Abstract
Objectives:
A small but significant proportion of patients with peripheral neuropathic pain (NeP) are refractory to the typical treatments applied in clinical practice, including amitriptyline and gabapentin. Thus, they continue to suffer the debilitating effects of NeP. This study aimed to evaluate the cost-effectiveness of pregabalin in comparison to usual care, in patients with refractory NeP, from a third party payer’s perspective (NHS).
Methods:
A stochastic simulation model was constructed, using clinical data from four non-randomized studies, to generate pain pathways of patients receiving usual care and pregabalin. Treatment effect (pain reduction) was converted to quality-of-life (QoL) data, using a regression analysis based on new utility data, collected from a survey of refractory NeP patients presenting to pain clinics in Cardiff, Wales. All relevant direct costs were estimated using resource use from the survey data (where available) and unit costs from the British National Formulary (BNF). The analysis was run over a 5-year time horizon, with costs and benefits discounted at 3.5%.
Study limitations:
The use of non-randomized (observational) data to characterize the effectiveness of treatments for NeP. Exclusion of productivity costs and consequences from the analysis.
Results:
In the base case analysis, an incremental cost-effectiveness ratio (ICER) of £10,803 per quality adjusted life year (QALY) was attained. This result was found to be reasonably insensitive to variations in the key input parameters, with ICERs ranging from £8505 to £22,845 per QALY gained.
Conclusions:
The analysis shows that pregabalin is a cost-effective alternative to usual care in patients with refractory NeP, with an ICER well below the threshold typically adopted by UK health technology assessment groups, such as NICE.
Introduction
Neuropathic pain (NeP) is a debilitating condition that affects a substantial proportion of the UK population. The prevalence of neuropathic pain is difficult to ascertain, but a UK primary care survey estimated that 8% of the UK population experience pain of predominately neuropathic originCitation1. The type of NeP is varied, most commonly associated with diabetes (painful diabetic neuropathy) and following shingles (post-herpetic neuralgia). As a condition, NeP is associated with a marked reduction in patients’ quality-of-life (QoL), losses in economic productivity, co-morbidities such as anxiety and depression, and high direct cost from the considerable use of health service resourcesCitation2. Indeed, a recent study showed that the average number of healthcare-related visits (the figure includes visits to practice nurse, GP and social sevices as well as home visits) in a 6-week period was 1.18 in patients with very severe pain compared to 0.47 in patients with no painCitation3.
Treatments for NeP typically consist of one, or a combination of, anti-depressants, anti-convulsants, weak opioids, strong opioids, NSAIDs, and/or analgesicsCitation4. These treatments can be effective in reducing NeP and the associated burden on healthcare resources and patients’ QoL. Response to these medicines is variable, however, and this is especially true in patients with refractory NePCitation5.
There is no consensus on the definition of refractory NeP. For this study we defined ‘refractory’ patients as those who do not achieve adequate pain relief from, or who are intolerant of, common early therapies including amitriptyline and gabapentin. The prognosis of refractory patients has been poor to date, and pregabalin may be a useful treatment option for these patients.
Pregabalin has a similar mechanism of action to other agents such as gabapentin; binding potently to the alpha2-delta sub-unit of the voltage-dependent calcium channel in the central nervous system. This reduces calcium influx at nerve terminals and therefore reduces the release of neurotransmitters such as glutamate and noradrenalineCitation6. Through these activities, pregabalin exhibits analgesic, anti-convulsant, and anxiolytic effects. However, pregabalin may improve upon current therapies by relieving pain as early as the first week of treatmentCitation6.
To date no studies have examined the value of pregabalin compared to other treatments for refractory NeP. This study sets out to assess the cost-utility of pregabalin in the treatment of patients with refractory NeP, from the perspective of a public healthcare provider. We describe the analytical framework used to assess cost-utility, the characteristics of the patient populations in terms of their clinical and demographic profiles, the results of the economic model and a discussion of the implications for the treatment of refractory NeP.
