Abstract
Objective:
To examine resource utilization and healthcare costs associated with the use of exenatide versus glargine in type 2 diabetes (T2D) patients.
Methods:
A retrospective analysis comprised of patients with T2D initiating exenatide (n = 7,255) or glargine (n = 2,819) between 04/01/2005 and 06/30/2007. Propensity score matching was used (2,506 matched pairs) to control for baseline demographic, clinical, resource use, and cost variables to balance treatment groups. Mean medical costs and other cost components were estimated using nonparametric bootstrapping.
Results:
Exenatide-treated patients had 19% lower likelihood of all-cause hospitalizations (odds ratio [OR]: 0.81, p = 0.009) compared to glargine-treated patients.
Exenatide-treated patients had significantly lower total medical costs of $2,597 (p = 0.008). Exenatide-treated patients had significantly lower inpatient costs of $1,968 (p = 0.004) and outpatient costs of $1,324 (p = 0.011), but higher prescription costs of $706 (p < 0.001). Exenatide-treated patients further incurred lower hospitalization costs of $1,910 (p = 0.005) and physician office visit costs of $608 (p = 0.008).
Key limitations:
Lack of availability of clinical measures including duration of diabetes, severity of T2D and lack of control for unmeasured confounding.
Conclusions:
Patients initiating exenatide treatment had significantly lower healthcare resource utilization and total medical costs. Cost offsets were observed in inpatient and outpatient costs despite higher prescription costs.
Introduction
Type 2 diabetes is a leading cause of morbidity and mortality in the United StatesCitation1. There are approximately 23.6 million people (7.8% of the population) in the United States with diabetes with an estimated 1.6 million new cases diagnosed each year in adults aged 20 years and olderCitation1. Type 2 diabetes accounts for 90% to 95% of all the diabetes cases. The economic burden of type 2 diabetes on the healthcare system is substantialCitation2. Type 2 diabetes was estimated to cost $174 billion in 2007, of which $116 billion was spent for direct medical costs and $58 billion for indirect costs (disability, work loss, and premature mortality)Citation2. Most of the preceding medical costs were due to hospitalization (approximately 50%)Citation2.
An appropriate management of type 2 diabetes is important to reduce diabetes-related complications and further reduce healthcare expenditures. The optimal management of glycemic control is a critical part of diabetes care. Poor glycemic control has been associated with a significantly higher rate of complicationsCitation3–5 and greater healthcare costsCitation6,Citation7.
Exenatide BID (exenatide) and insulin glargine are commonly prescribed injectable medications for the management of type 2 diabetes in patients who fail to achieve glycemic control on oral antidiabetic therapiesCitation8. The clinical efficacy of these two medications are well established for the treatment of type 2 diabetesCitation9,Citation10. A recently published meta-analysis reported that exenatide treatment over 16–52 weeks offers similar benefits to various insulin regimens for glycemic control (pooled difference in estimated HbA1c: −0.04%, 95% confidence interval [CI], −0.14 to 0.06, p = 0.41), but was advantageous over insulin with respect to weight loss (3–6 kg loss at up to 52 weeks of follow-up)Citation11. Insulin therapy is usually reserved for patients with type 2 diabetes who have lost a significant amount of beta cell function. On other hand, exenatide significantly improves beta cell functionCitation12. Despite these reported clinical benefits, the relative economic and utilization impact of these therapies is still uncertain.
Several pharmacoeconomic models have shown that exenatide is a cost effective alternative compared to insulin glargine for the treatment of type 2 diabetesCitation13,Citation14. However, to the authors’ knowledge there is only one study that directly compared the medical costs of exenatide to insulin glargineCitation15. Misurski et al. showed that exenatide was associated with significantly lower total direct medical costs including inpatient, outpatient, emergency room costs, and diabetes-related costs. However, this study did not examine the potential drivers of the differences in the total healthcare costs as well as healthcare resource utilization among these patients.
Exenatide is a first-in-class glucagon-like peptide-1 (GLP-1) receptor agonist used for the treatment of type 2 diabetes. Although the clinical benefits of exenatide use are well established, it is important to understand the economic benefits associated with the use of exenatide when compared with other therapies. Hence, the intent of this research is to perform a follow-up study to a previous researchCitation15 to further explore the comparative economic outcomes of exenatide and insulin glargine therapies for the management of type 2 diabetes. The objective of this study was to examine healthcare costs, healthcare resource utilization, and concomitant medication use in patients initiating exenatide compared to insulin glargine in a large US managed-care patient population.
