Abstract
Objective:
Chronic infection with Pseudomonas aeruginosa (PA) is the primary cause of pulmonary deterioration in cystic fibrosis (CF). This study describes healthcare costs and resource utilization among CF patients following PA infection in the US.
Methods:
This retrospective study utilized data from MarketScan claims database. CF patients with an initial PA infection were identified, and their healthcare utilization, medical and pharmacy costs were extracted for 12 months, pre- and post-PA infection. Descriptive and pair-wise non-parametric statistical analyses compared healthcare utilization and costs before and after infection.
Results:
Three hundred and fifty-eight CF patients met study criteria (mean age 20.1 years; 48% female). Mean annual per-patient costs following initial PA infection increased by an estimated $18,516 (outpatient: $3113; inpatient: $10,123; pharmacy: $4943). Overall healthcare costs were significantly higher (p < 0.0001) following PA infection, as were overall inpatient visits, outpatient visits, and unique prescriptions (p < 0.0001).
Conclusions:
PA infection in cystic fibrosis creates a significant economic burden and the cost is not uniformly distributed across the healthcare components.
Limitations:
Key limitations of this study include the absence of clinical parameters to characterize PA infections and data on indirect costs such as loss of productivity or caretaker-related burden.
Introduction
Cystic fibrosis (CF) is a multi-organ disease caused by the mutation of a single gene and characterized by excessive mucus clogging of the organs. Approximately 30,000 people are currently affected by this lethal autosomal recessive genetic disease, which is most common among Caucasians. About one out of every 3500 children born each year in the US has cystic fibrosis genetic mutationCitation1. At present there is no cure for CF, although on-going improvements in patient care have dramatically increased the median predicted survival age from 27 years to almost 36 yearsCitation2.
Although CF is a multi-system disorder, lung disease and pulmonary infection are the leading causes of CF morbidity and mortalityCitation3. Studies have documented the prevalence of various respiratory pathogens in patients with cystic fibrosisCitation4. Pseudomonas aeruginosa (PA) is the most commonly observed bacterial pathogen, infecting ∼52% of the CF populationCitation2,Citation4. The long-term survival of these patients, which depends on effective treatment of the PA infection, is reported to be ∼25% in children aged 2–5 years and ∼80% among adults aged 25–34 years in 2009Citation2. Chronic lung infection with PA is a major cause of pulmonary deterioration in CF patients and is associated with higher risk of mortalityCitation5. With chronic infection, PA transitions to a mucoid phenotype which is less accessible for antibiotics and has a strong ability to mutate. Once the transition to the mucoid phenotype has occurred, eradication of the pulmonary infection is generally no longer possibleCitation6. Chronic infection with PA leads to progressive CF airway disease, pulmonary insufficiency and early mortalityCitation7,Citation8. CF patients infected with PA have poor growth, more rapid deterioration in lung function, reduced quality-of-life, increased hospitalization, and increased need for antibiotic treatmentCitation9,Citation10.
The complications associated with PA infection frequently necessitate additional medical attention for afflicted patients. Although a study conducted previously reported increased economic burden associated with PA infection among a managed care CF population, no data exists on healthcare utilization pattern and key components of care that interpret increased economic burdenCitation11. The initiation of PA infection among CF patients necessitates information on associated costs and resource utilization.
In this study, we attempt to expand our understanding of medical cost drivers within the cystic fibrosis population by describing the PA infection-associated healthcare utilization and costs across the spectrum of care among cystic fibrosis patients in the US managed care population.
Patients and methods
Study design and sample selection
The data for this study were obtained from the MarketScan Commercial Claims and Encounters database for 2005–2008. The MarketScan research databases are proprietary databases maintained by Thomson Reuters from paid medical and prescription drug claims of individuals covered by employer-sponsored health insurance plans that cover active employees, early retirees (virtually all younger than 65 years of age) and their dependents. MarketScan databases capture de-identified, person-specific healthcare data including clinical utilization, expenditures, insurance enrollment, inpatient, outpatient, and prescription information from ∼150 payers.