Methods
A fixed time increment stochastic simulation model was constructed to estimate the cost-utility of pregabalin, compared to usual care, in patients with refractory NeP. The perspective taken was that of the UK National Health Service (NHS).
Model description
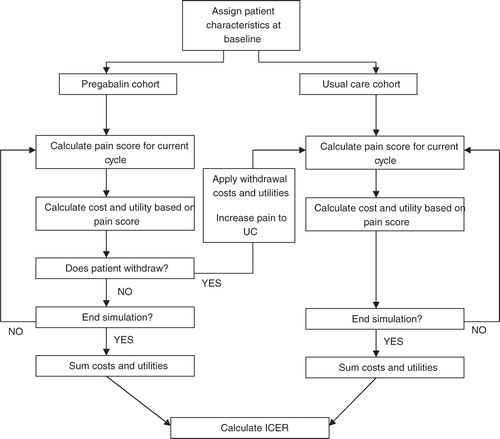
Previous economic evaluations of neuropathic pain have typically employed Markov models, which have defined categorical ‘mild’, ‘moderate’, and ‘severe’ health states according to pain score intervalsCitation7–10. This evaluation has used a fixed (weekly) time increment stochastic simulation model. The cost-utility model was constructed using Microsoft Excel 2007 and Visual Basic for Applications to assess the clinical and economic outcomes relevant to patients in the treatment and comparator cohorts. The decision criterion used to assess cost-utility was the willingness-to-pay (WTP) for a quality-adjusted life year (QALY). The model’s structure is described in the flow diagram in .
The model begins by allocating equivalent baseline pain scores to cohorts of 10,000 patients, receiving pregabalin and usual care. The model first simulates the treatment pathway of those patients in the usual care arm. Patients follow the pain pathway defined by the clinical data describing patients receiving usual care. At each cycle, patients accrue the utility associated with their current pain score and the costs associated with usual care treatment. The length of each cycle was set at 1 week, selected to appropriately reflect the fast-acting nature of pregabalin in the early stages of treatment.
The treatment pathway of patients in the pregabalin arm is then simulated, in a similar fashion. However, unlike the usual care simulation, pregabalin patients can incur an adverse event (AE) or withdraw from treatment for non-AE reasons. AEs and withdrawals were considered only in the pregabalin arm because the evaluation compares pregabalin plus usual care to usual care alone: any AEs and withdrawals occurring as a result of usual care treatment are assumed to be the same in both cohorts and so do not need to be considered in the analysis.
There is a probability at each cycle that a patient can incur an AE, in which case there is a transitory (one cycle length) cost and utility decrement assigned to that patient. There is a conditional probability of the patient withdrawing from treatment, once the event has occurred, in which case they will incur an additional cost and utility decrement of the same magnitude.
There is an additional probability at each cycle that a patient may withdraw from treatment for reasons not associated with AEs, in which case a transitory cost is applied. It is assumed that there is no immediate utility decrement associated with the withdrawal itself, but withdrawing patients do adopt the same pain, utility, and cost profile as patients in the usual care arm. Thus, their utility will be reduced because of the lower utility in this arm. The remainder of the cohort, which do not withdraw from treatment, continue to accumulate the cost and benefit profiles associated with their pain levels over the remainder of the modelled time horizon.
Patient histories are recorded at each cycle and summed over a 5-year time horizon, chosen to reflect a reasonable period over which costs and benefits are likely to differ between the comparator and treatment arms. It is assumed that there is no mortality in either cohort, which seems reasonable since there is unlikely to be mortality differences between the treatments and the modelled time horizon is short.
The difference between the simulated outcomes of the treatment and comparator arms is used to estimate the expected difference in costs and QALYs gained. Costs and benefits were discounted at 3.5% annuallyCitation11.
Individual patient-level data were not available and so the variability of baseline pain scores could not be modelled: a mean baseline pain score was used for each patient in the treatment and usual care cohorts, respectively. All pregabalin patients follow exactly the same pain ‘trajectory’ over the lifetime of the model; inter-patient variability exists because of the (random) probability that patients will experience adverse events and/or withdraw from therapy. In the same way, all patients in the ‘usual care’ cohort follow the same pain trajectory over the model’s lifetime; there is no inter-patient variability for this cohort as patients are assumed not to withdraw from therapy.
