Abstract
Background:
Studies examining outcomes of different insulin delivery systems are limited. The objective of this study was to compare healthcare utilization, costs, adherence, and hypoglycemia rates in patients with type 2 diabetes mellitus (T2DM) initiating rapid-acting insulin analog (RAIA) using prefilled pen versus vial/syringe.
Methods:
A retrospective analysis was conducted using a US claims database (1/1/2007 to 12/31/2008). Inclusion criteria were: ≥18 years old, with T2DM, ≥12 months of continuous eligibility, and new to RAIA. Difference-in-difference analyses after propensity score matching were conducted to compare changes in outcomes from 6 months prior to and 6 months after initiating RAIA with a prefilled pen versus vial/syringe (Wilcoxon rank-sum test for costs and t-test for other outcomes). Categories of utilization and costs (2009 USD) included total and diabetes-related inpatient, outpatient, and emergency room. Adherence was measured by proportion of days covered (PDC). Hypoglycemia was identified using ICD-9-CM codes.
Results:
Baseline characteristics were similar between the prefilled pen (n = 239) and vial/syringe (n = 590) cohorts after matching. Adherence to RAIA was greater in the prefilled pen cohort than the vial/syringe cohort (PDC: 54.6 vs. 45.2%, p < 0.001). While the increase in diabetes-related pharmacy costs from before to after initiating RAIA was greater in the prefilled pen cohort than the vial/syringe cohort (+$900 vs. +$607, p < 0.001), the prefilled pen cohort was associated with greater reductions in the total diabetes-related costs (–$235 vs. +$61, p = 0.006) and the utilization of oral anti-hyperglycemic agents (–1.3 vs. –0.7, p = 0.016). There were no significant differences in other outcomes.
Limitations:
Claims databases do not provide optimal measures for adherence or T2DM severity, and only capture hypoglycemia events requiring clinical intervention.
Conclusion:
Initiating RAIA with a prefilled pen was associated with better adherence and greater reduction in total diabetes-related costs than a vial/syringe. There was no significant difference in total healthcare costs.
Key words::
Introduction
Diabetes mellitus is the most common metabolic disorder and the global prevalence has been increasing at an alarming rateCitation1. In the United States (US), the estimates for diabetes incidence and prevalence in 2007 were 1.6 million and 23.6 million, respectively, with projected prevalence being about 30 million by 2030Citation2,Citation3. Type 2 diabetes mellitus (T2DM) comprises 90–95% of all diagnosed diabetes cases in adultsCitation2. The economic impact of diabetes on the US healthcare system is substantial. In 2002, the total costs attributable to diabetes were estimated to be about $132 billionCitation4 and the estimated costs increased to $174 billion in 2007, including $116 billion and $58 billion attributed to direct and indirect costs, respectivelyCitation5. Hospitalization was the most expensive cost component of direct costs (50% of total costs)Citation5.
Insulin is an effective and long-standing anti-hyperglycemic therapy (AHT). Over the years, several studies encouraged an earlier use of insulin therapy, including the latest consensus statement by the American Diabetes Association and the European Association for the Study of DiabetesCitation6–8. However, approximately half of the patients diagnosed with T2DM have sub-optimal glycemic controlCitation9,Citation10. Also, non-adherence to AHTs is widespread. According to Cramer et al., adherence to oral anti-hyperglycemic agents (OHAs) and insulin therapy was in the range 36–93% and 62–64%, respectivelyCitation11. Several treatment-, patient-, and physician-related factors have been identified to be associated with non-adherence to AHTs including the complexity of drug regimen, incorrect dose titration, fear of side-effects, and concern that patients may not be able to use therapy properlyCitation11–14.
Various insulin delivery systems have been introduced since the mid-1980s to ease patient burden associated with self-injection. According to a literature review by Molife et al., patient preference and satisfaction were greater with insulin pen delivery systems than vial/syringe systemsCitation15. Patients also reported greater convenience, flexibility, ease of use, self-efficacy, and dosing accuracy with a pen than with a vial/syringeCitation15.
