Abstract
Objective:
The spectrum of diseases caused by Streptococcus pneumoniae and non-typeable Haemophilus influenzae (NTHi) represents a large burden on healthcare systems around the world. Meningitis, bacteraemia, community-acquired pneumonia (CAP), and acute otitis media (AOM) are vaccine-preventable infectious diseases that can have severe consequences. The health economic model presented here is intended to estimate the clinical and economic impact of vaccinating birth cohorts in Canada and the UK with the 10-valent, pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared with the newly licensed 13-valent pneumococcal conjugate vaccine (PCV-13).
Methods:
The model described herein is a Markov cohort model built to simulate the epidemiological burden of pneumococcal- and NTHi-related diseases within birth cohorts in the UK and Canada. Base-case assumptions include estimates of vaccine efficacy and NTHi infection rates that are based on published literature.
Results:
The model predicts that the two vaccines will provide a broadly similar impact on all-cause invasive disease and CAP under base-case assumptions. However, PHiD-CV is expected to provide a substantially greater reduction in AOM compared with PCV-13, offering additional savings of Canadian $9.0 million and £4.9 million in discounted direct medical costs in Canada and the UK, respectively.
Limitations:
The main limitations of the study are the difficulties in modelling indirect vaccine effects (herd effect and serotype replacement), the absence of PHiD-CV- and PCV-13-specific efficacy data and a lack of comprehensive NTHi surveillance data. Additional limitations relate to the fact that the transmission dynamics of pneumococcal serotypes have not been modelled, nor has antibiotic resistance been accounted for in this paper.
Conclusion:
This cost-effectiveness analysis suggests that, in Canada and the UK, PHiD-CV’s potential to protect against NTHi infections could provide a greater impact on overall disease burden than the additional serotypes contained in PCV-13.
Introduction
Streptococcus pneumoniae is an important cause of respiratory disease worldwide, accounting for a range of illnesses in young, elderly, and immunocompromised individuals. These conditions include disseminated invasive diseases (ID) (e.g., bacteraemia and meningitis), non-invasive lower respiratory tract infections (e.g., pneumonia), and non-invasive upper respiratory tract infections (e.g., sinusitis and acute otitis media [AOM])Citation1.
Haemophilus influenzae is another major cause of infection, particularly in young childrenCitation2. It is often transmitted via contact with respiratory droplets emitted from asymptomatic nasopharyngeal carriersCitation2; and carriage rates of H. influenzae can be increased in places of prolonged close contact, such as daycare centres and care homesCitation3,Citation4. Non-typeable H. influenzae (NTHi) strains (those without a polysaccharide capsule) are significant pathogens that can affect both adultsCitation5 and childrenCitation6. NTHi is most commonly linked with mucosal diseases, such as otitis media (OM) (that tends to predominate in children) and sinusitis (that occurs across all age groups)Citation7. However, NTHi can also cause ID. Previously, ID caused by NTHi was believed to only occur in children with immunological or anatomical defects that pre-disposed them to bacterial infectionCitation8. However, it has now become clear that NTHi may cause bacteraemia and meningitis in otherwise healthy childrenCitation8; although it is more likely to affect young children, children with underlying disease, and adults aged >65 yearsCitation9,Citation10. The relative importance of invasive NTHi infections has increased in parallel with the decline in invasive H. influenzae serotype b disease achieved through the routine immunization of infantsCitation11. In some populations, NTHi is now responsible for over 50% of ID cases caused by H. influenzaeCitation9,Citation12,Citation13.
Pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV; GlaxoSmithKline Biologicals, Rixensart, Belgium) is a 10-valent pneumococcal conjugate vaccine that includes serotypes 1, 5, and 7F in addition to 4, 6B, 9V, 14, 18C, 19F, and 23F in the heptavalent pneumococcal conjugate vaccine (PCV-7; Pfizer/Wyeth, USA). Serotypes 1 and 5 are associated with complicated pneumonia or empyema, as well as other syndromes, in children aged 2–10 yearsCitation14–17. The serotypes included in PHiD-CV should cover ∼85% of all ID in children (up to 90%) in EuropeCitation18. PHiD-CV employs a novel carrier protein (protein D) derived from NTHi for eight of the 10 pneumococcal serotypes included in the vaccine. By virtue of this carrier protein, PHiD-CV is designed to offer the potential for protection against disease caused by NTHi. Prymula et al.Citation19 found that a predecessor of PHiD-CV that contained pneumococcal polysaccharides from serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F conjugated individually to NTHi-protein D reduced the overall incidence of OM by 33.6%, and the incidence of OM caused by H. influenzae (including NTHi) by 35.6%.
Here we describe a health economic analysis that compares the estimated economic impact of PHiD-CV with the recently licensed 13-valent pneumococcal conjugate vaccine (PCV-13; Pfizer Inc., New York) in the UK and Canada, under base-case conditions that include minimal estimations of NTHi infection rates in ID. PCV-13 contains serotypes 3, 6A, and 19A in addition to the 10 serotypes in PHiD-CVCitation20. An assessment of health economic models for PCVs has identified two important parameters that should ideally be incorporated in all future pharmacoeconomic analyses, namely indirect (herd) protection of non-vaccinated people and the ecological replacement of vaccine-type pathogens by other serotypes (serotype replacement)Citation21. Other important factors to consider include the potential for cross-reactivity between related S. pneumoniae serotypes and the impact of protection against NTHiCitation22. The study presented here aims to meet these requirements for pharmacoeconomic models of pneumococcal conjugate vaccination while estimating the cost-effectiveness of PHiD-CV vs PCV-13 in Canada and the UK. Results from these two countries are presented together due to the relative similarity of their healthcare systems (citizens of both countries are eligible for free [tax-funded] visits to doctors and specialists, and free hospital treatment, but must pay towards prescription drugs).
Materials and methods
The model described here is a Markov cohort model, built to simulate the epidemiological burden of pneumococcal- and NTHi-related diseases (ID, community acquired pneumonia [CAP] and AOM) within hypothetical birth cohorts of 348,000 and 772,500 newborns in CanadaCitation23 and the UKCitation24, respectively, in 2007 (). Cohort-based analyses represent one of the most common forms of health economic modelling and are particularly useful for determining the direct impact of medical interventions (as described previously by Sonnenberg and BeckCitation25 and Beck et al.Citation26).
Table 1. Country-specific model parameters.
In a Markov model, the individuals of the birth cohort move between Markov states according to estimated transition probabilitiesCitation25,Citation26. In this model, a birth cohort is followed over a lifetime from birth to death with a cycle length of 1 month (1128 month-long cycles, corresponding to 94 years). Our model has a number of mutually exclusive disease-related outcomes including meningitis, bacteraemia, CAP, AOM, no pneumococcal infection, and death. During each cycle, the probability of entering a specific health state is calculated using incidence rates of disease caused by S. pneumoniae and NTHi. These incidence rates are then used to estimate the probability of disease-management options for each of the diseases considered ().
Figure 1. Markov cohort model design. The cohort model is Markov-based with three exclusive health states: no disease, sequelae, and death. The transition from ‘no disease’ to ‘sequelae’ or ‘death’ is calculated based on this decision tree. In the model, only meningitis can lead to long-term sequelae; meningitis and bacteraemia include NTHi meningitis and NTHi bacteraemia, respectively; and non-consulting AOM are accounted for in the quality-of-life impact calculation. AOM, acute otitis media; GP, general practitioner; PCP, primary care physician; NTHi, non-typeable Haemophilus influenzae.