Patients and methods
Study design
A retrospective database analysis was performed using a large administrative claims database from i3 InVision. This database (i3 InVision) included data for over 30 million patients from October 2004 to March 2009 and was comprised of patients’ medical and prescription claims. For the purpose of this study patient claims with dates of service from October 1, 2004 through June 30, 2008 were examined. The study analysis was focused on comparing healthcare costs and resource utilization between patients who newly initiated exenatide or insulin glargine.
Patient population
The following inclusion and exclusion criteria were used (). Patients were included in this study if they received at least one new prescription for exenatide or insulin glargine between April 1, 2005 and June 30, 2007. The first prescription date for index or study medication was identified as the index date. Pre-index period was defined as a 6-month period prior to index date (i.e., 6 months before the first prescription of index medication) and the post-index period was defined as a 12-month follow-up period after the index date. Patients needed to have continuous insurance coverage during the study period (6 months prior to and 12 months following the index date). Patients must have had at least one diagnosis for type 2 diabetes identified using International Classification of Disease, Ninth Revision, Clinical Modification [(ICD-9-CM codes): (ICD-9-CM: 250.x0 or 250.x2)] during the pre-index period. Patients were required to have at least two prescriptions for metformin (MET), a sulfonylurea (SULF), a thiazolidinedione (TZD), MET-SULF, or MET-TZD in the pre-index period to ensure the on-label use of exenatide. All patients were at least 18 years of age at the index date. To ensure a patient population with type 2 diabetes, patients with a diagnosis of type 1 diabetes (ICD-9-CM: 250.x1 or 250.x3) or gestational diabetes (ICD-9-CM codes: 648.0x) were excluded. If prescribed exenatide, no use of insulin product was allowed during the study period. Similarly, if using glargine, no use of any other insulin product or exenatide was allowed during the study period. These criteria were to ensure that the two cohorts were not cross-contaminated. Finally, patients who had inpatient costs of more than $500,000 in the pre- and post-index periods were excluded to eliminate the influence of costs due to catastrophic illnesses and errors in coding.
Outcomes measurement
Healthcare costs
The healthcare costs associated with the use of exenatide or insulin glargine treatment, total direct medical costs and several other costs components were examined. Pre-index costs were summarized over the 6-month pre-index period. Post-index costs were summarized over the 12-month follow-up period. These costs included inpatient, outpatient, emergency room (ER), and prescription drug costs. To better understand the drivers of the cost differences, medical costs were further broken down into following categories: inpatient costs (hospitalizations including macrovascular and microvascular complications, nursing home/residential facility), outpatient costs (costs of physician office visits, hospital outpatient/ambulatory surgical center, ambulance services, home health visits, hospice care, laboratory and other ambulatory care). Pharmacy costs were divided into costs of antidiabetic medications including index medication and non-index antidiabetic medications, use of other concomitant medications, and diabetes supplies including glucose self-monitoring. All medical costs (total and several costs components) were converted to 2008 US dollars using the medical component of the Consumer Price IndexCitation16.
Resource utilizations
Resource utilization was largely divided into pharmacy and medical utilizations. The pharmacy utilization categories included use of oral antidiabetic medications (metformin, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors [AGIs], meglitinide, dipeptidyl peptidase-4 inhibitors [DDP-4]), and fixed-dose combination therapy). This study also examined other commonly used concomitant medications such as antihypertensives including diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, and lipid lowering medications including clopidogrel, digoxin, loop diuretic, coumadin, and lastly use of antidepressants. The medical utilization was categorized as outpatient utilization (visits to different healthcare providers), inpatient utilization (rate of hospital admissions), ER visits (rate of ER visits), and rate of hypoglycemic events. Inpatient utilization was further classified as macrovascular complications for the following events: myocardial infarction, ischemic heart disease, congestive heart failure, peripheral vascular disease, and stroke and microvascular complications for the following events: diabetic retinopathy/eye disease, renal failure, neuropathy, and amputation and/or foot ulceration/gangrene. These complications were identified using ICD-9-CM codes recorded in the medical claims database. Medical utilization was identified using place of service from medical claims. The hypoglycemic events were identified using ICD-9-CM codes recorded in the medical claims database. All the ICD-9-CM codes used to identify complications and comorbidities are listed in .