Interventions
The analysis compared the use of pregabalin combined with usual care (treatment) to usual care alone (comparator). Pregabalin doses of 150–600 mg per day were considered in the analysis, reflecting the licensed indication for treating NeP patients of mixed aetiologyCitation12. Usual care was defined as treatment with one or more weak opioids, strong opioids, NSAIDs, analgesics, and is reflective of a variety of medications typically used by patients with treatment-refractory NePCitation13.
The clinical goal of treatment of NeP syndromes is a reduction in the pain experienced, which is usually expressed by patient valuations on a pain scale (typically a 0–10 point scale, where 0 represents no pain and 10 represent the maximum possible pain). A treatment effect is seen as a reduction in pain score, which is associated with an improvement in patient QoL.
Data sources
Randomized controlled trials (RCTs) may provide unbiased estimates of treatment effects and are often considered the ‘gold standard’ of clinical data. On this basis we first reviewed RCTs that included pregabalin as a treatment option. Upon review, the majority of pregabalin RCTs were found to include a mixed treatment-naïve and refractory patient population; many had only a placebo comparator, and some excluded patients who had previously been treated with gabapentin. Crucially, none of the pregabalin RCT data were specific to refractory patients, and so they could not be used as input data for this model of treatment-refractory NeP.
Consequently, this economic evaluation is based on published studies that most closely fulfil the requirements of the economic model, namely to evaluate a population of patients with refractory NeP treated with pregabalin and usual care compared to usual care alone. Keyword searches using ‘pregabalin’ and ‘refractory’ were carried out using PubMed and Google between January and March 2008 to identify full publications and conference abstracts of studies assessing the use of pregabalin in patients who were refractory to previous pain medications. Six prospective, non-randomized studies were identified following review and were considered to be suitable for the purposes of this evaluationCitation14–19. However, only four of the six non-randomized studies provided data on patients’ mean pain scores, and so these four were chosen to provide clinical data describing the pregabalin arm of the economic model ()Citation14–17.
Table 1. Details of clinical studies describing the effectiveness of pregabalin in the management of NeP.
The four non-randomized studies included only treatment-refractory patients (with a variety of NeP syndromes) who had failed on a variety of previous treatmentsCitation14–17. The studies characterized pain using a 0–10 scale, where 0 represented no pain and 10 represented the maximum pain possible. The effect of pregabalin was evaluated in each study by comparing pain levels after pregabalin treatment with baseline pain levels.
The uncontrolled nature of the four studies meant there was no direct treatment comparator, making it difficult to model the treatment pathway of the usual care cohort. However, since the studies did not employ a ‘washout’ period prior to the start of treatment, the baseline pain levels reflect those associated with usual care. In addition, the study design for Stacey et al.Citation14 allowed ‘drug holidays’, in which pregabalin was discontinued while background usual care was maintained. These ‘drug holidays’ allowed the pain profile of the usual care arm to be modelled over time.
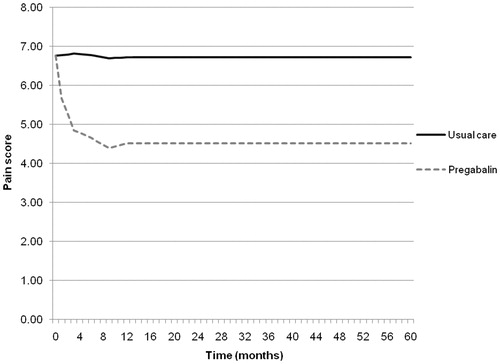
The data from the contributing non-randomized studies are shown in , which shows the pain scores at different time points for the comparator and treatment arm in each of the four studies. It can be seen that there is a clear trend in the initial treatment effect of pregabalin (weeks 0–12) in terms of a reduction in pain, and a general trend thereafter to 15 months, where the end-point of 15 months is dominated by the only longer-term follow-up study of pregabalin, i.e., Stacey et al.Citation14.
