recorded extensively in the files, including those performed in an ambulatory setting and administration costs of injectable medications at home.
An Excel-based decision analytic model was constructed. It compared two groups of patients with AML or MDS in the proportions of the randomised clinical trial (RCT)Citation3. Because the duration of the follow-up period was restricted to 100 days in the RCT and because there is a significant difference in survival between posaconazole and standard azole agents, a time extrapolation was performed to estimate the lifetime cost effectiveness. This time extrapolation took into consideration the fact that patients with AML and MDS have a shorter life expectancy than the general population.
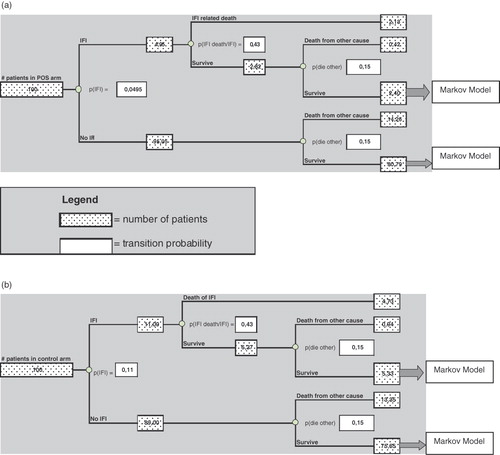
The model for the short-term phase used findings from the RCT. Transition probabilities were directly extracted from results as were the survival rates during the period. The duration of prophylaxis treatments was also issued from the RCT, with 29 days in the posaconazole arm and 25 days in the itraconazole or fluconazole arm. Surviving patients entered the second phase of the model that estimated cost-effectiveness ratios for the lifetime of the patients. A Markov model was used to calculate their life expectancy ().
Figure 1. Transition probabilities and number of patients for each strategy (on the basis of 100 patients) (a) in the posaconazole group, (b) in the control group.
The model calculated the costs of each strategy by multiplying the number of patients with the average cost of prophylaxis treatment for the entire population and the average cost of IFI for the patients contracting IFI. Incremental cost-effectiveness ratios can be estimated for this period by dividing the extra costs by the gain in life expectancy.
The Markov model estimated life expectancy during the lifetime of the patients taking into account the fact that patients with AML have only a 20.9% 5-year survival probability as compared with the survival probability of the corresponding general population according to SEER data (Surveillance Epidemiology and End Results) of the National Cancer InstituteCitation16. This 5-year survival probability is only 8% for patients with MDSCitation17. The proportion of patients with AML was 86% and 14% had MDS, as in the RCT.
Mortality tables from the French National Institute of StatisticsCitation18 were introduced into the Markov model. A discount rate of 3% was applied to cost and efficacy. One-way sensitivity analyses were performed calculating the economic results with the baseline value ± SD of the relevant parameters. Multivariate sensitivity analyses were performed using a probabilistic method with the distribution law of each parameter of interest. These analyses were computed with Excel.
For each iteration, the model picked up a value in the distribution of each parameter at random and estimated cost and efficacy to calculate the incremental cost-effectiveness ratio.
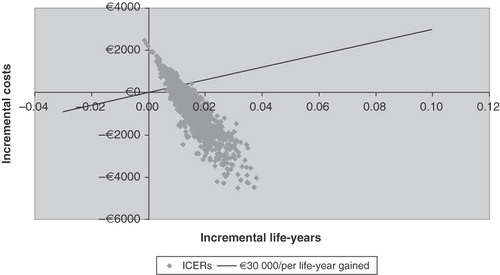
Results of the sensitivity analyses are shown in a with the 1000 incremental cost-effectiveness ratios.
Results
Cost study
A total of 50 patients were included in this cost study. Sex ratio was 2.12 males to 1 female, and mean age was 56.2 ± 14.1 years; 46% of the patients were over 60 years of age. Of these 50 AML patients, 12 have been treated with bone marrow graft during the follow-up, all after the occurrence of the IFI. IFI was verified in 44% of the patients and was considered probable in 56%.
Aspergillus was the most frequently (80%) identified fungus, being found alone in 67% of patients and Candida was present in 24% of the patients and found alone in 12%. Aspergillus and Candida were combined in 8% and the remaining six patients were infected with Absidia, Aspergillus plus a zygomycete, Candida plus Aspergillus plus Fusarium, Candida plus Aspergillus plus Fusarium, Fusarium and Geotrichum. The main sites of infection were lungs (76%), blood (18%) and sinuses (8%).
The average time between AML diagnosis and IFI occurrence was 6.2 ± 9.3 months. Of the 50 patients, 32 were followed-up until death. The average follow-up duration from IFI occurrence was 297.8 days. This duration was longer for surviving patients (566.3 days) than for deceased patients (146.7 days).
The reasons for admission during the index hospitalisation, where IFI was diagnosed, was AML treatment for 82% of the patients, bone marrow transplant for 8%, and fever and chest disorders for 10%. The LOS of index hospitalisations was 45.6 days on average (median 41 days).