Analysis
In the overall sample population, descriptive statistics showed considerable differences in baseline characteristics between those patients who initiated exenatide versus insulin glargine (). Since patients were not randomly assigned to exenatide or glargine treatment, there were significant differences in baselines characteristics of these patient groups. Propensity score matching technique was deemed appropriate to balance these differences and to make the study cohorts comparable. The propensity score matching led to a subset of patients where bias in the comparison of the two cohorts was reduced by controlling for the pre-index imbalance on factors assumed to affect costs and resource utilization. The propensity to be treated with exenatide or insulin glargine was estimated using a logistic regression model with relevant baseline measurements used as predictor variables. The Hosmer–Lemeshow goodness-of-fit test was used to assess lack of fit for the propensity model. Covariates included in the model were patient demographics, general health status (as measured by Charlson Comorbidity Index [CCI])Citation17, medical comorbidities, diabetes-related complications, concomitant medication use, healthcare resource utilization, and medical costs during the pre-index period (detailed list of variables provided in ). The glargine-treated patients were matched 1:1 to the exenatide-treated patients on the estimated propensity score using a greedy matching algorithmCitation18. The baseline characteristics for both the pre-matched population and the matched population were compared using independent groups t-tests for continuous variables and independent groups chi-square tests for categorical variables. A nonparametric bootstrap was used with the matched sample data to estimate the mean post-index period costs associated with the treatmentCitation19. The concomitant medication use and resource utilization in the post-index period for the matched population were examined using McNemar statistics. Logistic regression models were used to estimate the odds ratio (OR) for resource utilization including hospitalizations, ER visits, and hypoglycemic events in the post-index period. All the outcome variables and analyses were defined a priori. No adjustments were made for multiple inferences. The results were considered statistically significant for p < 0.05. All analyses were completed using SAS Version 9.1 (SAS Institute, Cary, NC, USA).
Table 1. Demographic and clinical characteristics of patients in the pre-index period.
Sensitivity analysis
Several analyses were conducted to help assess the sensitivity of the results to the sample. A propensity bin bootstrapping approachCitation19, was used to estimate the mean total medical costs with the entire sample of patients (i.e., 2,819 of glargine-treated patients and 7,255 of exenatide-treated patients) to ensure the generalizability of the matching results to the entire study sample. In addition, a generalized linear model (gamma function with log link) was fit to the entire sample to help confirm the generalizability of the results observed in matched sample to the entire study population. Finally, a separate generalized linear model (gamma distribution with log link function) was fit to the subgroup of patients (n = 2,524) for whom a pre-index HbA1c value was available. HbA1c (as a continuous variable) was used as a covariate in the model to estimate the mean total medical costs when controlling for patients’ blood glucose values at the baseline.
Results
Sample characteristics
Demographic and clinical characteristics of the study sample in the pre-index period are summarized in . In this database, a total of 43,119 patients were using exenatide and 145,359 were using insulin glargine during the study follow-up period. After applying all inclusion and exclusion criteria, a total of 7,255 patients using exenatide and 2,819 patients using insulin glargine were retained (detailed patient attrition table is enclosed as ). There were significant differences in the baseline characteristics (including demographics, clinical characteristics, diabetes-related complications and comorbidities etc.) of patients who initiated exenatide or insulin glargine (). After applying propensity score matching, a total of 2,506 pairs of patients were obtained for the analysis. The baseline characteristics of the matched sample were well balanced in the pre-index period (all p-values > 0.05) (detailed are listed in ). The key baseline characteristics of the matched cohorts in the pre-index period are listed below. Nearly three-quarters of the exenatide- (74.0%) and insulin glargine- (72.5%) treated patients were adults 45–64 years of age. More than one-half of exenatide- (58.1%) and insulin glargine-(56.3%) treated patients were male. Exenatide- and insulin glargine-treated patients had similar diabetes-related complications (). Similarly, there were no significant differences between matched cohorts for other common comorbidities including depression, dyslipidemia associated with diabetes and also in their CCI scores.
