Abstract
Background:
Percutaneous pulmonary valve implantation (PPVI) using the Melody transcatheter pulmonary valve is a new procedure introduced in 2000 as a less invasive treatment for right ventricular outflow tract (RVOT) dysfunction. The aim of this new procedure is to restore pulmonary valve competence without the need of open-chest operation. By prolonging the conduit lifespan, it delays surgical pulmonary valve replacement (PVR) and it can therefore potentially reduce the number of open-chest interventions over a patient’s lifetime. PPVI has been shown to be feasible and safe and can be performed with a low complication rate.
Objectives and Methods:
The aim of this study is to assess the cost of PPVI and the cost of surgical pulmonary valve replacement (PVR) in patients with right ventricular outflow tract dysfunction using a cohort simulation model applied to the UK population.
Results:
The model resulted in an estimate of mean cost per patient of £5,791 when PPVI is unavailable as a treatment option and in an estimate of mean cost per patient of £8,734 when PPVI is available over the 25-year period of analysis. After sensitivity analysis was undertaken the results showed that the mean per patient cost difference in implementing PPVI over 25 years as compared to surgical PVR lies somewhere between £2,041 and £3,913.
Limitations:
Given the lack of long-term data on treatment progression, the cost estimates derived here are subject to considerable uncertainty, and extensive sensitivity analysis has been used to counter this. Consequently this study is merely indicative of the levels of cost which can be expected in a cohort of 1,000 patients faced with a choice of treatment with PPVI or surgery. It is not a cost-effectiveness study but it helps place current knowledge on short-term benefits into context.
Conclusions:
As this analysis shows PPVI is associated with a relatively small increase in treatment management costs over a long time period. It is left entirely to the reader to value whether this inferred increase in long-term cost is worthwhile given the known short-term benefits and any personal judgement formed over long-term benefit.
Introduction
Remarkable progress in surgical management of complex congenital heart conditions means that over 85% are now reaching adulthood. In fact, the prevalence of congenital heart disease in the adult population is currently equal to, and will soon outweigh, that in the paediatric population. One of the major problems for this expanding population is dysfunction of the right ventricular outflow tract (RVOT). Initial surgical repair for complex conditions or repeated surgery for free pulmonary regurgitation or stenosis often includes the creation of an artificial right ventricle to main pulmonary artery connection. Over time, these conduits are prone to develop valvular incompetence or obstruction. There is clear evidence that pulmonary stenosis and regurgitation are associated with exercise intolerance, arrhythmias and an increased risk of sudden death. Timely pulmonary valve replacement can halt and may reverse such unfavourable outcomes. However, this means that patients have to undergo multiple open-heart surgeries in order to reduce the haemodynamic burden on the right ventricleCitation8.
PPVI using the Melody transcatheter pulmonary valve is a new procedure introduced in 2000 as a less invasive treatment for right ventricular outflow tract (RVOT) dysfunction. The aim of this new procedure is to restore pulmonary valve competence without the need of open-chest operation. By prolonging the conduit lifespan, it delays surgical PVR and potentially reduces the number of open-chest interventions over a patient’s lifetime. PPVI has been shown to be feasible and safe and can be performed with a low complication rateCitation1–7. It has also been shown that over a median follow-up period of 28.4 months, freedom from re-operation post-PPVI implantation was 93% (±2%), 86% (±3%), 84% (±4%) and 70% (±13%) at 10, 30, 50 and 70 months, respectively and freedom from transcatheter re-intervention was 95% (±2%), 87% (±3%), 73% (±6%) and 73% (±6%) at 10, 30, 50 and 70 months, respectively. Survival at 83 months was 96.9%Citation7.
Introduction of such new interventional technology naturally raises concerns about the cost implications for any national or private health system.
Methods
Against this background the aim of this study is to assess the cost of percutaneous pulmonary valve implantation (PPVI) using the Melody transcatheter pulmonary valve and the cost of pulmonary surgical valve replacement (PVR) in patients with right ventricular outflow tract dysfunction using a cohort simulation model applied to the UK population. The specific objectives of the analysis are:
To estimate the cost of percutaneous pulmonary valve implantation (PPVI) in patients with right ventricular outflow tract dysfunction using existing observational data. The data were based on the same patients as reported by Lurz et al.Citation7. Given the impact of learning curve, data on the first 50 patients were excluded from the analysisCitation7. The analysis was therefore based on those patients who underwent PPVI after the design of the device was changed to reflect the completion of the learning curve with regards to technical experience and patient selection. This resulted in a total of 141 patients who had undergone PPVI until 1st June 2008.