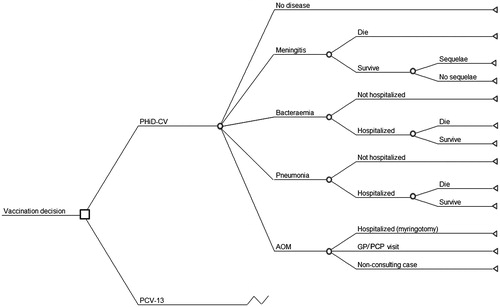
The model utilizes serotype-specific distributions per disease type and per age group ( and Appendix Table A1) for both countriesCitation27–30. Furthermore, since factors such as disease frequency, contact matrix, and hospital uptake differ across geographical regions, parameters concerning epidemiology and disease management are also specified in a country-specific manner. Age-specific overall monthly mortality rates (Appendix Table A2) were extracted from country-specific national databasesCitation24,Citation31. Finally, specific unit costs and quality adjusted life years (QALYs) were estimated for each age–time unit so that an accumulated cost and QALY estimate for the birth cohort was reported by summing all of the unit estimates over the cohort’s lifetime.
Serotype and pathogen replacement are both defined as the substitution of vaccine serotypes/pathogens by non-vaccine serotypes/pathogens in a vacant niche. Replacement has an individual component, which is the increased risk of nasopharyngeal colonization by a non-vaccine serotype/pathogen when exposed, and a collective component resulting from the increased circulation of non-vaccine serotypes/pathogens in a partially vaccinated population (the opposite of herd protection). Replacement may be observed in clinical trials (the individual component) and in post-marketing observational studies (a combination of individual and collective components). In the model presented here, the individual component of replacement is applied by reducing direct vaccine effectiveness estimates, while the collective component is combined with herd protection to estimate an indirect vaccine effectiveness rate, reported as net indirect effect, and applied directly to the number of predicted health outcomes (see ‘Net indirect effect’ section).
The analysis described here estimated the expected improvements in health outcomes provided by PHiD-CV and PCV-13 using background epidemiology data from Canada and the UK prior to the introduction of PCV-7. Currently, the UK employs PCV-13 for routine childhood immunizationCitation32, whereas in Canada either PHiD-CV or PCV-13 is used, depending on province or territoryCitation33. While using pre-PCV-7 epidemiology as a basis to estimate the impact of PHiD-CV or PCV-13 may not accurately reflect the current status of routine vaccination in Canada and the UK, the pre-PCV-7 vaccine era epidemiology can be considered to be relevant for decision-making. This assumption simplified the calculation of vaccine impact, first by removing issues surrounding the heterogeneous deployment of vaccines in Canada; second, by removing the need to model the epidemiological ramifications of multiple transitions from one PCV to another; and finally, by removing the possibility of under-estimating the value of any of the vaccine formulations if that country had already been using PCV-7 for some time. The assumption of a vaccine-naïve setting was not expected to significantly influence the overall conclusions of the model, in terms of deciding which vaccine is expected to provide the greatest impact on disease and costs.
The model was used to compare two independent 3 + 1 regimens (doses at 2, 4, 6, and 13 months in Canada and at 2, 3, 4, and 13 months in the UK) of PHiD-CV and PCV-13. Although a 2 + 1 schedule for PCV-13 is currently used in the UK (given at 2, 4, and 13 months)Citation32 and in Quebec (the rest of Canada uses a 3 + 1 schedule), we simulated the impact of a 3 + 1 schedule for both vaccines in both countries, as vaccine efficacy is highest using a 3 + 1 schedule, and this is what was used in the vaccine efficacy trial from Prymula et al.Citation19. For each vaccination scenario modelled in the analysis, the model estimates the expected effect of vaccination on invasive pneumococcal disease (acute episodes of meningitis and bacteraemia, and sequelae of meningitis), all-cause CAP (hospitalized and non-hospitalized cases) and all-cause AOM (hospitalized [myringotomies] and non-hospitalized cases). The residual burden of disease calculated by the model includes the number of pneumococcal/NTHi-related outcomes, the number of deaths and the number of survivors with sequelae. These estimates are then used to compute the total life-years and QALYs gained for all individuals in the cohort (lifetime gains). The model is also capable of calculating direct healthcare costs for the health system, families, and third-party payers, and the societal productivity gained as a result of preventing acute disease, early mortality, and disabilities. This is done using the human capital approachCitation34.
Since cohort models follow a group of individuals over time, it is generally accepted that outcomes should be discounted to allow a proper assessment of the benefit from the start of the interventionCitation35. Country-specific discount rates on cost and effect are applied as the cohort is evaluated over a lifetime. For the UK, these discount rates were taken as 3.5% for both cost and effectCitation36, whereas in Canada a discount a rate of 3% was usedCitation37 ().
A summary of all the data used to configure the model, including references to the original sources, is given in and in Appendix Tables A3–A6. Wherever possible, robust country- and vaccine-specific data were used to configure the model. However, when this was not possible, reliable published estimates from other countries, or from studies with the PCV-7 vaccine, were used instead.
Epidemiological data
Demographic input data for the birth cohorts and age-specific overall mortality rates were extracted from the published literature and from country-specific national sources, such as population censuses, life tables, and surveys wherever possible. Age-specific annual incidence data and case fatality ratios (CFRs) for each type of pneumococcal/NTHi disease were obtained from published literature or national databases from the pre-PCV-7 period ( and Appendix Tables A3–A6)Citation28,Citation38–46. For acute infectious disease events such as meningitis, bacteraemia, pneumonia, and AOM, prevalence rates over 1 year were estimated from corresponding annual incidence rates. These data were used because they are readily available in the literature. The prevalence of long-term sequelae was estimated as a percentage of the acute events with or without the vaccine programme. Sequelae were only considered when accurate surveillance data could be obtained. As such, sequelae were only estimated for meningitis (and not for bacteraemia, CAP, or AOM) using UK incidence rates for both countriesCitation38 (). Due to a lack of data, the general mortality of individuals with long-term sequelae was assumed to be the same as that of the general population.
Vaccine efficacy parameters
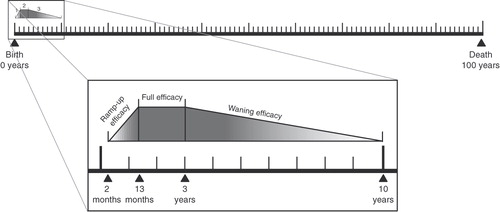
In both countries, vaccination coverage was assumed to be 100%. To simulate how vaccination protection alters over time, the model considers three distinct periods of protection: pre-vaccination, vaccination, and post-vaccination. Individuals of pre-vaccination age (0–2 months) and post-vaccination age (≥10 years) are considered to be not directly protected by the vaccine. Those within the vaccination age range (2 months to 10 years) are further sub-divided into three additional time periods: an initial ramp-up phase that occurs over the course of vaccine administration (2–13 months), a full efficacy phase (13 months to 3 years) and a waning efficacy phase (3–10 years) ()Citation47. This estimation of waning efficacy was based on the opinion of a board of experts and previous observations of efficacy with PCV-7Citation47. To simulate waning over these time periods, the model linearly adjusts vaccine efficacy each month. Vaccine efficacy is also adjusted to account for the different serotypes covered by each vaccine and the serotype distribution for the various clinical manifestations of pneumococcal and NTHi disease in each country.
Figure 2. Modelled age compartments and vaccine efficacy periods. Reproduced from De Wals et al.Citation47.