Resource utilization
Medical utilization
presents the medical utilization of matched cohorts in the post-index period. Exenatide-treated patients had 19% lower likelihood of all-cause hospitalization (OR: 0.81, p = 0.009). Furthermore, exenatide-treated patients were associated with a 30% lower likelihood of hospitalizations associated with macrovascular complications (OR: 0.70, p = 0.005) (shown in ). Among the different types of macrovascular complications, exenatide-treated patients were associated with a 26% lower likelihood of hospitalizations associated with ischemic heart disease (OR: 0.74, p = 0.039), 46% lower likelihood of hospitalizations associated with congestive heart failure (OR: 0.54, p = 0.005), and 52% lower likelihood of hospitalizations associated with peripheral vascular disease (OR: 0.48, p = 0.044). In addition, exenatide-treated patients had a lower mean number of hospital admissions per year (0.2 vs. 0.3, p = 0.005) and a lower number of hospitalized days per year (0.3 vs. 0.4 days, p < 0.001). The study found no significant differences between the two cohorts for the rate of hospitalizations associated with microvascular complications, ER visits, or hypoglycemic events.
Table 2. Resource utilization for matched cohorts in the post-index period.
Pharmacy utilization
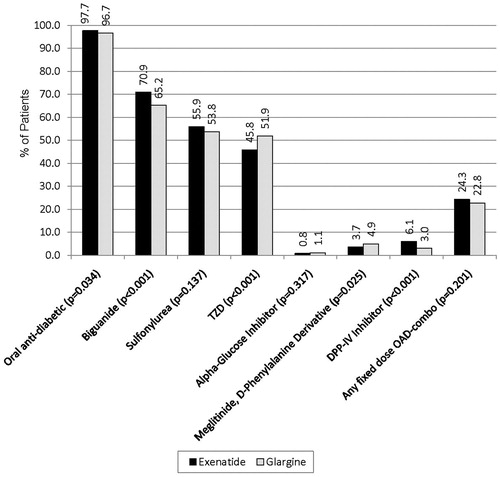
illustrates concomitant use of oral antidiabetic medications in matched patient cohorts in the post-index period. In matched cohorts, most of the patients were concomitantly using at least one oral antidiabetic medication (97.7% for exenatide vs. 96.7% for glargine, p = 0.034) (). A higher percentage of exenatide-treated patients were using metformin (70.9 vs. 65.2%, p < 0.001), and a lower percentage of exenatide-treated patients were using a TZD (45.8 vs. 51.9%, p < 0.001) ().
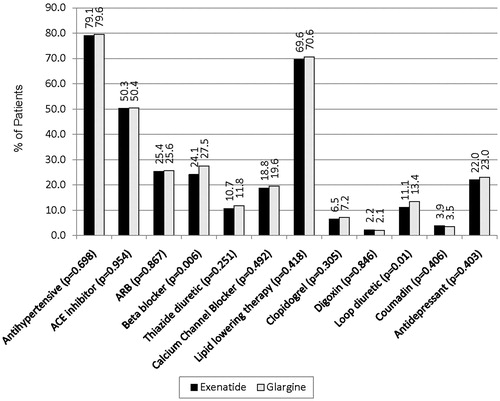
illustrates concomitant use of other commonly used medications (such as antihypertensive and lipid lowering agents) in matched patient cohorts in the post-index period. Over three-quarters of exenatide-treated (79.1%) and glargine-treated (79.6%) patients were also being treated with antihypertensive agents (p = 0.698) (). Approximately one-half of both exenatide- and glargine-treated patients were using angiotensin-converting enzyme (ACE) inhibitors (50.3 vs. 50.4%, p = 0.954), and approximately one-fourth were using β-blockers (24.1 vs. 27.5%, p = 0.006) (). More than two-thirds of exenatide-treated (69.6%) and glargine-treated (70.6%) patients were using lipid lowering agents (p = 0.418) (). A significantly lower percentage of exenatide-treated patients were using loop diuretics compared to glargine-treated patients (11.1 vs. 13.4%, p = 0.01).