Table 2. Pain scores and patient numbers of the four studies providing clinical data input to the model.
In order to generate complete efficacy data inputs, assumptions were made regarding the missing data points in the four studies. Intervening values were estimated by interpolating between the known points in a linear fashion, while data points beyond the scope of the study were estimated using the last observation carried forward (LOCF) method. The estimated pain profiles for each study were then combined by taking the mean pain score at each time point. A combined pain profile was calculated for both the treatment and comparator arm over a 5-year time horizon, as shown in .
Probability of developing adverse effects and withdrawal
Following a review of the four non-randomized refractory studies, Stacey et al.Citation14 was the only long-term study to provide comprehensive data on the total number of treatment-related AEs, withdrawals due to treatment-related AEs, and non-AE related withdrawals (including withdrawal for lack of efficacy, lack of compliance, and other reasons). The rates reported were associated exclusively with pregabalin treatment and covered the entire study period (15 months). To be consistent with the cycle length of the economic model (), the rates were converted to weekly probabilities.
Table 3. Adverse event and withdrawal profile of the treatment cohort.
Utilities
The measure of health benefit used in this economic evaluation is the QALY. Therefore, the clinical benefit of NeP treatment, i.e., a reduction in pain, needs to be expressed as a change in QoL. In order to link pain to QoL, a mapping exercise was undertaken using data collected from a bespoke survey of outpatients (n = 284), with a broad range of refractory and non-refractory NeP conditions, presenting to the Cardiff and Vale Local Health Board (LHB) NHS Trust pain clinics. The utility data collected in this survey were considered appropriate for use in this evaluation as they were both (i) generated in the UK, and (ii) collected from a refractory population. This type of survey is routinely carried out by the Cardiff & Vale LHB and has the necessary ethics approval.
In the survey, patients were asked to characterize the severity of their pain on a scale of 0–100 (where 0 represents no pain at all and 100 represents the greatest pain imaginable) for four different aspects of pain. These scores were averaged and divided by 10 to produce a pain score on the 0–10 scale. Utility was measured in the survey using the five domains of the EQ-5D questionnaire: mobility, self-care, pain/discomfort, usual activities, and anxiety/depression. For each domain there were three possible answers: no problems, moderate problems, or severe problems. Using standard UK time-trade- off (TTO) EQ-5D tariffs, patient responses were mapped to an overall utility score ranging from 0 (worst health scenario) to 1 (best health scenario)Citation20. Scores less than 0 are possible and essentially reflect a state worse than dead.
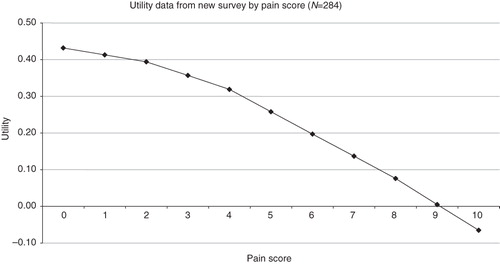
We use a generalized linear modelling framework where we fit a multiple linear regression equation to the data, in which the utility values formed the dependent variable and pain scores (conditioned into groups; ‘1–4’, ‘4–8’, ‘8–10’) were used to explain changes in utility, as well as any significant demographic variables (such as age). Mean EQ-5D scores for each pain score for the whole survey cohort were predicted, as well as a baseline utility for the cohort. A general-to-specific selection methodology was employed with non-significant covariates measured from the survey excluded at the 5% level of significance. All interaction effects between the pain categories and other independent variables were found to be insignificant. A ‘refractory’ patient was included in the regression, defined as having a duration of NeP ≥ 5 years, and a number of other pain medications ≥2. The EQ-5D data for each pain score, including the baseline score (i.e., no pain), was then adjusted by this refractory coefficient. The utility equation for the Cardiff pain survey is given by equation (1):
Using this equation, if pain is 0 (the reference case) the mean utility of patients in the new survey is 0.432 (0.53343 – 0.0999 − 0.00150). Movements from baseline mean utility are determined by changes in pain scores over time. As pain scores increase or decrease, the utility decrement/increment applied is described by the coefficient estimate in the equation. The ‘adjusted’ refractory data applied in the model base case are shown in , in which an inverse relationship between pain and utility can be clearly observed.