Invasive disease
The model estimates the number of cases of pneumococcal meningitis and bacteraemia separately ( and Appendix Tables A3 and A4). Vaccine effectiveness for PHiD-CV and PCV-13 against ID caused by S. pneumoniae serotypes included in PCV-7 was estimated from serotype-specific efficacies taken from a large case-control study using data from the US Centers for Disease Control and Prevention (CDC) Active Bacterial Core Surveillance on the effectiveness of PCV-7 in preventing ID ()Citation48. Vaccine efficacy rates for ID caused by serotypes not included in PCV-7 were estimated using the mean serotype efficacy observed in the case-control trial (94.7%)Citation48. Serotype 3 has been associated with an atypically abundant expression of capsular polysaccharide, which could make it less susceptible to anti-polysaccharide antibody defence mechanismsCitation49. However, when estimating the efficacy of PCV-13, the decision was taken to include the full efficacy for serotype 3.
Table 2. Vaccine-specific model parameters.
The model assumes the same efficacy for meningitis and for hospitalized and non-hospitalized bacteraemia. Mortality from ID was estimated using age-specific CFRs for hospitalized cases of pneumococcal meningitis and bacteraemia ( and Appendix Tables A3 and A4). All cases of meningitis were assumed to be hospitalized in both countries. However, for bacteraemia, all cases in the UK were assumed to be hospitalized, as was done by De Wals et al.Citation47; while in Canada 62% of cases were assumed to be hospitalized, as reported by Petit et alCitation43. For meningitis, neurological sequelae and severe hearing loss were estimated separately based on reported methodsCitation38. The model allows for sequelae specific to bacteraemia; however, due to a lack of data, bacteraemia sequelae were set to zero in the base-case analysis.
The model calculates the number of ID cases in children caused by NTHi, which was estimated to be 5% of the incidence of meningitis and bacteraemia caused by S. pneumoniae ()Citation50. This figure was based on data from the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) in 2007, which reported the percentage of NTHi meningitis (median 6%; range 4–7%) and NTHi bacteraemia cases (median 7%; range 4–10%) related to the incidence of both pneumococcal meningitis and bacteraemia in children aged <10 yearsCitation50. These estimates were based on isolates collected during 2002–2006. Mortality rates for NTHi ID in children aged <10 years were extrapolated from the 2006 European Union Invasive Bacterial Infections Surveillance Network (EU-IBIS) report and assumed to be 10%Citation12. No vaccine efficacy has been reported for invasive NTHi diseases, so the same efficacy shown for AOM was used ()Citation19. This was a conservative assumption, as vaccine efficacy against ID is often higher than in mucosal diseasesCitation51,Citation52.
Table 3. Model parameters common to both countries.
Community-acquired pneumonia
Identifying the influence of PCVs on pneumonia is challenging: serum sampling is unreliable and, while lung aspirates offer greater diagnostic sensitivity, this is an invasive procedure with a risk of pneumothoraxCitation6. As such, vaccine effectiveness estimates against pneumonia were not available by serotype. The Northern California Kaiser Permanente study with PCV-7 identified overall effectiveness rates of 4.3% for ambulatory pneumonia () and 20.5% for hospitalized pneumoniaCitation52. The model assumes that the vaccine effectiveness of PHiD-CV and PCV-13 is the same as PCV-7 for ambulatory pneumonia. However, for hospitalized pneumonia caused by S. pneumoniae, estimates of effectiveness were taken from a previous health economic analysis of 7-, 10-, and 13-valent pneumococcal conjugate vaccines in The NetherlandsCitation53. Hence, the model uses vaccine efficacies of 22.9% and 24.7% for PHiD-CV and PCV-13, respectively, to calculate the percentage reduction in all-cause pneumonia hospitalization attributable to vaccine efficacy against S. pneumoniaeCitation53 (). Vaccine efficacy for PHiD-CV against CAP caused by NTHi was not considered.
The model assumes that no deaths are related to ambulatory pneumonia cases. Country-specific hospitalization rates, general practitioner/primary care physician (GP/PCP) consultation rates, and CFRs were obtained from published sourcesCitation28,Citation38,Citation41,Citation42 ( and Appendix Table A5).
Acute otitis media
The model estimates the annual number of GP/PCP visits due to AOMCitation28,Citation43,Citation46 and inpatient myringotomy proceduresCitation28,Citation43–45 ( and Appendix Table A6). These estimates were then used to calculate the resource use and costs under each of the vaccine scenarios. In the UK, there has been a large decrease in the rate of GP consultations for AOMCitation54,Citation55, hence an adjustment for non-consultation was used to calculate the additional impact on QALYs incurred by infants with AOM who are not seen by GPs. This adjustment was derived from the ratio of total AOM cases as estimated in a previous cost-effectiveness analysis of pneumococcal vaccination in England and WalesCitation42 and the estimated total number of GP consultations for AOM, derived from a case-linked cohort study in the UKCitation46. The adjustment for non-consultation does not consider the economic impact of missed cases of AOM, but instead aims to capture the full impact of AOM on quality-of-life, which is known to be considerableCitation56.
The model assumes that 35.9% of AOM cases are attributable to S. pneumoniae and that 32.3% of AOM cases are attributable to NTHi (), both of which were calculated as weighted averages based upon published estimatesCitation57. Published surveillance data were then used to estimate the percentage of overall AOM cases covered by vaccine serotypes, which was assumed to be 64.3% for PHiD-CV without 6A cross-reactivity, 71.6% with 6A cross-reactivity (see ‘Cross-reactivity’ section), and 82.2% for PCV-13 ()Citation58.
Vaccine efficacy is modelled for vaccine type pneumococcal serotypes, non-vaccine type serotypes, and H. influenzae (including NTHi). The efficacy of PHiD-CV against AOM caused by vaccine-type S. pneumoniae serotypes and H. influenzae including NTHi was based upon the POET trialCitation19 of the 11-valent predecessor vaccine and assumed to be 57.6% and 35.6%, respectively (). Due to a lack of data, the vaccine efficacy of PCV-13 against AOM caused by vaccine-type S. pneumoniae serotypes was assumed to be equivalent to that of PHiD-CV. Although the POET trial reported an efficacy of 8.6% against pneumococcal non-vaccine serotypesCitation19, a conservative assumption of serotype replacement was made, and PHiD-CV was assumed to result in the same level of serotype replacement in non-vaccine serotypes as that reported for PCV-7 (i.e., −33% efficacy or a 33% increase in non-vaccine serotypes; )Citation59. This assumption was based on a previous modelling analysis of pneumococcal vaccination in the UKCitation47. Lastly, the efficacy of PCV-13 against AOM caused by H. influenzae including NTHi was assumed to be zero ().
Using the aforementioned estimates of vaccine efficacy, total (maximal) efficacy against AOM was calculated as the summed products of the vaccine’s efficacy against the pathogen, and the pathogens’ distribution, as described in the following equation:
where VEmax = maximal efficacy, VEVT = vaccine efficacy against vaccine serotypes, VENVT = vaccine efficacy against non-vaccine serotypes, and VENTHi = vaccine efficacy against disease caused by H. influenzae including NTHi.
The number of myringotomies prevented by each vaccine was estimated from the predicted number of AOM cases using a ratio of observations made by Black et al.Citation52, who reported that PCV-7 had 20.1% efficacy in preventing myringotomies and 7.0% efficacy in preventing AOM cases overall.
Net indirect effect
It is currently very difficult to predict the degree of herd protection that will be afforded by each vaccine in the adult population because of the lack of information about the dynamic processes of serotype replacement and interactions within a well-defined population structure. In practical terms, herd protection and serotype replacement may be hard to disentangle from each other when examining epidemiological data, since they apply contrasting influences on vaccine efficacy. Therefore, fixed values of net indirect effect, independent of vaccine type, were used to reduce the estimated ID frequency amongst the target age group (<5 years) and the rest of the population as a means of dispensing with the need to separate the two effects.