To date, four retrospective database analyses have been conducted to examine the outcomes of patients with T2DM using a prefilled pen versus a vial/syringe in administering insulin therapyCitation16–19. The pen delivery system of focus in all four studies was the insulin aspart and aspart mix (Novolog, Novolog Mix70/30 and Novolog Mix50/50) FlexPen. Study results, as well as study design and population, were different across the four studies. Lee et al. and Cobden et al. reported that pens were associated with improved medication adherence and healthcare cost reduction versus vials/syringesCitation17,Citation18. These studies examined outcomes prior to and after patients converted from a vial/syringe to a pen, without a control group. A study by Pawaskar et al. conducted two separate analyses: (1) an insulin-naive analysis: patients were naive to insulin therapy and its delivery systems, and (2) a pen-naive analysis: patients who switched from a vial/syringe to a pen were compared to those who remained on a vial/syringeCitation19. The authors concluded that diabetes-related medication adherence was significantly greater with a pen than with a vial/syringe for the pen-naive analysis, but not for the insulin-naive analysisCitation19. Both diabetes-related and total costs were significantly lower with a pen than with a vial/syringe for the insulin-naive analysis, but not for the pen-naive analysisCitation19. Last, Baser et al. compared outcomes between patients who switched from a vial/syringe to a pen and those who remained on a vial/syringeCitation16. The pen delivery system was associated with greater medication adherence than the vial/syringe, but no significant difference in healthcare costs or hypoglycemia rates was observedCitation16.
In January 2008, a new, easy-to-use prefilled pen delivery system, the KwikPen, was launched prefilled with insulin lispro (Humalog) in the USFootnote†. To date, the economic outcomes of the KwikPen have not been compared to those of the vial/syringe. The purpose of this study is to compare US healthcare resource utilization, costs, medication adherence, and hypoglycemia rates in patients with T2DM initiating rapid-acting insulin analog (RAIA) with the KwikPen versus vial/syringe.
There are several ways in which the current study is different from previous insulin pen delivery system outcomes studies. The current study is not only the first study to compare real-world outcomes between the KwikPen and vial/syringe, but also includes healthcare resource utilization as a key outcome measure, and the study population includes T2DM patients who are naive to index insulin therapy as well as to the index pen delivery system.
Methods
Data source and study design
A retrospective analysis was conducted using a large US claims database (MarketScan)Footnote‡. This dataset contains medical and pharmacy claims of >20 million US residents who were insured with a variety of commercial health plans. Data from January 1, 2007 to December 31, 2008 were used to compare the changes in outcomes 6 months prior to (pre-index) and 6 months after (post-index) initiating a RAIA using the KwikPen (for insulin lispro) versus vial/syringe (for insulin lispro or aspart) in patients with T2DM. Another form of RAIA, insulin glulisine (Apidra)Footnote§ was not included in the primary analysis because the prefilled pen delivery system for insulin glulisine was not available during the study period. However, the authors conducted a sensitivity analysis with insulin glulisine included in the vial/syringe cohort to test the robustness of the primary results. Approximately 5% of patients who initiated RAIA using vial/syringe initiated with insulin glulisine.
Sample selection
A previously validated algorithmCitation20 was used to identify patients with diabetes, in which patients were identified as having diabetes if they had ≥2 outpatient claims with a diagnosis code of diabetes (International Classification of Disease, 9th Edition, Clinical Modification [ICD-9-CM] codes 250.xx) on two different days or ≥1 inpatient claim with a diagnosis code of diabetes during the study period. Patients with T2DM were further identified by excluding patients with diagnosis of type 1 diabetes mellitus (ICD-9-CM codes 250.x1 or 250.x3).
