Abstract
Objective:
To perform an economic evaluation of duloxetine, pregabalin, and both branded and generic gabapentin for managing pain in patients with painful diabetic peripheral neuropathy (PDPN) in Mexico.
Research design and methods:
The analysis was conducted using a 3-month decision model, which compares duloxetine 60 mg once daily (DUL), pregabalin 150 mg twice daily (PGB), and gabapentin 600 mg three-times daily (GBP) for PDPN patients with moderate-to-severe pain. A systematic review was performed and placebo-adjusted risk ratios for achieving good pain relief (GPR), adverse events (AE), and withdrawal owing to intolerable AE were calculated. Direct medical costs included drug acquisition and additional visits due to lack of efficacy (poor pain relief) or intolerable AE. Unit costs were taken from local sources. Adherence rates were used to estimate the expected drug costs. All costs are expressed in 2010 Mexican Pesos (MXN). Utility values drawn from published literature were applied to health states. The proportion of patients with GPR and quality-adjusted life years (QALY) were assessed.
Results:
Branded-GBP was dominated by all the other options. PGB was more costly and less effective than DUL. Compared with branded-GBP and PGB, DUL led to savings of 1.01 and 1.74 million MXN (per 1000 patients). The incremental cost per QALY gained with DUL used instead of generic-GBP was $102 433 MXN. This amount is slightly lower than the estimated gross domestic product per capita in Mexico for 2010. During a second-order Monte Carlo simulation, DUL had the highest probability of being cost-effective (61%), followed by generic-GBP (25%) and PGB (14%).
Limitations:
Study limitations include a short timeframe and using data from different dosage schemes for GBP and PGB.
Conclusions:
This study suggests that DUL provides overall savings and better health outcomes compared with branded-GBP and PGB. Administering DUL rather than generic-GBP is a cost-effective intervention to manage PDPN in Mexico.
Introduction
Pain is the most common reason for healthcare visits worldwide and can be broadly classified on the basis of the pathophysiology into four categories: nociceptive, inflammatory, neuropathic, and functionalCitation1. The International Association for the Study of Pain (IASP) defines neuropathic or neurogenic pain as ‘pain initiated or caused by a primary lesion or dysfunction in the nervous system’Citation2. Neuropathic peripheral pain occurs when the lesion or dysfunction affects the peripheral nervous system. Painful diabetic peripheral neuropathy (PDPN) is a chronic neuropathic pain condition that affects patients with diabetes mellitus and can be manifested as either mononeuropathy or polyneuropathy. The main symptoms of PDPN typically include aching, burning, stabbing or tingling sensations which generally begin in the feet and are often worse at nightCitation3,Citation4. The prevalence of pain in the diabetic population has been estimated at 8–25% and reaches 40–50% in those patients with diabetic neuropathyCitation5–9. Epidemiological data indicates that PDPN is more frequent in type 2 than in type 1 diabetes mellitusCitation3,Citation10.
PDPN imposes a substantial economic and social burden. Patients suffering from this condition experience poor health-related quality-of-life and show a functional capacity level lower than would normally be expected for his/her ageCitation1,Citation11,Citation12. Pain disrupts sleep patterns causing anxiety, depression, and disabilityCitation3,Citation12–15. The presence of PDPN in the diabetic population is associated with more comorbidities, a notorious increase in the consumption of medical resources, and significantly higher treatment costsCitation1,Citation7,Citation16,Citation17.
The treatment of such a complex entity along its different etiologies besides the high impact of this condition on both diabetes evolution and patient functionality warrant medical pathways to be taken following an evidence-based approach on analgesic effect. There are several consensus documents and guidelines recently published that aimed to inform healthcare professionals about the sequential management of PDPN and the rational for using a polypharmacy strategy in some casesCitation3,Citation18–20.
A wide range of pharmacological agents from different therapeutic classes are commonly used to treat neuropathic pain. These classes include selective serotonin and noradrenaline re-uptake inhibitors (SNRI: duloxetine and venlafaxine); tricyclic antidepressants (TCA: amitriptyline, imipramine, desipramine, nortriptyline and clomipramine); anticonvulsants (gabapentin, pregabalin, valproic acid, topiramate, carbamazepine, oxcarbazepine and lamotrigine); and opioids (morphine, oxycodone and tramadol). However, only two drugs (duloxetine and pregabalin) are formally approved for the treatment of PDPN in Europe as well as in the USCitation9,Citation21.