Antifungal agents were consumed widely by the patients. Duration of antifungal agent use was 198.2 days on average with a minimum of 11 days and a maximum of 613 days. An average of 3.7 lines of treatment were prescribed, of which 1.1 was carried out with a combination of two or more agents. The most widely used antifungal agent was voriconazole, representing 59.9% of the total cumulative consumption. Other agents were utilised: caspofungine (14.8%), itraconazole (7.1%), posaconazole (5.4%), amphotericin B (4.6%), fluconazole (4.2%) and liposomal amphotericin B (3.9%), respectively.
Of the 50 patients, 11 were admitted for 13 additional hospitalisations directly related to IFI. Reasons were fever or infection which accounted for seven stays, surgical cure (mainly pulmonary) for three and diagnosis (n = 1) or relapse of IFI during a hospitalisation for AML treatment.
Costs of IFI consisted are primarily in three parts:
Costs of index hospitalisations;
Costs of re-hospitalisations;
Costs of antifungal therapies.
The average total cost of a probable or proven IFI episode can then be estimated at: €13,721 + €1,345 + €35,967 = €51,033.
Results of the model
The results of the baseline model are shown in . Posaconazole strategy is more efficacious and less costly because the cost of avoided IFI episodes exceeds the extra cost of posaconazole strategy over that of standard azoles.
Table 4. Results of the model according to the payer (average cost per patient).
The benefit related to avoiding an IFI will be essentially for the French sickness fund, as costs of IFI are mainly due to antifungal agents and costs of hospitalisations, and prophylaxis costs are paid by hospitals.
One-way sensitivity analysis results are the following:
When varying the IFI probability in the control arm (p = 0.11 ± 0.0181) the economic benefit of the posaconazole arm ranged from €351 to €1,368 and the effectiveness was respectively 0.013 and 0.016 life-years gained.
The IFI probability uncertainty (0.0495 ± 0.012) provided results from −€242 to −€1,477 in favour of posaconazole associated with life-years gained of 0.013 and 0.019, respectively.
The other important parameter that is the average cost of the IFI episode during index hospitalisation variation was assumed to be ± 15% of the €27,497. With this variation, benefit of posaconazole ranged from €651 and €1,068 without variation of the effectiveness results.
Results (n = 1000) of the probabilistic sensitivity analysis are presented in .
The average benefit was €839 (median €836) which was associated with an average increase in life expectancy of 0.016 years (median 0.015).
In all, 75.6% of the simulations show posaconazole to be more efficacious and less expensive than standard azole agents and only 0.4% show standard azole to be a superior strategy. Approximately 17% of the results are over €30,000 per life-year gained, usually an upper acceptable limit in France
Discussion
The aim of this work was to estimate the cost effectiveness of posaconazole in the primary prevention of IFI in AML patients undergoing chemotherapy. First, this study showed that costs related to IFI in these patients were extremely high, especially because of the long duration of the drug treatment.
Antifungal agents represented 72% of the costs – far more than hospitalisation costs.
This average cost estimate was higher than the IFI costs retrieved from the literature.
This is mainly due to the study design, which followed-up on patients until death, taking into account the long-term costs of IFI, with secondary prophylaxis and additional hospitalisations. Results for the short-term were similar to those analysed in the US and Germany, around €25,000. Long-term costs doubled this estimate, even if some of these patients died prematurely. As surviving patients were followed-up on average for more than 1½ years, and as almost all of them were treated with new antifungal agents, these long-term costs were probably underestimated.
However, some limitations of this cost study should be taken into account.
The design is retrospective and only information retrieved in the medical files could be used. Some information was not adequately included in the file for economic purposes. For example, number and types of exams were not systematically filled in on the chart, especially those performed in an ambulatory setting. This could lead to an underestimation in the costs of IFI.
Some information needs to be interpreted to allocate the resources to the IFI. This was also the case for additional hospitalisations, which were systematically reviewed by a physician. The low number of additional hospitalisations was the consequence of a conservative approach to attribute the stays to IFI.
Secondly, the cost-effectiveness model derived from the results of the clinical trial is in favour of the posaconazole strategy. This is a consequence of the high medical benefit for the patient and of the high expenses attributed to IFI episodes. This efficacy result was considered very important and convincing by the physicians but they were facing economic pressures from their management in France. Generalisation of the results of the clinical trial to the current clinical practice was considered to be complete as some of the experienced clinicians were prescribing prophylaxis without an evidence baseCitation19 because of the important consequences of the IFI occurrence in those patients.
The key point in the results of the model is the estimate of the costs of IFI to be compared with the extra costs of posaconazole over standard azole therapy. Other researchers presented a lower benefit (€183 per patient in their baseline scenario) for the posaconazole prophylaxis strategyCitation20 because of lower estimated costs of avoided IFI episodes.
The break-even point can easily be calculated by dividing the extra cost of posaconazole (€2,228) by the difference in the IFI rates of the two strategies (11–4.5%). If the average IFI cost is higher than €34,276, than, the posaconazole strategy is preferred in France.