Costs
presents healthcare costs for matched cohorts in the post-index period. Exenatide-treated patients had lower mean total direct medical of 2,597 ($19,978 vs. 22,575, p = 0.008), inpatient costs of $1,968 ($4,419 vs. 6,387, p = 0.004), and outpatient costs of $1,324 ($8,956 vs. 10,280, p = 0.011) compared to glargine-treated patients (). There was no significant difference in ER costs ($94 vs. 105, p = 0.529) for either cohort. Total inpatient costs were divided into hospitalization and nursing home costs. Exenatide-treated patients had significantly lower all-cause hospitalization costs of $1,910 ($4,397 vs. 6,307, p = 0.005) compared to glargine-treated patients. When specific types of hospitalization costs were examined, exenatide-treated patients had significantly lower costs of hospitalizations associated with macrovascular complications (difference of $1,001; $1,745 vs. 2,746, p = 0.034); however, no significant differences in hospitalization costs were associated with microvascular complications. When specific types of outpatient visits were examined, exenatide-treated patients also had significantly lower physician office visits costs of $608 ($2,974 vs. 3,582, p = 0.008) compared to glargine-treated patients. However, no significant differences were observed in other types of outpatient visits such as hospital outpatient, ambulance services and home health visits.
Table 3. Inpatient, outpatient, and prescription breakdown of costs for matched cohorts in the post-index period.
Although total prescription costs for exenatide-treated patients were higher than those of insulin glargine-treated patients (difference of $706; $6,509 vs. 5,803, p < 0.001), exenatide-treated patients incurred slightly lower costs of concomitant antidiabetic medications ($1,592 vs. 1,661, p = 0.049) and lower costs of other prescription medications ($2,895 vs. 3,084, p = 0.056) but this did not reach statistical significance compared to glargine-treated patients ().
Sensitivity analysis
Several sensitivity analyses on treatment cohorts were performed and similar estimates were found of the mean total medical costs for each cohort, with the glargine-treated patients having higher total costs than the exenatide-treated patients. A propensity bin bootstrapping method used to estimate the mean total medical costs with the entire sample of patients (i.e., 2,819 of glargine-treated patients and 7,255 of exenatide-treated patients) showed that exenatide-treated patients had significantly lower total medical costs compared to glargine-treated patients ($20,821 vs. 24,118, p < 0.001), ensuring the generalizability of the propensity score matched results to the entire study sample. In addition, a generalized linear model performed on the entire study sample also showed that exenatide-treated patients had significantly lower total medical costs compared to glargine-treated patients ($21,188 vs. 23,654, p < 0.001). Finally, a separate generalized linear model performed on the subgroup of patients (n = 1,892 for exenatide-treated patients and n = 631 for glargine-treated patients) for whom a pre-index HbA1c value was available also showed that exenatide-treated patients had significantly lower total medical costs compared to glargine-treated patients ($20,541 vs. 22,866, p = 0.017).
Discussion
Several clinical trials have demonstrated the clinical efficacy and safety of exenatide and insulin glargine and their use in the treatment of type 2 diabetesCitation9–11. This study compared the differences in the total direct medical costs, potential drivers of costs, and healthcare resource utilization associated with patients initiating exenatide or insulin glargine. Use of exenatide was associated with significantly lower total medical costs including inpatient and outpatient costs but higher prescription costs compared to insulin glargine. The findings of this study are consistent with a previous researchCitation15. Misurski et al.Citation15 showed that the use of exenatide was associated with significantly lower annual total direct medical costs including inpatient, outpatient, and ER visits costs compared to insulin glargineCitation15. The present study delves deeper to better understand the potential drivers of these costs (especially inpatient, outpatient and prescription costs) differences. This is the first study to examine the different components of total medical costs, and has also assessed the healthcare resource use such as medical utilization (including hospitalizations, ER visits, and hypoglycemic events) as well as pharmacy utilization (including use of antidiabetic medications and other commonly used medications) in patients with type 2 diabetes. This study further explored possible differences in hospitalizations mainly due to macrovascular (including myocardial infarction, ischemic heart disease, congestive heart failure, peripheral vascular disease, and stroke) and microvascular complications. In addition, this study used advanced techniques such as propensity score matching algorithm to balance the differences between baseline characteristics of patients. This methodology, along with other sensitivity analyses, gives further confidence to the important findings of this study and significant costs differences.