For the target population, a fixed net indirect effect of 15.4% was appliedCitation60 (). This was based on the reduction in cases of ID among children aged <5 years in the US following the introduction of PCV-7, as estimated by the CDC National Immunization SurveyCitation60. The net indirect effect on ID amongst those aged ≥5 years (29%) was also based on CDC data from 2005Citation61 (). A net indirect effect was not applied when estimating the disease burden of AOM; however, serotype replacement was included in the calculations of vaccine efficacy for this disease (as described in the ‘Acute otitis media’ section) based on observations reported in the FinOM trial of PHiD-CV in FinlandCitation59.
Cross-reactivity
Cross-protection (i.e., vaccine efficacy against non-vaccine serotypes in the same serogroup as those covered by the vaccine) was included for serotypes 6A and 19A and was estimated from a trial of PCV-7 reported by Whitney et al.Citation48. Based on data from immunogenicity studies and the opinion of a board of experts, the same level of cross-protection was assumed for PHiD-CV as that observed for PCV-7Citation62–65. The model therefore assumes the efficacy of PHiD-CV against ID to be 76% for serotype 6A and 26% for serotype 19A ()Citation48. This assumption was fairly conservative since PHiD-CV has demonstrated improved opsonophagocytic activity relative to that reported for PCV-7 against serotype 19ACitation65. The model also assumes that PHiD-CV will have an efficacy of 76% against AOM caused by serotype 6A (based on data from Prymula et al.Citation19 and Eskola et al.Citation59), but no efficacy against that caused by serotype 19A (due to low case numbers and wide confidence intervals).
Health outcomes
The model estimates the overall impact of disease on quality-of-life by combining QALY losses due to acute episodes and disutility attributable to long-term sequelae. QALYs lost as a result of acute episodes of disease are presented in and were derived from published studiesCitation38,Citation66,Citation67. These figures take into account the disutility value associated with the disease and the duration of time in the disease health state (in years). These were applied only once for each acute episode. QALYs lost because of long-term sequelae were estimated by applying a proportion of the disutility (equal to 1/12th) due to long-term sequelae () each month during the person’s remaining lifespan.
Disutilities for meningitis, bacteraemia, and pneumonia were taken from a study by Bennett et al.Citation66, which used computer-based utility assessment interviews to calculate utilities under several pneumococcal disease states. In this study, utilities were estimated from parents’ responses to a series of sequential or chained ‘standard gamble’ options, designed to produce meaningful estimates of utility by comparing increasingly severe outcomes of pneumococcal infection. Since this parental assessment of utility compared acute yet recovering cases of hospitalized meningitis or local pneumococcal infection (e.g., pneumonia) against more severe disease outcomes (e.g., severe brain damage), the disutility of acute pneumococcal disease without permanent sequelae was comparatively small. Given the serious long-term consequences that can occur from pneumococcal infection, this parental assessment of disutility may provide a more realistic estimation of disutility than one based on the responses of children, who may be more likely to focus on short-term disutility. Moreover, this approach follows previous analyses that have assumed that the quality-of-life of two people (the patient and one caregiver) would be equally affected by the disease during its acute phaseCitation37. Disutilities for AOM were taken from a cost-utility analysis of second-line antibiotics, which estimated disutility from a survey of paediatricians performed by Oh et al.Citation67, and used in the analysis by Melegaro and EdmundsCitation42. The disutilities of long-term neurological or hearing-related sequelae arising from meningitis were the same as those used in a study of pneumococcal disease burden in CanadaCitation38.
It is important to note that disutility values greater than those used in this analysis have been estimated in a time trade-off analysis of parents and adults in the communityCitation68. However, concerns have been raised by other authors that the values presented in this study may over-estimate the true impact of acute episodes of diseaseCitation42,Citation69. Accordingly, we use more conservative figures consistent with other work in this areaCitation42,Citation66,Citation69.
Resource use and costs
Details of estimated unit costs and the references from which they were taken are available in Appendix Table A7. The model was used to estimate only direct costs of pneumococcal and NTHi disease; and direct medical costs were generally estimated as the product of the number of resource units and their unit costs. This included vaccine and administration costs, disease-related treatment costs, and costs associated with long-term sequelae incurred over survivors’ lifetimes.
Unit costs for acute episodes in Canada are weighted averages inflated to 2007 prices based on data from Morrow et al.Citation38. For the UK, unit costs were estimated using a micro-costing approach that specified detailed resource use categories and unit prices for each disease. Healthcare resource utilization for acute cases of meningitis, bacteraemia, CAP, and AOM were derived from the literatureCitation42 or based on assumptions validated by expert opinion. Unit costs were taken from available public sourcesCitation70,Citation71 and updated to 2007 UK pounds. Where multiple Health Research Group (HRG) codes exist for a disease, a weighted average was constructed using the proportion of hospital admissions for each HRG code as the weights.
Costs related to long-term sequelae were derived from the literatureCitation37,Citation72–75. The lifetime costs of the long-term sequelae of meningitis were weighted by their respective age-specific probabilities of occurrence: 0.07 for children and 0.19 for adults for neurologic sequelae, and 0.133 for children and 0.254 for adults for hearing loss, as reported by Morrow et al.Citation38.
Although indirect costs and productivity loss were not included, these could be considerable. Indirect costs and productivity loss could result from lost earnings due to premature mortality from pneumococcal/NTHi-related diseases as well as a loss of earnings associated with time missed from work for employed adults with the disease or for parents caring for affected children.
The price for PCV-13 was assumed to be equivalent to the PHiD-CV list price per dose in Canada (Canadian $70) and the UK (£27.60). The costs of vaccine administration were based on the assumption that vaccination occurs as part of country-specific primary vaccination schedules. All financial costs prior to 2007 were adjusted to 2007 costs using price indices from the respective countries.
Sensitivity analysis
To evaluate the effects of uncertainty in the key parameters of the model, two forms of sensitivity analysis were undertaken. A one-way sensitivity analysis was performed using realistic ranges for each of the base-case parameters derived from published sources, as far as was possible. The ranges for each parameter in the model are summarized in Appendix Table A8. Mainly, one of three approaches were used: data were varied up and down for all age groups at the same time by ±20% (or ±50%) of the base case value; data were varied to the reported 95% confidence intervals; or a weighted average of studies was calculated to derive suitable values.
In addition, a probabilistic sensitivity analysis (PSA) was performed by recording the results of 1000 Monte Carlo simulations, each of which simultaneously sampled each of the model’s input parameters from an appropriate probability distribution. These distributions were determined using the same source information as the one-way sensitivity analysis and are provided in Appendix Table A8.
Results
Estimated impact of vaccination on disease burden and costs
Canada had ∼33.0 million inhabitants in 2007, with a birth cohort of 348,000 ()Citation23. The corresponding figures for the UK were 61.0 million and 772,500Citation2Citation4 (). The estimated impact of vaccination on disease burden in Canada and the UK is presented in . For both countries, the estimated impact of PHiD-CV and PCV-13 were broadly similar for all-cause meningitis, bacteraemia, and pneumonia under the base-case conditions. Indeed, since the model assumes that PHiD-CV and PCV-13 have equal efficacy against preventing outpatient pneumonia, the impact of both vaccines was predicted to be identical when considering health outcomes related to this condition (PCP/GP visits, ). While both vaccines were predicted to have an approximately comparable influence on ID and CAP under the base-case conditions, PHiD-CV was projected to have a much greater impact than PCV-13 on all AOM-related outcomes, including hospitalized myringotomy procedures GP/PCP visits.