To estimate the cost of pulmonary surgical valve replacement (PVR) in a similar group of patients using a cohort simulation model populated with data drawn from the literature and expert opinion.
The analysis is undertaken from the perspective of the UK National Health Service using a UK population.
Model and assumptions
The model is a cohort simulation model and assesses the cost of PPVI and the cost of surgical PVR using a hypothetical population of 1,000 individuals with right ventricular outflow tract dysfunction starting when their first valved biological conduit was surgically placed and following them for a period of 25 years assuming that (1) PPVI is not available as a treatment option and (2) that PPVI is available for those eligible for it. Under the first assumption the model simulates this patient population in the absence of PPVI and it is therefore used to assess the cost per patient of treating this patient population in the absence of PPVI while under the second assumption the model simulates the same patient population in the presence of PPVI and it is used to assess the cost per patient of treating this patient population in the presence of PPVI.
The model was developed using a hypothetical population of 1,000 individuals with right ventricular outflow tract dysfunction who have already undergone surgical valve replacement (PVR) with valved biological conduits with annual cycles over a period of 25 years. The choice of duration for the model was based on observational data being available for a population of 505 patients with congenital malformations (median age 4.0 years, range 2 days ± 31 years) who received their first valved conduit between January 1974 and August 1999 and were observed for a 25-year periodCitation3. This is the longest observational study on this patient population to date.
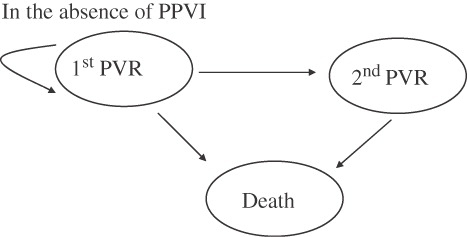
Under the first assumption, that is in the absence of PPVI technology, in each cycle the model assumes that a proportion of these individuals will undergo surgical valve replacement (PVR), a proportion will die and the remaining individuals will remain free of re-operation (). The respective probabilities of re-operation and death for each cycle were obtained from Homann et al.,Citation3 and from expert opinion and they are reported in . The cost of each cycle consists of the cost of the surgical PVR procedure and the cost of complications following the procedure. The cost of surgical PVR was assumed to be £13,000 and the cost of within-year complications following surgical PVR was assumed to be £1,500. These costs were based on data from the Great Ormond Street Hospital and are reported in and . The total cost in any given cycle was discounted at an annual rate of 3.5% as recommended by the UK National Institute for Health and Clinical Excellence (NICE).
Table 1. Probabilities of death in each cycle following PVR (based on expert opinion) and of 2nd PVR in cycle (based on Homann et al.,Citation3 and expert opinion).
Table 2. Unit costs for PPVI and PVR (Source: Great Ormond Street Hospital).
Table 3. Complication costs within the first year (Source: Great Ormond Street Hospital).
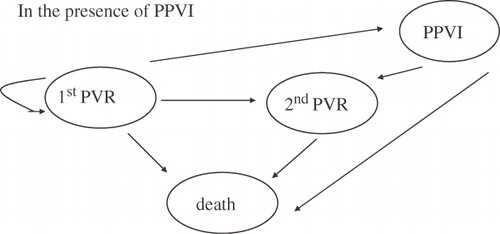
Under the second assumption, that is the availability of PPVI technology, in each cycle the model assumes that of the proportion of these individuals who would otherwise undergo pulmonary surgical valve replacement (PVR) (the same proportion as above), a constant proportion of 80% is eligible for PPVI and will receive PPVI instead (as based on the clinical experience to date at Great Ormond Street Hospital), the remaining 20% will undergo surgical PVR, a proportion will receive a second PPVI, a proportion will die and the remaining individuals will remain free of re-operation and PPVI. The assumption that of the people who had initial PVR 80% are eligible for PPVI in each year following initial surgery, i.e. a drop-out of 20%, was based on the following reasons: risk for coronary compression, unfavourable branch pulmonary artery anatomy, and too small a conduit with the associated risk of high residual gradients post PPVI implant. Similar drop-out was seen in the short-term observational study by Lurz et al.Citation7. Given the indicative nature of these assumptions the model is obviously heuristic and it can be readily seen that extrapolation to incorporate long-term clinical benefit would be unsupportable. The cost assumptions are also subject to extensive sensitivity analysis. Finally, a break-even analysis provides an estimate of the cost of the PPVI device that results in the PPVI procedure and the surgical pulmonary valve replacement (PVR) having the same cost per patient over the period of analysis considered.