The period from January 1, 2008 to June 30, 2008 was used to capture the index RAIA and respective delivery system of KwikPen or vial/syringe (denoted as ‘index RAIA’ from here on). If patients had at least one pharmacy claim for a new RAIA and the KwikPen was the first pen device used for RAIA, then the patients were categorized as the KwikPen cohort. Otherwise, if the vial/syringe was the delivery system associated with the first RAIA prescription, then those patients were categorized as the vial/syringe cohort. Patients were required to be continuously enrolled in the health plan during both the pre- and post-index periods.
Patients with the following criteria were excluded from the study: <18 years of age at index date; evidence of pregnancy (ICD-9-CM codes 761.5x, V22.xx, V72.40, V2.32, V23.86) or gestational diabetes (ICD-9-CM codes 648.0x or 648.8x) during the entire study period; any pharmacy claims of insulin pump, pump supplies, or inhaled insulin during the entire study period. Because the analysis only included those who were RAIA naive, patients were excluded if they had pharmacy claims of any RAIA or any insulin therapy used with the KwikPen during the pre-index period. Patients with total costs during the study period that were >99% of the sample were also excluded to minimize cost outliers. Patients who switched RAIA or the delivery system during the post-index period were further excluded from the study.
Outcome measures
Baseline characteristics included patient age, gender, geographic region of residence, provider type and time period associated with the index RAIA, insurance information, complications, comorbidities, medication use, and adherence. Diabetes-related complications included microvascular diseases (neuropathy, nephropathy, and retinopathy) and macrovascular diseases (cardiovascular diseases and peripheral circulatory diseases). Comorbidities included hypertension, disorders of lipid metabolism, obesity, depression, dexterity-impacting diseases, and diseases of the esophagus, stomach, and duodenum. Both complications and comorbidities were identified using ICD-9-CM codes or Current Procedural Terminology (CPT) codes.
Resource utilization was largely divided into pharmacy and medical utilizations. Both were reported as number (frequency) per patient in the 6-month pre- and post-index periods, separately. The pharmacy utilization was measured by number of claims and the categories included OHAs (metformin, sulfonylureas [SUs], thiazolidinediones [TZDs], alpha-glucosidase inhibitors [AGIs], non-SU secretagogues, dipeptidyl peptidase-4 [DPP-4] inhibitors, and the fixed-dose combination therapies), insulin, exenatide/pramlintide (considered together), self-monitoring blood glucose (SMBG), and other drugs of interest (diuretics, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, β-blockers, calcium-channel blockers, anticoagulants, antiplatelets, statins, and the fixed-dose combination therapies). Insulin use during the pre-index period was categorized as ‘insulin-naive’ if a patient did not have any pharmacy claims of insulin therapy; ‘basal pen’ if a patient used long-acting insulin (insulin glargine or insulin detemir) with a pen; ‘basal vial’ if a patient used long-acting insulin with a vial/syringe; ‘other pen’ if a patient used any other insulin (e.g., neutral protamine Hagedorn [NPH], premixed insulin, etc.) with a pen; or ‘other vial’ if a patient used any other insulin with a vial/syringe. A separate variable was created to identify analog versus human insulin during the pre-index period if a patient used any insulin therapy.
The medical utilization was categorized as outpatient utilization (counts of provider and facility visits on different days), inpatient utilization (count of hospital admissions), ER visits (count of ER visits on different days), and number of HbA1c tests on different days. The medical utilization was further identified as diabetes-related if it had ≥1 medical claim with a diagnosis of diabetes (ICD-9-CM codes 250.xx).
Cost variables were created using the amount reimbursed in the claims database during the 6-month pre- and post-index periods, separately. Costs were analyzed as total direct costs, total diabetes-related costs, and the following subgroups: pharmacy, outpatient, inpatient, and emergency room costs. All costs were adjusted to 2009 US Dollars (USD) using the Consumer Price Index. The diabetes-related costs for outpatient service, inpatient service, and ER were defined as costs associated with a claim with diabetes diagnosis codes (ICD-9-CM 250.xx). Diabetes-related pharmacy costs included costs of AHTs (OHAs, insulin, exenatide/pramlintide) and SMBG.