Table 4. Estimated impact of PHiD-CV and PCV-13 on lifetime disease burden in Canada and the UK.
reports the estimated direct costs by disease type and vaccination regimen for Canada and the UK. In both countries, the greatest burden of cost was derived from pneumonia and AOM outcomes. While PCV-13 was expected to provide a greater quantity of savings associated with pneumonia and ID than PHiD-CV, the magnitude of these savings was dwarfed by the expected impact of PHiD-CV on AOM-related costs. Moreover, under the assumption of price parity for PHiD-CV and PCV-13, the model predicted considerable cost savings associated with the use of PHiD-CV in both countries.
Table 5. Direct costs in Canada and the UK by disease and vaccine.
Under base-case conditions, the model’s projections indicate that PHiD-CV is the dominant intervention in both Canada and the UK, due to the greater number of QALYs gained and the substantially greater amount of costs saved. Since this analysis identified a dominant comparator, the calculation of incremental cost-effectiveness ratios (ICERs) was not possible.
Sensitivity analysis
A one-way sensitivity analysis was performed using realistic ranges for each of the base-case parameters derived from published sources where possible (please see Appendix Table A8). In this analysis, each of the variables in the health economic model was independently varied and the corresponding cost and effectiveness results documented. The presentation of a univariate analysis for a dominant base case (i.e., where the comparator is the most effective and least costly intervention) can be difficult to interpret (e.g., using a traditional tornado diagram) due to the inability to calculate an ICER. Therefore, present the impact of the nine most influential variables on a cost-effectiveness plane generated by comparing PHiD-CV to PCV-13 in Canada and the UK, respectively.
Figure 3. Univariate sensitivity analysis for (a) Canada and (b) the UK, comparing PHiD-CV to PCV-13 (discounted). * Most variables are ±20% of the base-case value, but PCV-13 efficacy against AOM caused by H. influenzae including NTHi was assumed to be 0%, so this value was varied to +10% and −10%, based on the −11% efficacy against H. influenzae reported by Eskola et al.Citation59. AOM, acute otitis media; CI, confidence interval; GP, general practitioner; LL, lower limit; NTHi, non-typeable Haemophilus influenzae; nVT, non-vaccine type; PCP, primary care physician; PCV-7, 7-valent pneumococcal conjugate vaccine; PCV-13, 13-valent pneumococcal conjugate vaccine; PHiD-CV, pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine; QALY, quality adjusted life year; Sp, Streptococcus pneumoniae; UL, upper limit.
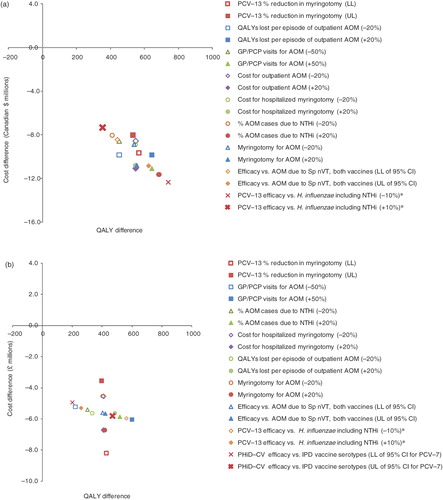
While the majority of the model’s variables were found to exert very little influence over the conclusion of dominance in either Canada or the UK (i.e., that PHiD-CV was both less costly and more effective than PCV-13), parameters relating to AOM-related outcomes were found to be particularly influential in the analysis of both countries. This observation was not surprising, since the potential of PHiD-CV to provide protection against AOM caused by NTHi is a major difference between the two vaccines.
In the PSA, PHiD-CV was found to dominate PCV-13 both in terms of cost and impact on quality-of-life in 95% and 90% of the 1000 simulations in Canada and the UK, respectively. Simulations that ran into quadrants with less QALYs gained were predominantly attributed to samples in which only a small proportion of AOM was attributed to NTHi.
As a further sensitivity analysis, we used new, as yet unpublished, serotype distribution data for England and Wales that indicated a higher proportion of 19A serotype in children <2 years compared to the one used in this analysis. This resulted in decreases in discounted QALYs gained (from 374 to 297) and discounted cost savings (£4.9 to £4.3 million) for PHiD-CV vs PCV-13.
Discussion
The cohort model presented here demonstrates the potential impact of two different vaccines (PHiD-CV and PCV-13) in Canada and the UK over the lifetime of a birth cohort. Interestingly, the model predicted that both vaccines would have a broadly similar impact on the burden of morbidity and mortality from ID and pneumonia in both countries. This can be explained by the vaccine formulations: while PHiD-CV includes three fewer pneumococcal serotypes than PCV-13, it has demonstrated cross-reactivity against the invasive serotypes 6A and 19A, and has the potential to target NTHi. This attribute was predicted to balance the prevention of overall ID when the combined disease burden from the two pathogens was considered.
In contrast to ID and pneumonia, the model predicted a substantial difference in the number of AOM outcomes prevented under the two vaccination regimens in both countries. The efficacy of PHiD-CV against AOM was based on a study by Prymula et al.Citation19, which compared a predecessor vaccine to PHiD-CV with a control vaccine. In this study, pneumococcal vaccine efficacy was 33.6% against all clinical episodes of AOM (p < 0.001), 51.5% against culture-confirmed pneumococcal episodes (p < 0.001), and 35.6% against episodes caused by H. influenzae including NTHi (p = 0.032)Citation19. However, there was no clear correlation between efficacy and enzyme-linked immunosorbent assay (ELISA) carrier protein D antibody concentrations. Therefore, it is possible that the vaccine effect on H. influenzae AOM could be an indirect effect, i.e., reducing the number of episodes of pneumococcal AOM could have reduced the number of infants subsequently vulnerable to H. influenzae infectionCitation19. However, this seems unlikely, as PCV-7 has been found to result in an increase in H. influenzae AOM episodes, rather than a decreaseCitation59.
In the UK, PCV-13 was predicted to result in ∼20% more myringotomies and 4% more AOM-related visits to GPs than an equivalent PHiD-CV regimen. In Canada, these proportional differences were twice as substantial, with ∼46% more myringotomies and 7% more PCP visits occurring under a PCV-13 regimen compared with PHiD-CV. Considering the marginal improvements in ID and CAP estimated under the PCV-13 regimen, it was this substantial difference in AOM disease burden that contributed to the greater number of QALYs predicted to be saved with PHiD-CV. Furthermore, since this analysis only considered sequelae for meningitis, and not for AOM, the reported QALYs did not include the considerable disutility accrued during long-term AOM-related sequelae.
In Canada and the UK, the estimated direct cost of AOM-related disease was much less than that for pneumonia. However, the model predicts that the impact of PHiD-CV on the costs of AOM will far outweigh that of PCV-13 on pneumonia-related costs. Considering the model parameters, this proportionally greater saving in AOM costs (compared with pneumonia costs) can be attributed to the large volume of AOM cases prevented by vaccination, rather than high individual treatment costs. By the age of 3 years, ∼75% of children are expected to have had at least one episode of AOMCitation76, and it is therefore not surprising that any intervention that targets this highly prevalent disease is likely to produce a considerable reduction in economic burden and improvement in quality-of-life. Moreover, sub-optimal management of AOM may result in high levels of antibiotic consumption that could lead to increases in resistance which may lead, in turn, to higher disease management costs and decreased quality-of-life.