In each cycle, of those individuals who receive PPVI, a proportion will undergo a second surgical PVR, a proportion will die and a proportion will receive a second PPVI implantation (). The respective probabilities of re-operation and death for each cycle for those individuals who undergo PVR were again obtained from Homann et al.,Citation3 and from expert opinion, while the probabilities of re-operation and death for those individuals who receive PPVI were based on the patient level data held at Great Ormond Street Hospital as were the probabilities of a patient receiving a second PPVI implantation. These probabilities are critical to the modelling of costs and they are fully presented in to show the assumed changes over time.
Table 4. Probabilities of receiving PVR after PPVI, 2nd PPVI and probability of death in cycle (Data obtained from Great Ormond Street Hospital).
The cost of each cycle consists of the cost of the surgical PVR procedure, the cost of complications following the surgical procedure, the cost of PPVI, the cost of complications following PPVI implantation, the cost of a second PPVI and the cost of complications following the second PPVI. The cost of surgical PVR and within-year complications following surgery was assumed as above, the cost of PPVI was assumed to be £20,000 plus an additional £3,000 × 0.80 representing 80% of cases who needed pre-stenting during the PPVI implantation (data from Great Ormond Street Hospital presented in ) and the cost of within-year complications following PPVI was assumed to be £348 and half that following a second PPVI within the same cycle (data from Great Ormond Street Hospital presented in ).
Results
Based on the assumptions stated above, the model resulted in an estimate of mean cost per patient of £5,791 in the absence of PPVI and in an estimate of mean cost per patient of £8,734 in the presence of PPVI over the 25-year period of analysis. The mean difference in cost in using PPVI is therefore £2,943.
Sensitivity analysis
Given the range of assumptions used to populate the model sensitivity analysis was undertaken to assess the impact of varying the following parameters on the cost estimates. First, the probabilities of death in the year following initial surgical PVR were reduced by 10% and then increased by 10% and so were the probabilities of undergoing a second surgery in the year following initial surgery. When the low values were used for both sets of probabilities the resulting mean cost estimates were £5,537 per patient in the absence of PPVI and £8,351 per patient in the presence of PPVI over the 25-year period of analysis. When the high values were used for both sets of probabilities the resulting mean cost estimates were £6,005 per patient in the absence of PPVI and £9,057 per patient in the presence of PPVI over the 25-year period of analysis. Secondly, the proportion of patients eligible for receiving PPVI was varied to 70% and 90% (from 80% in the base-case analysis). The respective estimates were £8,366 and £9,102 per patient in the presence of PPVI. Third, the cost of surgical PVR was increased by 10% resulting in a mean cost estimate of £6,310 per patient in the absence of PPVI and £8,838 per patient in the presence of PPVI over the 25-year period of analysis. Finally the cost of PPVI was increased by 10% resulting in a mean cost estimate of £9,450 per patient in the presence of PPVI over the 25-year period of analysis.
The largest estimated mean difference between surgical PVR and the PPVI cohort over the 25-year period of analysis was therefore £3,913 and this was observed when the cost of PPVI was increased by 10% and the probabilities of death in the year following initial surgical PVR and of undergoing a second surgery were reduced by 10% compared to the base-case analysis. The smallest estimated mean difference over the 25-year period of analysis was £2,041 and this occurred when the cost of surgery was increased by 10% and the probabilities of death in the year following initial surgery and of undergoing a second surgery were reduced by 10% compared to the base-case analysis. Finally, the break-even analysis showed that the PPVI and the PVR procedures resulted in a similar mean cost per patient over the 25-year period of analysis (of approximately £5,800) when the cost of the PPVI device is £11,100.
A probabilistic sensitivity analysis was also undertaken where the following parameters of the model were all varied: the cost of the surgical PVR procedure (between £11,000 and £15,000), the cost of complications following the surgical procedure (between £1,300 and £1,700), the cost of PPVI (between £20,000 and £25,000) the cost of complications following PPVI implantation (between £300 and £400) and the proportion of patients receiving PPVI implantation (between 70% and 90%). The estimated cost difference ranged between £1,401 and £4,785 compared to £2,943 of the base-case analysis.