A retrospective cross-sectional study was designed to assess the impact of PA infection in CF patients on their healthcare resource utilization and medical expenditure. Expenditures were determined from the managed care payer perspective. CF patients with PA infection were defined using International Classification of Diseases, 9th Clinical Modification (ICD 9 CM) codes of 277.0 and 482.1, respectively. Among CF patients with PA infection, PA-related subsequent medical events were defined as presence of a claim with therapeutic class 4 (antibiotic, aminoglycosides) or class 16 (Quinolones, NEC), or ICD-9-CM code 482.1 (pneumonia due to PA), which were used to determine PA-related resource utilization and expenditures. A medical claim for PA infection during the index period (May 2006–April 2007) but no PA-related claim during the 12 month baseline period (May 2005–April 2006) was defined as initial or new PA infection. The date of initial PA infection served as index date for the pre–post study design. To ensure the validity of the CF diagnosis, subjects were required to have a diagnosis claim of 277.0 during at least one inpatient admission or two outpatient visits that were more than 30 days apart during 13 months around the initial diagnosis of PA (i.e., 12 months baseline period through 1 month post-PA infection to allow capturing possible delays in claim submission). depicts the sample selection process. The study sample was restricted to cases continuously enrolled in a managed care plan for 12 months before and after the index date. Individuals with a prior PA infection, enrolled in “capitated” plans, without prescription drug coverage or not continuously enrolled with complete medical coverage were excluded to ensure that healthcare utilization was not missed due to disenrollment or partial medical coverage. The study protocol was approved by the institutional review board.
Outcome measures and analysis
Demographic information was retrieved and healthcare utilization and expenditures were calculated for three categories 12 months pre- and post-index date for eligible subjects:
Inpatient care: visits, hospital length of stay;
Outpatient care: physician visits, urgent care visits, emergency room visits; and
Prescriptions: total number of prescription claims, total number of unique claims (i.e., excludes refills).
Descriptive and pair-wise non-parametric statistical analyses were conducted to describe the study sample and compare annual healthcare resource utilization and expenditures before and after PA infection using SAS (version 9.2, SAS Institute Inc, Cary, NC).
Results
A total of 358 subjects met the study inclusion criteria. The study population had a mean age of 20.1 (SD = 15.5) years and 48% were females (). Pancreatic insufficiency (67%), chronic sinusitis (20%), and diabetes (15%) were the most common co-morbid conditions identified.
Table 1. Sample characteristics of CF patients with PA infection in the MarketScan claims and encounters database.
The annual per-patient healthcare expenditures and resource utilization data is presented in . Healthcare resource utilization significantly increased across all categories following a PA infection (p < 0.0001), with the exception of ER visits. The greatest magnitude of increase was observed in inpatient visits, which grew by a factor of 2.4 relative to baseline mean value (0.54, pre-infection −1.29, post-infection). Average outpatient visits and unique pharmacy prescriptions also increased significantly (18.27 vs 21.67 and 10.97 vs 12.72, respectively; p < 0.0001) following PA infection. In line with the increase in healthcare resource utilization, a statistically significant increase (p < 0.0001) was seen in all categories of medical expenditure following an initial PA infection, with the exception of expenditures on ER visits. In our study, pre-infection annual per-patient medical expenditures were $33,305. Subsequent to the PA infection, annual per-patient expenditures increased to $51,821, a jump of more than $18,500 relative to baseline value. Inpatient care was the single largest contributing factor, with an increase of $8580 over baseline expenditures. Increases in outpatient care expenditures ($5048) and prescription expenditures ($4834) following the initial PA infection were also substantial.
Table 2. Average annual per-patient healthcare costs and resource utilization among CF patients pre- and post-PA infection.