To better understand what influences total cost differences, this study categorized the total costs into several cost components. When inpatient costs were examined, exenatide-treated patients had significantly lower costs of hospitalization. Patients with type 2 diabetes commonly experienced microvascular and macrovascular complications which tend to be significant cost driversCitation1. This study showed that exenatide-treated patients had lower rates of hospitalizations associated with all-cause as well as macrovascular complications. Exenatide has shown cardioprotective effects in several clinical trialsCitation20–22. When the different type of macrovascular complications were examined, exenatide-treated patients had significantly lower likelihood of hospitalizations associated with ischemic heart disease, congestive heart failure, and peripheral vascular disease. In addition, exenatide-treated patients also experienced a significantly lower number of hospital admissions and a shorter length of stay. This lower resource utilization may have been translated into significantly lower hospitalization costs.
When different types of outpatient visits were examined, exenatide-treated patients had significantly lower costs of physician office visits. This observation may reflect the physician/patient experience of treatment with insulin therapy in actual clinical practice. Patients who initiate insulin for the first time typically require frequent physician office visits to learn about insulin administration, titration, and glucose monitoringCitation23–25. In addition, patients may need additional assistance due to other issues related to use of insulin therapy such as hypoglycemic episodes or weight gain, which also contribute to the total costsCitation23–25. The higher costs in the glargine cohort could be attributed to the self-care management associated with insulin therapy such as self-monitoring of blood glucose levels, treatment of hypoglycemia, and medical nutrition therapy. However, this could not be confirmed from the current study.
Although, this study found that total prescription costs and costs of index medication were significantly higher for patients initiating exenatide compared to insulin glargine, exenatide-treated patients had significantly lower total medical costs. Furthermore, exenatide-treated patients also incurred significantly lower costs of concomitant antidiabetic medications; costs of other prescription medications were also lower in exenatide-treated patients but this did not reach statistical significance. The lower prescription costs may be due to the decreased use of concomitant medications within this cohort; however, this conjecture could not be confirmed in the present study.
The results of the present study should be interpreted in the light of several limitations associated with the claims database. The severity of type 2 diabetes is not reported in the medical claims that may affect total healthcare costs between the two treatment groups. Although severity of type 2 diabetes could not be identified, surrogate markers were used to control for it in the propensity score matching such as diabetes-related complications, comorbidities, and concomitant medication use. In addition, sensitivity analysis performed in the subgroup of patients with HbA1c values further confirmed these findings. Associations between glycemic control and concomitant OAD medication use could not be examined in these patients due to a lack of available data (e.g., A1C, duration of diabetes) needed for such an analysis. Also, due to the lack of available data, this study could not control for other important potential covariates, such as certain socioeconomic factors (e.g., income, race), important clinical characteristics (e.g., duration of diabetes and body mass index, hypoglycemic events not requiring medical assistance), and behavioral factors (such as smoking and alcohol consumption). As this was a claims database analysis and patients were not randomized to treatment, there were significant differences in baseline characteristics of patients initiating exenatide or insulin glargine. To help balance the observed differences between groups, a propensity score matching technique was used to make a fair comparison. While the propensity score matching analysis reduces the biasing effect of imbalances in observed patient characteristics, it cannot account for bias resulting from variables not measured in the database. Insulin therapy is commonly used for patients with type 2 diabetes who have lost a significant amount of beta cell function. Over time, most patients with type 2 diabetes may require insulin. It is also important to mention that because glargine has been available for a long time, patients receiving glargine may have had diabetes for a longer time than those patients receiving exenatide. However, this study could not test this hypothesis as data regarding duration of diabetes were not recorded in the claims database. Lastly, the analytical approach allowed for associations to be observed but did not allow for causal inferences.
Despite these limitations, this study has important potential implications for payers and policymakers. Exenatide has demonstrated clinical effectiveness for the treatment of patients with type 2 diabetes, with a secondary benefit of weight loss and low risk of hypoglycemia (unless used with a sulfonylurea). In addition, this study also demonstrated potential cost savings when exenatide is used for the treatment of type 2 diabetes. Improving patients’ access to modern therapies, such as exenatide, may not only improve patients’ health outcomes but may also result in significantly lower healthcare resource utilization and cost savings for overall management of type 2 diabetes.