The predictions of the model are supported by both univariate and multivariate sensitivity analyses that demonstrate its robustness. While permutations to the majority of the model’s parameters had very little impact on its calculation of cost-effectiveness, those related to AOM (e.g., the percentage reduction in myringotomy expected with PCV-13 and the number of GP/PCP visits for AOM) were found to be particularly influential in determining the model’s final outcomes. However, since the difference in predicted cost-effectiveness between PCV-13 and PHiD-CV is predominantly derived from the differential impact of the two vaccines on AOM, it is difficult to determine whether the prominent influence of these parameters is attributed to the structure of the model or to the crucial importance of this disease area to the comparison being undertaken.
One of the challenges of modelling diseases caused by NTHi is identifying reliable surveillance data. In many countries, the lack of routine national surveillance for H. influenzae has limited previous attempts to quantify its pathogenic potential and to document shifts in its prevalence or resistance profile. The need for surveillance of this organism has been recognized by organizations such as EU-IBIS, which previously collected data on H. influenzae infection from 24 European countries, Australia and IsraelCitation12; a responsibility that has since passed to the European Centre for Disease Prevention and Control. One limitation of these surveillance data is that they are not stratified by immune status, and those who are immunocompromised may not respond well to vaccination. An additional concern is that epidemiological reports may under-estimate the prevalence of NTHi as a cause of diseaseCitation77, as H. influenzae may not be routinely distinguished from Haemophilus haemolyticus using standard methodsCitation78. This highlights the need for improved surveillance of H. influenzae, which would be valuable for future health economic models of NTHi disease.
Although NTHi is a recognized cause of pneumonia, the extent of its role remains unclear, as the typing of H. influenzae strains is not routine. It has previously been suggested that NTHi pneumonia may be under-detected as a cause of bacterial pneumoniaCitation79. However, there is considerable variation among estimates of NTHi pneumonia incidence reported in the literature. For example, results of lung tap studies in children from around the world have identified H. influenzae in ∼2–42% of aspiratesCitation6. Although PHiD-CV may have the potential to provide protection against disease caused by NTHi, pneumonia caused by this pathogen was not included in the present analysis due to the uncertainty surrounding its prevalence. However, in a separate analysis, we considered a scenario where the incidence of pneumonia hospitalizations due to NTHi was assumed to be at the lower end of the range of estimates reported in the literatureCitation6,Citation80–82. Accordingly, the incidence of NTHi pneumonia was estimated as approximately 3% of all-cause pneumonia incidence (which excludes NTHi) in children <10 years old. The potential efficacy of PHiD-CV against NTHi pneumonia was also considered, based on the efficacy of the PHiD-CV precursor vaccine against AOM caused by H. influenzae (including NTHi) as reported in the POET trial (35.6%)Citation19. Including the effects of NTHi pneumonia and the corresponding potential efficacy of PHiD-CV (in a 3 + 1 regimen) resulted in additional savings of three and four discounted QALYs and £240,000 and Canadian $590,000 in discounted costs in the UK and Canada, respectively, compared to estimates where NTHi pneumonia was not considered.
The limitations of cohort models, such as that presented here, are most apparent in their estimation of how indirect vaccine effects develop over time. More specifically, cohort analyses require the long-term prediction of changes in disease epidemiology that extend over the cohort’s lifetime. In real life, estimations of herd effect are obtained as static observations, thus making the abstraction of their temporal development a difficult process. Many studies have examined the magnitude of indirect effects (i.e., herd protection and serotype replacement), but the extent of these effects has varied considerably amongst published estimates. For instance, in Spain, the net indirect effect on those aged >65 years of PCV administration to infants has been estimated as an ∼23% increase in IDCitation83, while in the US the impact of net indirect vaccine effect has been estimated as a 38% reduction in disease amongst the same age groupCitation84. It is clear that there is a substantial decrease in vaccine serotypes in all cases, but changes in non-vaccine serotypes, whether due to replacement or secular trends, appear to vary (geographically and temporally), contributing to the observed differences in net impact. Many different factors influence the end result, for example vaccine and non-vaccine serotype coverage, duration of national immunization, epidemiology, vaccine schedule, immunization coverage, force of infection, time of measuring the effect, rate and level of serotype replacement, vaccine dosing, contact matrices, and demography. Moreover, the replacement of existing vaccines with new alternatives will make estimates of the net indirect effect per vaccine serotype even more difficult to disentangle; hence, estimating precise and independent contributions may be nearly impossibleCitation85.
Another limitation of this study is the input data for the model. There is limited clinical trial data for PHiD-CV and PCV-13 (and no head-to-head study) as most of the trials aimed to demonstrate immunogenicity, safety, and tolerability of these vaccines, and clinical effectiveness is inferred from these immunological data, based on earlier vaccine formulations. Therefore, vaccine efficacy data are largely based on studies of PCV-7Citation48,Citation52,Citation59 and a study on a predecessor vaccine to PHiD-CVCitation19. It is interesting to note that the efficacy against AOM of PCV-7 in FinOMCitation59 was much lower than that of the precursor to PHiD-CV in POETCitation19 (7% vs 34%). While these trials are not directly comparable, PHiD-CV would be expected to have a greater effect on AOM due to its activity against NTHi. In the POET trial, no non-vaccine type serotype replacement was observed with the PHiD-CV precursor; whereas this was observed for PCV-7 in FinOMCitation86. There were also different proportions of bacterial pathogens in the two studies. Palmu et al.Citation86, former investigators of FinOM, have assessed the impact of variations in case definition, design, and local epidemiology between the two trials, and concluded that these factors only had a slight impact on the estimated efficacy of the vaccines.
The GP/PCP consultation rates for AOM used in the study are ∼10-fold higher for CanadaCitation28,Citation43 than the UKCitation46. This may be due to a variety of factors, e.g., different ICD-9 codes, different age ranges included, and a lower rate of GP consultation for AOM in the UK. However, the key comparisons here are those between the two vaccines within either Canada or the UK, and not a between-country comparison. Therefore, these differences should not affect the results within each country.
A further limitation is the uncertainty surrounding the disutility value used for AOM. We used the same AOM disutility value as Melegaro and EdumundsCitation42, namely 0.005. This came from a study by Oh et al.Citation67, who derived it from physician responses to scenarios of AOM with combinations of adverse events. This value has been used in most economic evaluations of pneumococcal vaccinesCitation87–91. However, some other analyses have used a higher AOM disutility value of 0.011Citation92,Citation93, based on a study by Prosser et al.Citation94. Performing a sensitivity analysis using the estimate of 0.011 QALY decrement resulted in an increase in the difference of QALYs gained for PHiD-CV vs PCV-13 to 1063 for Canada and 828 for the UK.
Conclusion
Direct comparison of the antigenic content of PCV-13 and PHiD-CV cannot be used to differentiate the potential benefits of the two vaccines: while PCV-13 contains antigens for an additional three S. pneumoniae serotypes, PHiD-CV has the potential to provide protection against disease caused by NTHi. Computational models are therefore the only option available for those wishing to compare the predicted impact of the two vaccines. The heavy burden of pneumonia (in terms of mortality and costly medical interventions) and AOM (in terms of high prevalence of disease) suggest that decision-makers should also take the impact of vaccination on these diseases into account when choosing a pneumococcal conjugate vaccine for routine infant immunization. While the two vaccines are predicted to provide broadly comparable impacts on overall ID and CAP under base-case conditions, PHiD-CV is expected to provide a substantially greater reduction in clinical AOM compared with PCV-13. As such, the cost-effectiveness analysis reported here suggests that in Canada and the UK, PHiD-CV’s potential to protect against NTHi may provide a greater impact on overall disease burden than the additional serotypes contained in PCV-13.
Transparency
Declaration of funding
GlaxoSmithKline Biologicals was the funding source and was involved in all stages of the study and analysis. GlaxoSmithKline Biologicals also took responsibility for all costs associated with the development and publishing of the present manuscript. Gerhart Knerer was involved with the design of the model and validation of assumptions. David W Pearce and Afisi Ismaila were responsible for adapting the model to the UK and Canada.