Proportion of days covered (PDC) was used to measure adherence to the index RAIA during the post-index period. PDC was calculated by dividing the number of days with the index RAIA on hand (using the ‘days supplied’ field in pharmacy claims) in the post-index period by 6 months of the post-index periodCitation21. If there was an overlap in days supplied of the index RAIA, the patients were assumed to finish the current prescription before starting the next prescriptionCitation22. When days supplied was missing, average days supplied was imputed using days supplied of RAIA with the same NDC code and quantity dispensed in the study sample. PDC is a flexible adherence measurement, and PDC for a single drug, such as the index RAIA, can be considered as an adjusted medication procession ratio (adjusted MPR, total days supplied divided by number of days in the analysis period, capped at 1)Citation23. PDC is also recommended to measure adherence to a class of drugsCitation24,Citation25, and it was used to calculate the baseline adherence to AHTs and SMBG in this study.
The rate of hypoglycemia was counted during the pre- and post-index periods, separately. A hypoglycemia event was identified using ICD-9-CM codes 251.0x, 251.1x, 251.2x, and 250.3x for outpatient or inpatient services on different days. A hypoglycemia event during an ER visit followed a validated coding algorithmCitation26 as an ER visit with claims of the aforementioned ICD-9-CM codes and with claims of ICD-9-CM codes 251.8x, but excluding ICD-9-CM codes 259.8x, 272.7, 681.xx, 682.xx, 686.9x, 707.1x–707.9x, 709.3x, 730.0x–730.2x, and 731.8x.
Statistical analysis
The propensity score matching method was used to control for potential sample selection bias across the two study cohorts. The logistic regression model determined the propensity score of initiating vial/syringe versus the KwikPen. All patient characteristics in and baseline outcome variables (costs, medical and pharmacy utilization, rate of utilization, number of HbA1c tests, adherence to AHT and SMBG) were used in the logistic regression. To balance the variance and the bias in the sample, the caliper matching strategy was implemented. The matches were kept if the propensity score in the vial/syringe cohort was within a pre-defined radius (≤0.01) of the propensity score in the KwikPen cohort. Patients in the KwikPen and vial/syringe cohorts were matched on a 1:3 ratio. For each patient in the KwikPen cohort, at least one and up to three patients in the vial/syringe cohort were selected as long as the patients had a propensity score ≤0.01 that of the patients in the KwikPen cohort. The patient characteristics were compared across study cohorts using a t-test for continuous variables and the Pearson chi-square test for categorical variables, both before and after the matching process. The changes in outcomes were calculated by subtracting pre-index outcome from post-index outcome for each cohort. Then, the changes in outcomes were compared between the two cohorts using a difference-in-difference (DID) analysis that subtracted the difference in the KwikPen cohort from the difference in the vial/syringe cohort. Because cost data were skewed, the Wilcoxon rank-sum test was used to conduct the analysis on costs. Other outcomes (adherence, hypoglycemia rate, and utilization) and the corresponding changes were compared using t-tests.
Table 1. Patient characteristics before and after propensity score matching.
Post hoc and sensitivity analyses were conducted to examine the impact of the KwikPen and vial/syringe on sub-groups of cost outcomes and to test the robustness of the results. In the post hoc analyses, diabetes-related pharmacy and medical costs were further broken down into the following sub-groups: pharmacy (OHAs and the subgroups of metformin, SUs, TZDs, DPP-4 inhibitors, other single OHAs, and fixed-dose combination; insulin and the subgroups of long-acting analog, NPH, RAIA, short-acting human insulin [SAHI], premixed analog, and premixed human; exenatide/pramlintide; and SMBG), outpatient costs (healthcare provider costs and the subgroups of primary care physicians [PCPs], endocrinologists, cardiologists, and other providers; facility costs; and other outpatient costs), and inpatient costs (primary reason for hospitalization, including microvascular complications, macrovascular complications, and other hospitalization). Sensitivity analysis was conducted by including patients who initiated insulin glulisine in the vial/syringe cohort.