Conclusion
Patients with type 2 diabetes who initiated exenatide treatment had significantly lower total medical costs than those initiating glargine, mainly due to lower inpatient and outpatient costs despite having higher total prescription costs. Major cost savings were observed in exenatide-treated patients mainly due to lower costs of hospitalizations, physician office visits and concomitant medication use. In addition, the rates of all-cause hospitalizations, and hospitalizations associated with macrovascular complications were significantly lower for exenatide-treated patients. This study showed that the use of exenatide was associated with significant cost savings in management of type 2 diabetes compared to insulin glargine in patients who may be treated with either exenatide or insulin glargine.
Transparency
Declaration of funding:
This study was funded by Eli Lilly and Company. Eli Lilly and Company contracted the technical writing of this manuscript with i3 Statprobe.
Declaration of financial relationships:
M.P. and A.Z. are employees and stockholders of Eli Lilly and Company. T.S. is employed by MedFocus LLC and inventive Clinical Solutions LLC. L.S. is an employee of Tulane University and works as a consultant for Eli Lilly and Company.
Acknowledgments:
Eli Lilly and Company contracted the technical writing of this manuscript with i3 Statprobe. The authors thank Johnna Anderson for assistance in data analysis, Eli Lilly and Company and Lilly USA, Dr Jarrett Coffindaffer and Ms Teri Tucker, i3 Statprobe employees, for writing and editorial assistance, and John H. Holcombe, MD and Mark L. Hartman, MD, Eli Lilly and Company and Lilly USA, for reviewing and commenting on the manuscript.
References
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596-615
- Dall T, Mann SE, Zhang Y, et al. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:1-20
- Hirsch IB, Atchley DH, Tsai E, et al. Ascorbic acid clearance in diabetic nephropathy. J Diabetes Complications 1998;12:259-263
- Stratton IM, Adler AI, Neil HA, et al. Association of glycemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-412
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- Shetty S, Secnik K, Oglesby AK. Relationship of glycemic control to total diabetes-related costs for managed care health plan members with type 2 diabetes. J Manag Care Pharm 2005;11:559-564
- Caro J, Ward A, O’Brien J, et al. Impact of improving long-term glycemic control on US lifetime costs of complications. Presentation at the 61st Scientific Session of the American Diabetes Association, June 2001, Philadelphia, PA
- Edwards KL, Alvarez C, Irons BK, et al. Third-line agent selection for patients with type 2 diabetes mellitus uncontrolled with sulfonylureas and metformin. Pharmacotherapy 2008;28:506-521
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007;29:2333-2348
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559-569
- Norris SL, Lee N, Thakurta S, et al. Exenatide efficacy and safety: a systematic review. Diabet Med 2009;26:837-846
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients; a randomized controlled trial. Diabetes Care 2009;32:762-768
- Watkins JB, Minshall ME, Sullivan SD. Application of economic analyses in U.S. managed care formulary decisions: a private payer’s experience. J Manag Care Pharm 2006;12:726-735
- Ray JA, Boye KS, Yurgin N, et al. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin 2007;23:609-622
- Misurski D, Lage MJ, Fabunmi R, et al. A comparison of costs among patients with type 2 diabetes mellitus who initiated therapy with exenatide or insulin glargine. Appl Health Econ Health Policy 2009;7:245-254
- The United States Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (CPI-U): U.S. city average, by expenditure category and commodity and service group (table 1A). Available at: http://www.bls.gov/cpi/cpid08av.pdf. Accessed April 19, 2010
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-619
- Bergstralh EJ, Kosanke JL. Computerized matching of controls. Section of Biostatistics Technical Report 56. Rochester, MN: Mayo Foundation; 1995
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-2281
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006;8:436-447
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275-286
- Verge D, Lopez X. Impact of GLP-1 and GLP-1 receptor agonists on cardiovascular risk factors in type 2 diabetes. Curr Diabetes Rev 2010;6:191-200
- Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord 2002;26:S18-24
- Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care 2005;28:2673-2679
- Tibaldi J. Initiating and intensifying insulin therapy in type 2 diabetes mellitus. Am J Med 2008;121:S20-29
Appendix
eAppendix A. Inclusion/exclusion criteria.
Appendix B. ICD-9 codes for comorbidities.