Declaration of financial/other relationships
All authors are employees of GlaxoSmithKline Biologicals.
Supplementary Material
Download PDF (40 KB)Acknowledgements
Editorial assistance was provided by Ben Holtom (Fishawack Communications Ltd.) and Jenny Lloyd during the preparation of this manuscript. Abdelilah Ibrahimi (XPE Pharma and Science for GlaxoSmithKline Biologicals), Elhem Sbaa, Paul Kenny, Oleksandr Topachevskyi, and Julie Roiz of GlaxoSmithKline Biologicals provided assistance with manuscript coordination and editing and technical support. Financial support was provided by GlaxoSmithKline. The authors thank Laure-Anne Van-Bellinghen at Deloitte for help with the development of the model. The authors are grateful to the experts who attended the series of GlaxoSmithKline PHiD-CV Health Economics roundtable meetings (Dublin, October 2007; Montreal, March 2008; and Reykjavik, June 2008) that helped to inform the assumptions used during the course of the GlaxoSmithKline Pneumococcal Health Economic model-design process.
References
- Lynch 3rd JP, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 2009;30:189-209
- Erwin AL, Smith AL. Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol 2007;15:355-62
- Sarangi J, Cartwright K, Stuart J, et al. Invasive Haemophilus influenzae disease in adults. Epidemiol Infect 2000;124:441-7
- Shapiro ED, Ward JI. The epidemiology and prevention of disease caused by Haemophilus influenzae type b. Epidemiol Rev 1991;13:113-42
- Dworkin MS, Park L, Borchardt SM. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons > or = 65 years old. Clin Infect Dis 2007;44:810-6
- Vuori-Holopainen E, Peltola H. Reappraisal of lung tap: review of an old method for better etiologic diagnosis of childhood pneumonia. Clin Infect Dis 2001;32:715-26
- Murphy TF. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr Opin Infect Dis 2003;16:129-34
- Erwin AL, Nelson KL, Mhlanga-Mutangadura T, et al. Characterization of genetic and phenotypic diversity of invasive nontypeable Haemophilus influenzae. Infect Immun 2005;73:5853-63
- Heath PT, Booy R, Azzopardi HJ, et al. Non-type b Haemophilus influenzae disease: clinical and epidemiologic characteristics in the Haemophilus influenzae type b vaccine era. Pediatr Infect Dis J 2001;20:300-5
- Ladhani S, Slack MP, Heath PT, et al. Invasive Haemophilus influenzae disease, Europe, 1996–2006. Emerg Infect Dis 2010;16:455-63
- Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 2000;13:302-17
- European Union Invasive Bacterial Infections Surveillance (EU-IBIS) Network. Invasive Haemophilus influenzae in Europe 2006
- Perdue DG, Bulkow LR, Gellin BG, et al. Invasive Haemophilus influenzae disease in Alaskan residents aged 10 years and older before and after infant vaccination programs. JAMA 2000;283:3089-94
- Bekri H, Cohen R, Varon E, et al. Streptococcus pneumoniae serotypes involved in children with pleural empyemas in France. Arch Pediatr 2007;14:239-43
- Byington CL, Korgenski K, Daly J, et al. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J 2006;25:250-4
- Fletcher M, Leeming J, Cartwright K, et al. Childhood empyema: limited potential impact of 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2006;25:559-60
- Hausdorff WP, Bryant J, Paradiso PR, et al. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000;30:100-21
- PneumoADIP. Pneumococcal regional serotype distribution for Pneumococcal AMC TPP
- Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006;367:740-8
- Dinleyici EC, Yargic ZA. Current knowledge regarding the investigational 13-valent pneumococcal conjugate vaccine. Expert Rev Vaccines 2009;8:977-86
- Beutels P, Thiry N, Van Damme P. Convincing or confusing? Economic evaluations of childhood pneumococcal conjugate vaccination–a review (2002–2006). Vaccine 2007;25:1355-67
- De Wals P, Erickson L, Poirier B, et al. How to compare the efficacy of conjugate vaccines to prevent acute otitis media? Vaccine 2009;27:2877-83
- Statistics Canada. Available at: http://www.statcan.gc.ca. Accessed October 2008
- UK National Statistics. Population. Available at: http://www.statistics.gov.uk/hub/population/index.html. Accessed September 2009
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322-38
- Beck JR, Pauker SG, Gottlieb JE, et al. A convenient approximation of life expectancy (the "DEALE"). II. Use in medical decision-making. Am J Med 1982;73:889-97
- Bettinger JA, Scheifele DW, Halperin SA, et al. Invasive pneumococcal infections in Canadian children, 1998–2003: implications for new vaccination programs. Can J Public Health 2007;98:111-5
- Talbird SE, Taylor TN, Knoll S, et al. Outcomes and costs associated with PHiD-CV, a new protein D conjugate pneumococcal vaccine, in four countries. Vaccine 2010;28(6 Suppl):G23-9
- Health Protection Agency. Pneumococcal serotype distribution for samples referred for serotyping epidemiological years (July–June): 2000/1 – 2005/6. Available at: http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733824984?p=1203409671918. Accessed June 2009
- Denham BC, Clarke SC. Serotype incidence and antibiotic susceptibility of Streptococcus pneumoniae causing invasive disease in Scotland, 1999–2002. J Med Microbiol 2005;54:327-31
- Statistics Canada. Complete life table, Canada, 2000–2002. Table 2a males, Table 2b females. Catalogue No. 84-537-XIE. Available at: http://www.statcan.ca/english/freepub/84-537-XIE/tables.htm. [Last accessed September 2008]
- NHS. Routine childhood immunisations from Spring 2010. Available at: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_120269.pdf. Accessed November 2010
- Public Health Agency of Canada. Publicly funded immunization programs in Canada - Routine schedule for infants and children (including special programs and catch-up programs) 2010-09-23. Available at: http://www.phac-aspc.gc.ca/im/ptimprog-progimpt/table-1-eng.php. Accessed March 2011
- Spratt JS Jr. The relation of 'human capital' preservation to health costs. Am J Econ Sociol 1975;34:295-307
- Lipscomb J, Weinstein M, Torrance G. Time preference. In: Gold M, Siegel J, Russel L, et al, eds. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996. p 214-47
- National Institute for Clinical Excellence. Guide to the methods of technology appraisal. Issue date: June 2008. Available at: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf. Accessed November 2010
- Poirier B, De Wals P, Petit G, et al. Cost-effectiveness of a 3-dose pneumococcal conjugate vaccine program in the province of Quebec, Canada. Vaccine 2009;27:7105-9
- Morrow A, De Wals P, Petit G, et al. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol 2007;18:121-7
- Health Protection Agency. Number of laboratory confirmed invasive pneumococcal disease cases (including meningitis) in England and Wales, 1996–2005. Available at: http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733751944?p=1203409671918. Accessed April 2008
- Johnson AP, Waight P, Andrews N, et al. Morbidity and mortality of pneumococcal meningitis and serotypes of causative strains prior to introduction of the 7-valent conjugant pneumococcal vaccine in England. J Infect 2007;55:394-9
- Jette LP, Lamothe F. Surveillance of invasive Streptococcus pneumonia infection in Quebec, Canada, from 1984 to 1986: serotype distribution, antimicrobial susceptibility, and clinical characteristics. J Clin Microbiol 1989;27:1-5
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine 2004;22:4203-14
- Petit G, De Wals P, Law B, et al. Epidemiological and economic burden of pneumococcal diseases in Canadian children. Can J Infect Dis 2003;14:215-20
- Office for National Statistics. Time series data: Mid-2006 population estimates: United Kingdom; estimated resident population by single year of age and sex