Results
Patient characteristics
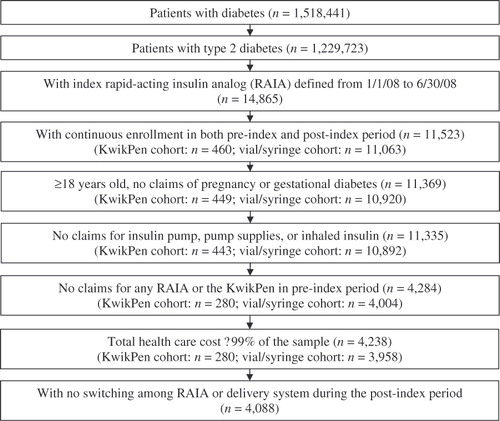
The flow of study sample selection is illustrated in . A total of 1,518,441 patients were identified as having diabetes, of which 1,229,723 patients were determine to have T2DM. Among patients with T2DM, 14,865 patients had ≥1 pharmacy claim for index RAIA, including insulin lispro KwikPen (n = 590), insulin lispro vial/syringe (n = 8,424), and insulin aspart vial/syringe (n = 5,851). The remaining sample selection criteria led to a final sample of 268 patients initiating insulin lispro KwikPen and 3,820 patients initiating insulin lispro or insulin aspart vial/syringe. Before propensity score matching, multiple patient characteristics were different between the two study cohorts (). Most notably, patients who initiated a RAIA using the KwikPen were younger than those who initiated using a vial/syringe (mean: 58 vs. 62 years, p < 0.001). A greater proportion of patients in the KwikPen cohort had endocrinologists as their index physician type and commercial health plan as their insurance source than those in the vial/syringe cohort (p < 0.001 for both comparisons). Pre-index insulin use was significantly different between the two cohorts. For example, fewer patients in the KwikPen cohort were insulin-naive during the pre-index period than the vial/syringe cohort (p = 0.002). Among patients who used insulin during the pre-index period, a greater proportion of patients in the KwikPen cohort used insulin with a pen (‘basal pen’ and ‘other pen’) than the vial/syringe cohort (p < 0.001 for both comparisons). These differences corroborate a previous study that examined the key predictors of pen versus vial initiation in patients with T2DMCitation27.
The propensity score matching method was applied successfully, with a success rate of 89%. A total of 590 vial/syringe cohort patients were matched to 239 patients in the KwikPen cohort. After matching, the two cohorts were balanced in terms of baseline characteristics. There were no significant differences across the matched cohorts across approximately 20 different variables (), except for the variable ‘basal pen’. The use of ‘basal pen’ during the pre-index period was greater in the KwikPen cohort than the vial/syringe cohort, although the magnitude of difference was reduced after matching (post-match: 25.5 vs. 15.8%, p = 0.001; pre-match: 32.1 vs. 3.3%, p < 0.001).
Resource utilization and costs
After matching, there were no significant differences in resource utilization across the two cohorts during the pre-index period (). In the post-index period, the KwikPen cohort had significantly lower utilization of OHAs compared to the vial/syringe cohort (mean count: 2.2 vs. 2.7, p = 0.021). From pre- to post-index periods, the OHA utilization decreased in both cohorts, but the reduction was greater in the KwikPen cohort than in the vial/syringe cohort (mean count: –1.3 vs. –0.7, p = 0.016, negative value representing reduction: DID = 0.6). The KwikPen and vial/syringe cohorts were similar in other post-index utilizations and changes in utilization from pre- to post-index periods.
Table 2. Results of adherence and difference-in-difference (DID) analysis on resource utilization and hypoglycemia rate.
There were no significant differences in costs across the two cohorts during the pre-index period (). The diabetes-related pharmacy costs increased from pre- to post-index periods in both cohorts, and the increase was greater in the KwikPen cohort by $294 than the vial/syringe cohort (+$900 vs. +$607, p < 0.001; DID = –$294). The total pharmacy costs also increased for both cohorts, but the difference in the increased amounts was not significant (+$1,171 vs. +$874, p = 0.058; DID = –$297). The KwikPen cohort showed a decrease in total diabetes-related costs of $235, while the vial/syringe cohort showed an increase in total diabetes-related costs of $61 (DID = $297, p = 0.006). There were no significant differences in other costs and changes in costs from pre- to post-index periods between the two study cohorts.