Referenced utility decrements for the modelled AEs could not be identified from the published literature. Thus, it has been assumed that the mean utility decrement associated with an AE is 0.06 in the base case analysis, which is approximately equivalent to the upper limit of the utility decrements observed in movements between pain states. This assumption is tested in the sensitivity analysis. Furthermore, the period over which a patient will experience a disutility due to an AE is uncertain: additional sensitivity analysis explored the effect of this parameter uncertainty on the modelled output.
Resources utilization and cost
The model considers the full direct costs of treatment in both the pregabalin and usual care arm, including drug acquisition costs (pregabalin and other medications), as well as NHS and Personal Social Services resources. Costs are applied weekly to be consistent with the cycle length of the model.
Resource usage was sourced from the survey of NeP patients attending the Cardiff and Vale NHS Trust pain clinic described earlier. Patients were asked a series of questions related to their NHS resource use, including GP visits, hospital referrals, etc., and their use of pain medications (pregabalin, gabapentin, paracetamol, etc.). For further details of the resource section of the survey, see Technical Appendix 1.
Survey data from patients with refractory neuropathic pain only (n = 144) were included in the resource use analysis. Two patient populations within the refractory cohort were considered:
Users of pregabalin and refractory patients (n = 34): Treatment population; and
Non-users of pregabalin and refractory patients (n = 110): Comparator population.
Within these two refractory populations (treatment and comparator), the mean quantity of resources used on a weekly basis was assessed. The results of the cost analyses are summarized as quantities of resources used ().
Table 4. Quantities and costs of treatment and treatment-related resources (weekly cost and quantities) in refractory patients.
The survey did not ask patients to specify the daily dose of pregabalin used, so the mean cost of twice daily (BID) and three times daily (TID) dosing was used, as only these two dose regimens are specified in the pregabalin Summary of Product Characteristics (SPC)Citation12. The daily cost of BID and TID dosing (£2.30 and £3.45, respectively) were obtained from the British National Formulary (BNF) and were combined to generate a mean daily cost for BID/TID dosing of £2.88Citation2. The weekly cost of pregabalin for BID, TID, and mean BID/TID dosing is therefore £16.10, £24.15, and £20.13, respectively. The mean cost (£20.13) is applied in the base case, with the impact of upper and lower costs explored in the sensitivity analysis.
The costs of other pain medications were based on an analysis of the average use of other pain drugs by refractory patients, as reported by patients in the Cardiff survey. The type and quantity of other pain drugs used by the treatment and comparator cohorts of the survey were very similar, so a mean cost for a ‘basket’ of other medications was calculated and used as cost inputs for both treatment and comparator arms of the model. A mean weekly cost was calculated by identifying the unit costs of each of the drug categories collected in the survey from the BNF and from the Prescription Pricing Authority scheduleCitation24,Citation25. In the base case, the cost of other medications was assumed to be the mid-point between the minimum and maximum daily drug costs based on licensed indications (£10.56)Citation2. The specific drugs collected in the survey, and their costing, are summarized in . Patients were asked only about the name of their medicine, not how much they were taking each day. As a result, there is some uncertainty in the cost of other medications, the impact of which is explored in sensitivity analysis.
Table 5. Pricing of other pain medication usage.
It is assumed that the cost associated with AEs, AE dropouts, and withdrawals is equivalent to the cost of a standard GP consultationCitation21. However, this is uncertain as the cost of managing treatment-related AEs is unknown. In addition, the cost of medicines to treat AEs may vary widely and is difficult to meaningfully estimate. As such, this assumption is tested in the sensitivity analysis.
Sensitivity analyses
A range of deterministic sensitivity analyses were undertaken to assess the structural and parameter uncertainties surrounding the model. Sensitivity of the modelled output was tested by varying the key input parameters in the manner described below.