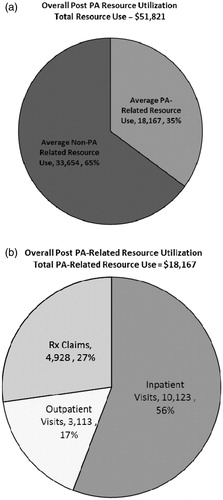
graphically depicts the manner in which medical spending post-PA infection was allocated. Our analysis indicates that in the 12-months following an initial infection with PA, 35% of annual medical spending was related to infection by this pathogen, while the remaining 65% was attributable to other causes. It was found that 56% of PA-related medical spending was attributable to inpatient visits, 27% was attributable to prescription drug claims, and 17% was attributable to outpatient visits.
Discussion
Initial PA infection in CF is well known to have a major impact on the clinical course of the disease. In this study, we have shown that initial PA infection had a significant impact on medical expenditures and resource utilization, with a more than 50% mean increase in annual treatment expenditures as compared to the pre-infection period. Furthermore, we found that this increase was significant across the various components of medical expenditures and resource utilization. Interestingly, the increase in the total per patient healthcare expenditure post-initial PA infection of $18,500 found in this study was comparable to the incremental cost due to chronic PA infection reported in a previous study of ∼$21,000Citation11. Our study did further explain the types of healthcare components that are associated with increased expenditures among CF patients with initial PA infection. Whether increased expenditures of chronic PA infection in CF patients have a similar distribution is not known.
We found a substantial increase in the inpatient visits after initial PA infection to become the largest single contributor of healthcare expenditures. Subsequent to a PA infection, inpatient visits increased by a factor of 2.4 relative to baseline values while associated expenditures increased by a factor of 1.9. Increases in the outpatient visits and prescription drug claims were other significant factors of higher expenditures, although the order of magnitude of change observed in these items was not as dramatic as seen with inpatient care. The disproportionate increase in inpatient visits suggest that efforts to prevent PA infections and, if infected, preventing subsequent exacerbations may have a marked effect on managing cystic fibrosis symptoms and controlling the associated economic burden to the managed healthcare system. A single course of oral antibiotic therapy in an outpatient setting may reduce the cost of inpatient resource utilization when effective and may not require course of IV therapyCitation12.
Because of morbidity and mortality associated with the establishment of PA infection in CF, studies have been performed where patients with CF are screened for the acquisition of PA by periodic sputum cultures. Early detection of PA leads to aggressive PA eradication with antibiotic regimens and success has been seen in randomized controlled trialsCitation13,Citation14. Our data suggests that dealing with PA in the asymptomatic phase could have substantial economic benefits also. As determined by our study, once symptomatic PA infection has occurred, healthcare expenditures spiral upwards despite treatment, and based on data from chronic PA infection, remain elevated.
Therapies to control established PA infection are widely used in CF patients. One such treatment regimen includes inhaled tobramycin given for 4 weeks, alternating with 4 weeks of antibiotic free periodsCitation15. Recently, another inhaled antibiotic, aztreonam has been approved for the same purpose with the same treatment regimenCitation16. Another approach has been the use of chronic macrolide treatment, which, by both anti-inflammatory and indirect antimicrobial effects, reduces exacerbations of CFCitation17,Citation18. Excessive increase in the in-patient visits after initial PA infection in our study may be an indication of either failure in early management of the infection or patients’ non-adherence to the prescribed treatment or both. Development of alternative drugs or approaches to further reduce the impact of PA infection in CF is clearly required.
Our study does have limitations that need to be considered. Although we attempted to exclude chronic PA infection from our study by requiring an absence of PA-related claims for a 12 month period prior to the index infection, due to limited clinical data available from the claims database, some of our patients may indeed have chronic PA infection. Our estimates of medical expenditures come from administrative claims data, and hence we were unable to account for out-of-pocket payments and indirect costs resulting from absenteeism, impact on job performance, and quality-of-life for the CF patient and/or caregivers. Data were limited to MarketScan Commercial Claims and Encounters Database; hence results may not be representative of CF patients in public insurance programs with supplemental insurance. Furthermore, if inpatient care was delivered near the end of the study period, an unknown portion of the treatment costs for that event may not have been captured. We were unable to differentially explain the contribution of anti-pseudomonal therapy towards overall PA-related resource use because parenteral therapy provided in the inpatient settings cannot be teased out separately. After our study period, an inhaled anti-pseudomonal antibiotic, which costs more than 5 K, has been approved and could affect the overall costs of PA treatmentCitation19.