Table 3. Results of difference-in-difference (DID) analysis on cost.
Adherence and hypoglycemia rate
In the matched sample, adherence to the index RAIA during the post-index period () was significantly greater in the KwikPen cohort than the vial/syringe cohort (PDC: 54.6 vs. 45.2%, p < 0.001). There were no significant differences in hypoglycemia rate between the two cohorts (episodes per patient over the 6-month pre-index period: 0.03 vs. 0.03, p = 0.905; episodes per patient over the 6-month post-index period: 0.03 vs. 0.02, p = 0.539; change from pre- to post-index period: +0.004 vs. –0.003, p = 0.707).
Post hoc and sensitivity analyses
In the post hoc analyses, significant differences were observed in several diabetes-related cost components between the KwikPen and vial/syringe cohorts (). Greater reduction in OHA costs was observed in the KwikPen than in the vial/syringe cohort (–$152 vs. –$60, p = 0.006), including greater reduction in the costs of SUs and DPP-4 inhibitors (–$22 vs. –$7, p = 0.003 and –$53 vs. –$10, p = 0.008, respectively). The KwikPen was also associated with a greater increase in insulin costs than the vial/syringe (+$982 vs. +$643, p < 0.001), including a greater increase in the costs of long-acting analog and RAIA (+$375 vs. +$201, p < 0.001 and +$669 vs. +$438, p < 0.001, respectively), with a slightly greater reduction in the cost of SAHI (−$9 vs. $0, p = 0.002). The increase in the cost of SMBG was significantly greater in the KwikPen cohort than the vial/syringe cohort (+$91 vs. +$41, p = 0.004). Among sub-categories of diabetes-related outpatient costs, the KwikPen was associated with a greater reduction in PCP costs (–$23 vs. –$1, p = 0.008) and outpatient facility costs (–$550 vs. –$314, p = 0.045). No significant differences were observed among sub-groups of diabetes-related inpatient costs across the cohorts.
Table 4. Results of post hoc analyses on diabetes-related pharmacy, outpatient, and inpatient cost breakdowns.
In the insulin glulisine sensitivity analysis, after matching, the final sample included 236 patients initiating insulin lispro using the KwikPen and 601 patients initiating insulin lispro, aspart or glulisine using vial/syringe. The results were consistent with the original results in this study. The KwikPen cohort was associated with a greater adherence than the vial/syringe cohort (PDC: 53.9 vs. 46.3%, p = 0.001). The diabetes-related pharmacy costs increased for both cohorts but the increase was greater in the KwikPen cohort than the vial/syringe cohort by $330 (+$942 vs. +$612, p < 0.001; DID = –$330). However, the reduction in the total diabetes-related costs was greater in the KwikPen cohort than the vial/syringe cohort by $110 (–$246 vs. –$136, p = 0.004; DID = $110) as well as the reduction in the OHA utilization (–1.2 vs. –0.7, p = 0.024; DID = 0.5).
Discussion
Using a large healthcare claims database, the current study evaluated the impact of differing insulin delivery systems on outcomes in patients with T2DM who newly initiated RAIA. Despite the greater increase in diabetes-related pharmacy costs associated with initiating RAIA with the KwikPen versus the vial/syringe, the use of the KwikPen was associated with several key positive outcomes: (1) there was a greater reduction in the total diabetes-related costs (potential cost offset) with the KwikPen than with the vial/syringe (DID: $297 per patient); (2) there was a greater reduction in the utilization of OHAs with the KwikPen than with the vial/syringe (DID in mean count: 0.6; mean cost: $92); and (3) medication adherence was also greater with the KwikPen than with the vial/syringe (difference in PDC: 9.5%). There were no significant differences in the rate of hypoglycemia; total direct costs; or diabetes-related outpatient, inpatient, and ER costs between the two cohorts.