Sensitivity analysis 1: Utility decrements of changes in pain score. The effect of implementing alternative utility sources in the model was explored using a Belgian cost-utility study, in which utility data were gathered from NeP patients using the SF-6D, with no adjustment for refractory statusCitation13.
Sensitivity analysis 2: Cost of comparator (usual care). Alternative usual care costs were explored: £0, the minimum cost (£5.33), and the maximum (£15.79) mean weekly cost of other pain drugs.
Sensitivity analysis 3: Cost of treatment (pregabalin). The cost of pregabalin is explored using the minimum licensed level of £16.10 per week (twice daily, BID) and the maximum level of £24.15 per week (three times daily, TID).
Sensitivity analysis 4: Frequency of adverse events (pregabalin arm only). The uncertainty in the AE data (probability of occurrence, and probability of withdrawal once AE is experienced) was explored by increasing each by 100%.
Sensitivity analysis 5: Utility decrement of adverse events (pregabalin arm only). Since evidence of the size of the AE utility decrement could not be found, it was assumed to vary by ±100%, which approximates the utility decrement associated with a one-unit change in pain score.
Sensitivity analysis 6: Withdrawal rate for non-AE reasons. This sensitivity analysis explored the sensitivity of the ICER to changes in this variable, from 0–4%, in the absence of any data to inform the uncertainty surrounding this parameter.
Sensitivity Analysis 7: Cost of AEs. This sensitivity analysis explores increasing the AE cost by 100% to account for the uncertainty in the base case costing assumption.
Sensitivity Analysis 8: Period over which AEs have an impact. To test the hypothesis that the effect of AEs could last for up to 1 month, the cost and utility decrement associated with AEs has been multiplied by four. This methodology was applied to approximate a structural change to the model. Since AEs apply only to the treatment arm, this analysis can be considered conservative—i.e., would be expected to over-estimate the ICER of pregabalin compared to usual care.
Sensitivity Analysis 9: Time horizon of model. The implication of modelling a time horizon from 1–4 years is explored.
Results
Base-case analysis
shows the result of the base case analysis, in terms of the simulated pain profiles of the treatment and comparator arm over the 5-year time horizon. A clear reduction in pain is observed in those patients receiving pregabalin treatment in comparison to usual care. However, following the initial reduction in pain, there is a gradual increase in the treatment pain profile towards the comparator arm. This is a result of patients withdrawing from pregabalin treatment (because of an AE or non-AE reason) and switching to the usual care cohort. As a result, over time, the average modelled pain profile of the treatment arm tends towards the comparator arm.
Figure 4. Simulated pain profiles of the treatment and comparator cohort over the model time horizon.
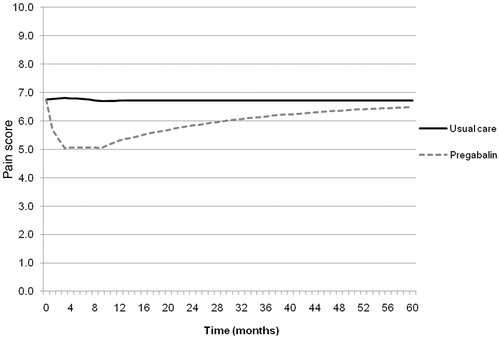
The model showed that the reduction in pain associated with pregabalin treatment, in comparison to usual care, resulted in an incremental utility gain of 0.25 (0.43 vs 0.68) at an additional cost of £2748 (£15,624 vs £18,372) per individual. The incremental cost-utility ratio (ICER) was £10,803 per QALY-gained ().
Table 6. Cost-effectiveness results (base case analysis).
Sensitivity analyses
The cost-utility results of the sensitivity analyses are reported in . It can be seen that the model was most sensitive to the use of alternative utility data inputs with an ICER value of £22,116 per QALY. The model was found to be relatively insensitive to changes in all other parameters, with ICERs ranging from £8505–£12,646 per QALY. Overall, the ICERs ranged from £8505–£22,116 per QALY-gained, across all sensitivity analyses.