On the other hand, the benefit expressed in life-years gained is not as great because of the current short life expectancy of these patients and because of the scarcity of information available concerning the expected survival of patients according to the presence or absence of IFI.
Nevertheless, limits of these models have been widely discussed and unit costs of some particular issues always represent a large part of these studies.
Conclusion
Posaconazole is an effective treatment in the prophylaxis of invasive fungal infections of patients with acute leukaemia presenting with neutropenia under chemotherapy and the costs consequences for the French healthcare system are fully acceptable.
Transparency
Declaration of funding:
This study was supported by Schering Plough.
Declaration of financial/other interests:
M.M., P.M. and P.B. have disclosed that they has no relevant financial relationships to disclose. D.C. has disclosed that he has been a consultant for Pfizer and Shering-Plough. B.D. has disclosed that he has received honoraria, grants and was member of speakers’ bureaus for Gilead, MSD, Schering-Plough, Astellas.
P.R. was a member of speakers' bureaus for Schering-Plough, MSD, Pfizer and Gilead.
R.H. has disclosed that he was a consultant for Astellas, Gilead, MSD, Pfizer, Schering-Plough and has been a member of speakers’ bureaus for Gilead, Pfizer, Schering-Plough and has received research grant from Pfizer. J.P. has disclosed that he has received research funding from Gilead, MSD, Pfizer and Schering Plough. A.L. has disclosed that he is an employee of Cemka Eval, a company that received funding from Schering Plough to conduct this study. A.K.O. has disclosed that she is employed by i3 Innovus, another company that received funding from Schering Plough to help conduct this study.
References
- Grosclaude P, Bossard N, Remontet L, et al. Survie des patients atteints de cancer en France. Étude des registres du réseau FRANCIM. Paris: Springer, 2007:406 pp
- Staber P, Langner S, Dornbusch HJ, et al. Antifungal management in cancer patients. Wien Med Wochenschr 2007;157:503-510
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348-359
- Cordonnier C, Pautas C, Maury S, et al. Chimioprophylaxie des infections fongiques en hématologie. Conférence de consensus commune SFAR, SPILF, SRLF (2004). Available at http://www.infectiologie.com/site/medias/_documents/consensus/antifongiques-court-04.pdf. Accessed 3 June 2009
- Cornely OA, Böhme A, Buchheidt D, et al. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Haematologica 2008 Dec 9
- Chapuis F, Thiebaut A, Bataillard A, et al. Conséquences économiques d'une infection à aspergillus ou à autre champignons filamenteux chez les patients traités pour leucémie aiguë myéloïde et modélisation des stratégies préventives. J Mycol Med 2001;11:67-72
- Wilson LS, Reyes CM, Stolpman M, et al. The direct cost and incidence of systemic fungal infections. Value Health 2002;5:26-34
- Jansen JP, Kern WV, Cornely OA, et al. Economic evaluation of voriconazole versus conventional amphotericin B in the treatment of invasive aspergillosis in Germany. Value Health 2006;9:12-23
- Wingard JR, Herbrecht R, Mauskopf J, et al. Resource use and cost of treatment with voriconazole or conventional amphotericin B for invasive aspergillosis. Transpl Infect Dis. 2007;9:182-188.
- Wenzel R, Del Favero A, Kibbler C, et al. Economic evaluation of voriconazole compared with conventional amphotericin B for the primary treatment of aspergillosis in immunocompromised patients. J Antimicrob Chemother 2005;55:352-61
- Collins CD, Stuntebeck ER, DePestel DD, et al. Pharmacoeconomic analysis of liposomal amphotericin B versus voriconazole for empirical treatment of febrile neutropenia. Clin Drug Investig 2007;27:233-241
- Collins CD, Ellis JJ, Kaul DR. Comparative cost-effectiveness of posaconazole versus fluconazole or itraconazole prophylaxis in patients with prolonged neutropenia. Am J Health Syst Pharm 2008;65:2237-2243
- de Vries R, Daenen S, Tolley K, et al. Cost effectiveness of itraconazole in the prophylaxis of invasive fungal infections. Pharmacoeconomics 2008;26:75-90
- Ascioglu S, Rex JH, de Pauw B, et al. Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer; Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7-14
- http://www.atih.sante.fr/openfile.php?id=1819 Accessed 3 June 2009
- http://seer.cancer.gov/ Accessed 9 March 2007
- Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer 2006; 106:1099-1109
- http://www.insee.fr Accessed 3 June 2009
- Al-Badriyeh D, Slavin M, Liew D, et al. Pharmacoeconomic evaluation of voriconazole versus posaconazole for antifungal prophylaxis in acute myeloid leukaemia. J Antimicrob Chemother 2010;65:1052-1061
- Stam WB, O'Sullivan AK, Rijnders B, et al. Economic evaluation of posaconazole versus standard azole prophylaxis in high risk neutropenic patients in the Netherlands. Eur J Haematol 2008;81:467-474