Post hoc analyses provided further differences among various cost sub-groups. In the post hoc analyses, the KwikPen was associated with greater reductions in OHA costs, specifically in the costs of SUs and DPP-4 inhibitors and increases in the cost of insulin therapies, including long-acting insulin and RAIA, when compared to the vial/syringe cohort. A potential hypothesis behind these changes (reduction in OHA costs and increase in insulin costs) is that the increased utilization (i.e., prescription fills) of insulin and intensification of insulin therapy would be associated with reduction in OHA use. When outpatient costs were broken down further into several sub-groups, KwikPen was associated with significant reductions in PCP visit costs and outpatient facility costs than the vial/syringe cohort. There are several potential hypotheses behind this study finding. For example, PCP visits may not be as frequent as expected since 70% of the KwikPen and 60% of the vial/syringe cohorts in this study (before propensity score matching) had experience in using insulin prior to initiating RAIA.
Selective positive outcomes in the current study corroborated findings from several previous studies comparing prefilled pen versus vial/syringe. A significant reduction in the diabetes-related healthcare costs was observed after patients either switched from vial/syringe to a prefilled pen or initiated the prefilled pen in studies by Lee et al., Cobden et al., and Pawaskar et al.Citation17–19. Adherence was also greater with the prefilled pen than the vial/syringe in these studies and in a study by Baser et al., except for the insulin-naive analysis in the Pawaskar et al. studyCitation16–19. Similar to the current study, Baser et al. estimated the hypoglycemia rate by counting the number of hypoglycemia events and found no significant differences between the prefilled pen and vial/syringe cohortsCitation16. In studies by Lee et al. and Cobden et al., there was significant reduction in the percentage of patients experiencing hypoglycemia events when patients converted from vial/syringe to prefilled penCitation17,Citation18. Hypoglycemia was not reported by Pawaskar et al.Citation19.
There are several limitations to this study, the majority of which are inherent to all retrospective database analyses involving patients with T2DM. First, adherence measure using pharmacy refill records is not optimal since medication use during a hospitalization, potential medication sharing or wastage cannot be assessed. However, PDC has been endorsed by the National Quality Forum to indicate the medication management qualityCitation25 and can measure adherence to one medication or a class of medicationsCitation24. In addition, PDC is commonly used in the literatureCitation21,Citation23,Citation25. Second, the degree of glycemic control (e.g., HbA1c) which may impact healthcare resource utilization is not identifiable in claims databases. The current study used surrogate markers such as diabetes-related complications, comorbidities, and concomitant medications to adjust for T2DM severity. Additional variables are not present in the claims database (e.g., smoking status). Applying advanced methods to control the unobserved as well as observed sample selection bias could provide an important contribution to treatment evaluation in patients with T2DM. Third, since hypoglycemia rates were calculated using ICD-9-CM codes, which require some clinical intervention, mild to moderate events may not be captured. Fourth, the results may not represent un-insured population or elderly patients with only basic Medicare coverage. The MarketScan database does not include all Medicare claims for those who are ≥65 years old.
Conclusion
The study findings suggest that patient outcomes vary by different insulin delivery systems (prefilled pen versus vial/syringe) when patients with T2DM initiate RAIA. Initiating RAIA with the KwikPen was associated with a greater increase in diabetes-related pharmacy costs but better RAIA adherence, greater reduction in total diabetes-related costs, and OHA utilization than vial/syringe. There were no differences in the changes to the total healthcare costs.
Declaration of interest
This study was financially supported by Eli Lilly and Company (Indianapolis, IN, USA). L.J.L. and M.D.P. are employees and stock owners of Eli Lilly and Company. S.M.C. is an employee of Lilly USA, LLC and a stock owner of Eli Lilly and Company. Q.L. and M.W.R. are employees of United BioSource Corporation (Lexington, MA, USA). Eli Lilly and Company contracted with United BioSource Corporation for the conduct of this study.