- Hospital Episode Statistics (HES). The NHS Information Centre for Health and Social Care. Primary diagnosis: 4 character. Available at: http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=214. [Last accessed September 2008]
- Williamson I, Benge S, Mullee M, et al. Consultations for middle ear disease, antibiotic prescribing and risk factors for reattendance: a case-linked cohort study. Br J Gen Pract 2006;56:170-5
- De Wals P, Black S, Borrow R, et al. Modeling the impact of a new vaccine on pneumococcal and nontypable Haemophilus influenzae diseases: a new simulation model. Clin Ther 2009;31:2152-69
- Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006;368:1495-502
- Poolman J, Kriz P, Feron C, et al. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 2009;27:3213-22
- Netherlands Reference Laboratory for Bacterial Meningitis (AMC/RIVM). Bacterial meningitis in the Netherlands; annual report 2007. Amsterdam: University of Amsterdam, 2008
- WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec 2006;81:445-52
- Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002;21:810-5
- Vemer P, de Greeff S, Schouls L, et al. Seven, ten, or thirteen? The cost-utility of infant vaccination with a 7-, 10- or 13-valent pneumococcal conjugate vaccine in the Netherlands. Value Health 2009;12:A228 [abstract VA1]. Abstract available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1524-4733.2009.00592_1.x/pdf. Presentation available at: https://www.ispor.org/awards/12euro/VA1.pdf. [Last accessed August 2011]
- Williamson I, Benge S, Mullee M, et al. Consultations for middle ear disease, antibiotic prescribing and risk factors for reattendance: a case-linked cohort study. Br J Gen Pract 2006;56:170-5
- Thompson PL, Murray ML, Sharland M, et al. Up-to-date findings show change in acute otitis media consultation trend. Br J Gen Pract 2006;56:379-80
- Rovers MM. The burden of otitis media. Vaccine 2008;26(7 Suppl):G2-4
- Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J 2004;23:1142-52
- Hausdorff WP, Yothers G, Dagan R, et al. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr Infect Dis J 2002;21:1008-16
- Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-9
- Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction–eight states, 1998–2005. MMWR Morb Mortal Wkly Rep 2008;57:144-8
- Centers for Disease Control and Prevention (CDC). Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998–2003. MMWR Morb Mortal Wkly Rep 2005;54:893-7
- Bermal N, Szenborn L, Chrobot A, et al. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr Infect Dis J 2009;28(4 Suppl):S89-96
- Vesikari T, Wysocki J, Chevallier B, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009;28(4 Suppl):S66-76
- Wysocki J, Tejedor JC, Grunert D, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J 2009;28(4 Suppl):S77-88
- Hausdorff WP, Hoet B, Schuerman L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr 2010;10:4
- Bennett JE, Sumner 2nd W, Downs SM, et al. Parents' utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med 2000;154:43-8
- Oh PI, Maerov P, Pritchard D, et al. A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clin Ther 1996;18:160-82
- Prosser LA, Ray GT, O'Brien M, et al. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics 2004;113:283-90
- Rubin JL, McGarry LJ, Strutton DR, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine 2010;28:7634-43
- Curtis L. Unit costs of health & social care 2007. Canterbury: Personal Social Services Research Unit (PSSRU), University of Kent; 2007. Available at: http://www.pssru.ac.uk/pdf/uc/uc2007/uc2007.pdf. Accessed August 2011
- Department of Health. National tariff 2006/07: payment by results. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4127649. Accessed August 2011
- Ontario Ministry of Health and Long Term Care. Schedule of benefits: physician services under the Health Insurance Act (July 1, 2011). Available at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html. [Last accessed August 2011]
- Russell P. Disabled children, their families and child poverty: briefing paper. London: Council for Disabled Children. Available at: http://www.leeds.ac.uk/disability-studies/archiveuk/every%20child/disabled_children_and_child_poverty.pdf. [Last accessed August 2011]
- National Collaborating Centre for Women’s and Children’s Health. Surgical management of otitis media with effusion in children. Clinical Guideline February 2008. Available at: http://www.nice.org.uk/nicemedia/pdf/CG60fullguideline.pdf. [Last accessed August 2011]
- Kinane C, Gupta K. Residential care homes for the mentally ill. Implications for a catchment area service. Psych Bull 2001;25:61-6
- Klein JO. Otitis media. Clin Infect Dis 1994;19:823-33
- Murphy TF, Brauer AL, Schiffmacher AT, et al. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:266-72
- Murphy TF, Brauer AL, Sethi S, et al. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis 2007;195:81-9
- Shann F. Haemophilus influenzae pneumonia: type b or non-type b? Lancet 1999;354:1488-90
- De Schutter I, De Wachter E, Crokaert F, et al. Microbiological results in Belgian children with non-responding or recurrent community acquired lower respiratory tract infection (CA-LRTI). Brussels, Belgium: ESPID, 2009
- Chiang WC, Teoh OH, Chong CY, et al. Epidemiology, clinical characteristics and antimicrobial resistance patterns of community-acquired pneumonia in 1702 hospitalized children in Singapore. Respirology 2007;12:254-61
- Straus WL, Qazi SA, Kundi Z, et al. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus amoxycillin for pneumonia among children in Pakistan: randomised controlled trial. Lancet 1998;352:270-4
- Ardanuy C, Tubau F, Pallares R, et al. Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997–2007. Clin Infect Dis 2009;48:57-64
- Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis 2007;196:1346-54
- Chen I, Knerer G, Hausdorff W. Herd protection following conjugate pneumococcal vaccination: perception and evidence. 3rd Vaccine Global Congress, Singapore, 4–6 October 2009. Abstract P2.42
- Palmu A, Jokinen J, Kilpi T. Impact of different case definitions for acute otitis media on the efficacy estimates of a pneumococcal conjugate vaccine. Vaccine 2008;26:2466-70
- Rozenbaum MH, Sanders EA, van Hoek AJ, et al. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ 2010;340:c2509
- Chuck AW, Jacobs P, Tyrrell G, et al. Pharmacoeconomic evaluation of 10- and 13-valent pneumococcal conjugate vaccines. Vaccine 2010;28:5485-90
- Beutels P, Van Damme P, Oosterhuis-Kafeja F. Effects and costs of pneumococcal conjugate vaccination of Belgian children. Knowledge Centre (KCE); 2006. KCE reports 33C (D/2006/10.273/53). Available at: kce.fgov.be/Download.aspx?ID=633. [Last accessed August 2011]
- Salo H, Sintonen H, Nuorti JP, et al. Economic evaluation of pneumococcal conjugate vaccination in Finland. Scand J Infect Dis 2005;37:821-32
- Beutels P, Blommaert A, Hanquet G, et al. Rapport coût-efficacité des vaccins antipneumococciques conjugués 10-valent et 13-valent chez l’enfant. Health Technology Assessment (HTA). Bruxelles: Centre federal d’expertise des soins de santé (KCE), 2011. Reports 155B. D/2011/10.273/20. Available at: http://kce.fgov.be/index_fr.aspx?SGREF=3228&CREF=19992. Accessed August 2011
- Lieu TA, Ray GT, Black SB, et al. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. JAMA 2000;283:1460-8
- O’Brien MA, Prosser LA, Paradise JL, et al. New vaccines against otitis media: projected benefits and cost-effectiveness. Pediatrics 2009;123:1452-63
- Prosser LA, Ray GT, O'Brien M, et al. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics 2004;113:283-90