Table 7. Cost-effectiveness results (deterministic sensitivity analyses).
Discussion
This study sought to determine the cost-utility of pregabalin in comparison to usual care for treating patients with refractory NeP. To the authors’ knowledge, this is the first study that attempts to estimate cost-utility in a refractory population. Previous studies have considered the use of pain relief medications in non-refractory populations or at a different point in the therapy line.
The base case analysis showed that pregabalin is a cost-effective addition to usual care in refractory patients with NeP. The sensitivity analyses showed that the ICER remained relatively stable to considerable variation in most of the model’s input parameters, including cost of usual care, cost and utility decrement of adverse events, and the modelled time horizon. However, the model was found to be more sensitive to the use of alternative utility data inputs. Nevertheless, overall, pregabalin was associated with an ICER that remained below £23,000 per QALY in all analyses.
The results of this study are consistent with the limited data available from other cost-utility studies. For example, a cost-utility study of NeP comparing pregabalin to usual care reported pregabalin was more effective and less expensive (dominant), irrespective of pregabalin doseCitation7. However, the Annemans et al.Citation7 study was based upon clinical efficacy data which was not specific to a refractory populationCitation26. The present evaluation considers an evidence base which is more relevant to a refractory population and applies an alternative modelling approach.
The authors consider that the results of the bespoke NeP survey represent a valuable addition to the evidence base in refractory NeP. The ‘real-world’ clinical practice data gathered on QoL associated with refractory NeP, pain characterization, resource use, and demographics have been essential to this evaluation. These data alone may be useful to clinicians or in future evaluations.
There are several limitations to the study. First, the use of non-randomized data to characterize pain limits the internal validity of the clinical inputs in assessing the effects of pregabalin above and beyond the effects of usual care. However, this study set out to evaluate the cost-utility of pregabalin compared to usual treatments in a refractory population, which necessarily limited the available evidence. Furthermore, the use of real-world observational studies improves the generalizability of the model to the treatment observed in pain clinics.
Second, as with all model-based economic evaluations, several simplifying assumptions have been made regarding the natural history of NeP. Where assumptions have been made these have been chosen to reflect the salient features of NeP and important patient outcomes.
Third, other benefits of treatment of refractory NeP, such as productivity improvements, have not been considered, given the perspective of this evaluation. Consideration of improvements in labour productivity with pregabalin through a relative reduction in pain, compared to other available treatments, may be expected to improve the cost-utility of pregabalin.
This study has highlighted weaknesses in the NeP outcomes evidence base, and there is a clear need for additional research. In particular, comparative evidence of pregabalin’s benefit over other treatments in a refractory population would be of great value. Furthermore, given the chronic nature of NeP, there is a need for studies to assess the long-term retention rates and clinical benefit in patients prescribed pregabalin.
Conclusion
This is the first study to estimate the cost-utility of pregabalin compared to usual care in a refractory treatment setting. The results from this evaluation suggest that pregabalin represents a cost-effective use of healthcare resources at conventional UK norms of societal willingness-to-pay. We conclude that the clinical and cost-utility of pregabalin has been further supported by the collection of new evidence from actual clinical practice that summarizes the characteristics and health outcomes of people suffering from refractory NeP. Further studies are needed to strengthen estimates of the relative clinical and cost-utility of treatments for refractory NeP.
Transparency
Declaration of funding
This study was funded by Pfizer Ltd, Walton Oaks, UK.
Declaration of financial/other relationships
Zahava Gabriel is an employee at Pfizer Ltd. Anthony Tetlow is an employee at Cardiff Research Consortium. Jason Gordon, Phil McEwan and Matthew Prettyjohns were employees at Cardiff Research Consortium when this cost-utility study was carried out. Steven Lister was a former employee at Cardiff Research Consortium and Pfizer Ltd.
Acknowledgements
No assistance in the preparation of this article is to be declared. Preliminary abstract presented at the 2009 British Pain Society Meeting.