Transparency
Supplementary Material
Download MP3 Audio (5.1 MB)Acknowledgments
The authors would like to thank Khaled Sarsour, PhD, an employee of Eli Lilly and Company, for his input on the development of the study hypothesis.
Notes
* Novolog, Novolog Mix70/30, Novolog Mix50/50, and FlexPen are registered trademarks of Novo Nordisk Pharmaceuticals Inc. (Princeton, NJ, USA)
† Humalog and the KwikPen are registered trademarks of Eli Lilly and Company (Indianapolis, IN, USA)
‡ MarketScan is a registered trademark of Thomson Reuters (Ann Arbor, MI, USA)
§ Apidra is a registered trademark of Sanofi-Aventis US LLC (Bridgewater, NJ, USA)
References
- Iqbal N. The burden of type 2 diabetes: strategies to prevent or delay onset. Vasc Health Risk Manag 2007;3:511-20
- NIDDK (NIH). National Diabetes Statistics, 2007. Available at: http://diabetes.niddk.nih.gov/DM/PUBS/statistics/#i_people [Last accessed 12 April 2010]
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-53
- Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917-32
- Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596-615
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of Type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med 2008;25:924-32
- Riddle MC. Timely initiation of basal insulin. Am J Med 2004;116(Suppl 3A):3S-S
- Oglesby AK, Secnik K, Barron J, et al. The association between diabetes related medical costs and glycemic control: a retrospective analysis. Cost Eff Resour Alloc 2006;4:1
- Salas M, Hughes D, Zuluaga A, et al. Costs of medication nonadherence in patients with diabetes mellitus: a systematic review and critical analysis of the literature. Value Health 2009;12:915-22
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004;27:1218-24
- Odegard PS, Capoccia K. Medication taking and diabetes: a systematic review of the literature. Diabetes Educ 2007;33:1014-29; discussion 30-1
- Polonsky WH. Emotional and quality-of-life aspects of diabetes management. Curr Diab Rep 2002;2:153-9
- Polonsky WH, Fisher L, Guzman S, et al. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 2005;28:2543-5
- Molife C, Lee LJ, Shi L, et al. Assessment of patient-reported outcomes of insulin pen devices versus conventional vial and syringe. Diabetes Technol Ther 2009;11:529-38
- Baser O, Bouchard J, Deluzio T, et al. Assessment of adherence and healthcare costs of insulin device (FlexPen(R)) versus conventional vial/syringe. Adv Ther 2010;27(2):94-104
- Cobden D, Lee WC, Balu S, et al. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy 2007;27:948-62
- Lee WC, Balu S, Cobden D, et al. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther 2006;28:1712-25; discussion 0-1
- Pawaskar MD, Camacho FT, Anderson RT, et al. Health care costs and medication adherence associated with initiation of insulin pen therapy in Medicaid-enrolled patients with type 2 diabetes: a retrospective database analysis. Clin Ther 2007;29 Spec No: 1294-305
- Asghari S, Courteau J, Carpentier AC, et al. Optimal strategy to identify incidence of diagnostic of diabetes using administrative data. BMC Med Res Methodol 2009;9:62
- Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10:3-12
- Leslie R, Gwadry-Sridhar F, Thiebaud P, et al. Calculating medication compliance, adherence and persistence in administrative pharmacy claims databases. Pharmaceutical Programming 2008;1:13-19
- Karve S, Cleves MA, Helm M, et al. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care 2008;46:1125-33
- Martin BC, Wiley-Exley EK, Richards S, et al. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother 2009;43:36-44
- Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728-40
- Ginde AA, Blanc PG, Lieberman RM, et al. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:4
- Lee LJ, Anderson J, Foster SA, et al. Predictors of initiating rapid-acting insulin analog using vial/syringe, prefilled pen, and reusable pen devices in patients with type 2 diabetes. J Diabetes Sci Technol 2010;4:547-57