Future studies are recommended to determine treatment patterns and patients’ adherence to the treatments recommended for managing PA infection. Estimation of long-term expenditures of medical care, including indirect costs, among CF patients with chronic PA infections is essential. Determining whether the cost of PA-related infection varies across age groups and comorbid conditions will help target intervention efficiently.
Conclusions
PA infection was found to be associated with a significant increase in healthcare resource utilization, and, as consequence, increased annual per-patient medical expenditures by an average of ∼$18,500 relative to baseline costs. Early and effective management of initial PA infection to avoid exacerbation and subsequent inpatient hospitalizations may help contain increased medical expenditures post-PA infection among CF patients in the US.
Transparency
Declaration of funding
This study was supported by Bayer HealthCare Pharmaceuticals, Inc.
Declaration of financial/other relationships
SSS and SS have disclosed that they are consultants to Bayer HealthCare Pharmaceuticals Inc. VNJ and SB have disclosed that they are employees of Bayer Corporation. RKG and PC have no relevant financial relationships to disclose.
References
- Strausbaugh SD, Davis PB. Cystic fibrosis: a review of epidemiology and pathobiology. Clin Chest Med 2007;28:279-88
- Cystic Fibrosis Foundation Patient Registry. 2009 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation Patient Registry, 2009
- Elphick HE, Smyth RL. Infections in patients with cystic fibrosis: effects of a longer survival. The Microbe-Host Interface in Respiratory Tract Infections. England: Horizon Scientific Press Ltd, 2004
- Govan J, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Molr Biol Rev 1996;60:539
- Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002;34:91-100
- Deretic V, Schurr M, Boucher J, et al. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J Bacteriol 1994;176:2773
- Nixon GM, Armstrong DS, Carzino R, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 2001;138:699-704
- Winnie GB, Cowan RG. Respiratory tract colonization with Pseudomonas aeruginosa in cystic fibrosis: correlations between anti Pseudomonas aeruginosa antibody levels and pulmonary function. Pediatr Pulmonol 1991;10:92-100
- Britto MT, Kotagal UR, Hornung RW, et al. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002;121:64
- Jones AM, Dodd ME, Morris J, et al. Clinical outcome for cystic fibrosis patients infected with transmissible Pseudomonas aeruginosa: an 8-year prospective study. Chest 2010;137:1405
- Ouyang L, Grosse SD, Amendah DD, et al. Healthcare expenditures for privately insured people with cystic fibrosis. Pediatr Pulmonol 2009;44:989-96
- Briggs EC, Nguyen T, Wall MC, et al. Oral antimicrobial use in outpatient cystic fibrosis pulmonary exacerbation management; a single center experience. Clin Respir J 2011: published online 9 August 2011, doi:10.1111/j.1752-699X.2011.00246.x
- Lee T. Eradication of early Pseudomonas infection in cystic fibrosis. Chron Respir Dis 2009;6:99
- Taccetti G, Campana S, Festini F, et al. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J 2005;26:458
- Moss RB. Administration of aerosolized antibiotics in cystic fibrosis patients. Chest 2001;120(3 Suppl):107S
- Waknine Y. FDA approves inhaled aztreonam for cystic fibrosis. Medscape Today; 2010. Available at www.medscape.com/viewarticle/717553. [Last accessed 19 September 2011]
- Wolter J, Seeney S, Bell S, et al. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002;57:212
- Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. JAMA 2003;290:1749
- U.S. Food and Drug Administration approves Cayston(R) for the improvement of respiratory symptoms in cystic fibrosis patients with pseudomonas aeruginosa. Gilead Press Release. Available at: http://www.gilead.com/pr_1393831. Accessed October 30, 2011