Finally, an additional analysis was undertaken in order to derive estimates of the cost of the procedures over different time periods. When the period of analysis was assumed to be 10 years the mean cost difference between the two cohorts was estimated to be £1,615 and £719 when the period of analysis was assumed to be 3 years.
Discussion
Existing clinical studies show short-term clinical benefit from the adoption of PPVI using the Melody transcatheter pulmonary valve. Given the uncertainty associated with extrapolating to long-term clinical benefit, this study has used a 25-year observational study of surgical patients to estimate the possible cost consequences of implementing a treatment strategy based on PPVI versus surgical PVR. Moreover given the lack of long-term data on treatment progression the cost estimates derived here are subject to considerable uncertainty, and extensive sensitivity analysis has been used to counter this. Consequently this does not allow for cost-effectiveness inferences to be drawn. However the results have shown that the mean per patient cost difference in implementing PPVI over a 25-year period as compared to surgical PVR lies somewhere between £2,041 and £3,913. For a relatively small increase in cost, PPVI offers a less invasive treatment for RVOT dysfunction as an alternative to watchful waiting, with all the attenuate uncertainty for the patient, until surgery is deemed necessary. Clinical study has shown that PPVI can significantly postpone surgery in the majority of cases allowing patients to cope better with their condition and avoid for a longer period the risks associated with surgery itself.
This study is merely indicative of the levels of cost which can be expected in a cohort of 1,000 patients faced with a choice of treatment with PPVI or surgery. It is not a cost-effectiveness study but it helps place current knowledge on short-term benefits into a context. As this analysis shows PPVI is associated with a relatively small increase in treatment management costs over a long-time period. It is left to the reader to value whether this inferred increase in long-term cost is worthwhile given the known short-term benefits and any personal judgement formed over long-term benefit. Despite a good physician acceptance of PPVI for the management of RVOT dysfunction, there is still lack of knowledge about the best option to adopt during a patients’ lifetime. Complete understanding of the pros and cons of the available options, including true cost effectiveness and in particular with regards to quality of life, will be reached after several years of observation.
Conclusion
In conclusion, given the difficulty in extrapolating long-term clinical benefits from known short-term impact, this study has shown PPVI using the Melody transcatheter pulmonary valve to incur a relatively small increase in long-term costs as compared to surgical PVR. Given that the number of patients eligible for PPVI implantation is small, this suggests a relatively small budget impact for any provider. The methodology adopted here can be generalised across different health systems, although the relative resource implications may of course vary. It is left to personal judgement to adjudicate whether this cost difference is justified.
Transparency
Declaration of funding:
This study was funded through an educational grant from Medtronic International Trading SARL, Tolochenaz, Switzerland. One of the co-authors was an employee of the sponsor, Medtronic, and contributed to the study design and writing of the manuscript.
Declaration of financial/other relationships:
None.
Acknowledgements:
No assistance in the preparation of this article is declared.
Notes
* Melody transcatheter pulmonary valve manufactured by Medtronic International Trading SARL, Tolochenaz, Switzerland
* Medtronic International Trading SARL, Tolochenaz, Switzerland
References
- Coats L, Tsang V, Khambadkone S, van Doorn C, et al. The potential impact of percutaneous pulmonary valve stent implantation on right ventricular outflow tract re-intervention. Eur J Cardiothorac Surg 2005;27:536-543
- Frigiola A, Nordmeyer J, Bonhoeffer P. Percutaneous pulmonary valve replacement. Coron Artery Dis 2009;20:189-191
- Homann M, Haehnel JC, Mendler N, et al. Reconstruction of the RVOT with valved biological conduits: 25 years’ experience with allografts and xenografts. Eur J Cardiothorac Surg 2000;17:624-630
- Khambadkone S, Coats L, Taylor A, et al. Percutaneous pulmonary valve implantation in humans results in 59 consecutive patients. Circulation 2005;112:189-197
- Nordmeyer J, Coats L, Taylor AM, et al. Benefits of percutaneous pulmonary valve implantation are sustained after 1 year. Circulation 2006;114(Suppl S):390
- Nordmeyer J, Coats L, Lurz P, et al. Percutaneous pulmonary valve-in-valve implantation: a successful treatment concept for early device failure. Eur Heart J 2008;20:810-815
- Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation 2008;117:1964-1972
- Rosengart T, Feldman T, Borger M, et al. Percutaneous and minimally invasive valve procedures. A scientific statement from the American Heart Association Council on cardiovascular surgery and anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2008;117:1750-1767