References
- Torrance N, Smith BH, Bennett MI, et al. The epidemiology of chronic pain of predominately neuropathic origin. Results from a general population survey. J Pain 2006;7:281-9
- Jensen M, Chodroff M, Dworkin R. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 2007;68:1178-82
- Lister S, Gabriel Z, McEwan P, et al. Linking UK patients’ self-reported pain with healthcare resource use and quality of life. Presented at the British Pain Society Annual Scientific Meeting, Manchester, April 2010
- Dworkin RH, Corbin AE, Young JP, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo controlled trial. Neurology 2003;60:1274-83
- Freynhagen R, Strojek K, Griesing T, et al. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005;115:254-63
- Rosenstock J, Tuchman M, LaMoreaux L, et al. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004;110:628-38
- Annemans L, Caekelbergh K, Morlion B, et al. A cost-utility analysis of pregabalin in the management of peripheral neuropathic pain. Acta Clin Belg 2008;63:170-8
- Rodriguez MJ, Diaz S, Vera-Llonch M, et al. Cost-effectiveness analysis of pregabalin versus gabapentin in the management of neuropathic pain due to diabetic polyneuropathy or post-herpetic neuralgia. Curr Med Res Opin 2007;23:2585-96
- Tarride JE, Gordon A, Vera-Llonch M, et al. Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective. Clin Ther 2006;28:1922-34
- Smith KJ, Roberts MS. Sequential medication strategies for postherpetic neuralgia: a cost-effectiveness analysis. J Pain 2007;8:396-404
- National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal (reference N0515). 2004. http://www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guide_to_the_methods_of_technology_appraisal_reference_n0515.jsp. Accessed 30 June 2011
- Lyrica Capsules - Summary of Product Characteristics (SPC) - electronic Medicines Compendium (eMC). Datapharm Communications Ltd. http://www.medicines.org.uk/emc/medicine/14651. Accessed 30 June 2011
- Patient UK. Neuropathic pain. http://www.patient.co.uk/health/Neuropathic-Pain.htm. Accessed 30 June 2011
- Stacey BR, Dworkin RH, Murphy K, et al. Pregabalin in the treatment of refractory neuropathic pain: results of a 15-month open-label trial. Pain Med 2008;9:1202-8
- Douglas E, Forbes RD, Smith SC, et al. An audit of pregabalin for refractory neuropathic pain. Poster presented at British Pain Society, Liverpool, April 2007
- Allen S. Pregabalin – is it any better than gabapentin? Poster presented at 2005 World Congress of Pain, Sydney, Australia
- Freynhagen R, Grond S, Schuepfer G, et al. Efficacy and safety of pregabalin in treatment refractory patients with various neuropathic pain entities in clinical routine. Int J Clin Pract 2007;61:1989-96
- Hanu-Cernat, Harrison GR, Blaney LP, et al. Pregabalin, the first cases. Poster presented at 2005 IASP, Sydney, Australia
- Toth C. The utility of pregabalin in neuropathic pain patients – degree of benefit in responders and non-responders to gabapentin. Eur J Pain 2007;11(1 Suppl):S150
- Dolan P. Modeling valuations for EuroQol Health States. Medical Care 1997;35:1095-1108.
- Netten A. & Curtis L. Unit costs of health and social care. Personal Social Services Research Unit (PSSRU) 2004/05. http://www.pssru.ac.uk/uc/uc.htm. Accessed December 18, 2008
- Salisbury C, Hollinghurst S, Montgomery A, et al. The impact of co-located NHS walk-in centres on emergency departments. Emerg Med J 2007;24:265-9
- National audit office. NHS direct in England. http://www.nao.org.uk/publications/nao_reports/01-02/0102505.pdf. 2009. Accessed 7 July 2010
- Joint Formulary Committee. British National Formulary (BNF). 57 edn. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2009
- NHS Prescription Services. Electronic drug tariff. http://www.ppa.org.uk/ppa/edt_intro.htm. 2009. Accessed 7 July 2010
- Van Seventer R, Feister HA, Young JP, et al. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomised trial. Curr Med Res Opin 2006;22:375-84