The Neuropathic Pain Special Interest Group of the IASP recommended TCA, SNRI, calcium channel alpha(2)-delta ligands (i.e., gabapentin and pregabalin), and topical lidocaine as first-line treatment options for neuropathic painCitation18. The French-speaking Society of Diabetology stated that TCA, anticonvulsants, and SNRI are of equal value in treating PDPNCitation3. Another evidence-based guideline developed by the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation included pregabalin (level A), duloxetine (level B), and gabapentin (level B) into their list of recommended drugs to treat PDPNCitation19. The National Institute for Health and Clinical Excellence (NICE) in England and Wales has recently published a guideline for the pharmacological management of neuropathic pain in adults. Based on aspects such as efficacy, tolerability, and cost-effectiveness, NICE recommends duloxetine to be offered as the preferred option for first-line treatment of PDPNCitation20.
Three clinical randomized and placebo-controlled trials have demonstrated duloxetine to be both well-tolerated and effective in reducing levels of neuropathic painCitation22–25. A recently published meta-analysis suggests that duloxetine provides comparable efficacy and tolerability to gabapentin and pregabalin in PDPNCitation21. The once daily dosing of duloxetine represents a potential advantage over pregabalin and gabapentin, which in contrast need to be administered two and three times every day, respectivelyCitation26. A less frequent dose may improve compliance and can be more convenient for patientsCitation27,Citation28.
Examining the pharmacoeconomic profile of newer drugs is necessary to make an optimal allocation of available resources and to maximize the clinical and economic benefits to societyCitation29. The aim of the present study was to evaluate the cost-effectiveness of duloxetine as a first-line treatment of PDPN from the Mexican public healthcare system.
Materials and methods
Both a cost-effectiveness and a cost-utility analysis were performedCitation30. The target population consists of adult diabetic patients with diagnosis of PDPN that is causing moderate-to-severe pain.
The following competing interventions were evaluated: Duloxetine (DUL) 60 mg once daily, pregabalin (PGB) 150 mg twice daily (300 mg/day) and gabapentin (GBP) 600 mg (two 300 mg capsules) three times daily (1800 mg/day), each administered orally. This analysis uses the 300 mg/day PGB dose that is believed to work well for most PDPN patientsCitation19 and the lowest value of the clinically effective range dose of 1800–3600 mg/day for GBPCitation4. The DUL 60 mg once daily is the recommended starting dose for this drugCitation20,Citation26 and has been used as a base-case in previous cost-effectiveness studiesCitation4,Citation31–33. Two different types of GBP were analyzed: a branded version (brand-GBP) and a generic one (gen-GBP); it was assumed there are no relevant differences in efficacy and safety among these two options.
Time horizon
Cost and outcomes were evaluated for a 12-week timeframe, which is consistent with the duration of the blinded phase of the randomized placebo-controlled clinical trials of DUL in PDPNCitation22–24. Therefore, costs and benefits were not discounted as the analysis was conducted within a 1-year time horizonCitation34.
Model description
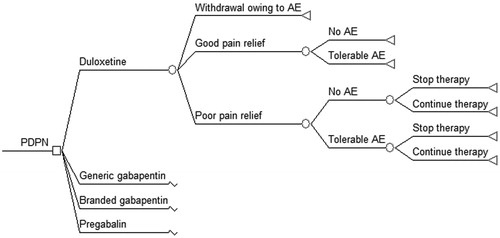
Since the time horizon is relatively short, a decision-tree can effectively represent the decision-making context. The structure of the decision-analytic model used in this analysis is shown in . A quite similar framework has been previously used by other authors and is detailed elsewhereCitation33,Citation35,Citation36. Briefly, the model consists of seven different pathways defined according to the magnitude of pain relief, the presence of adverse events (AE), and the possibility of withdrawal owing to intolerable AE or due to lack of efficacy. Treatment adherence depends on the daily frequency that the medication must be taken and estimates on adherence were used to calculate the expected costs of medicationsCitation27,Citation28. It was assumed that efficacy rates reported in clinical trials already reflect the effect of dosing frequency, and therefore they were not weighted by adherence. Poor pain relief and AE lead to additional costs and disutility. The model also assumes that all health states resulting from treatment are present for the entire horizon and that pain relief is related to a reduction in the symptoms, and not the duration, of painCitation31,Citation33.
Resource use and costs
According to the study perspective, only direct medical costs were analyzed. Following Drummond et al.Citation30 the analysis focuses on those items that may truly differ among the interventions, namely: (1) cost acquisition of the competitive drug’s schemes; (2) additional costs derived from managing AE; and (3) additional costs due to poor pain relief. Patients achieving good pain relief are assumed to complete the 12-week treatment; some patients with poor pain relief may also remain in therapy for the whole period even if they had tolerable AE. Mean treatment duration for patients that stopped therapy due to intolerable AE or because of lack of efficacy were set at 7 and 28 days, respectivelyCitation4. Cost of medication in each scheme was calculated as the product of three factors: the duration of therapy (expressed in days), the adherence rate, and the daily cost of that medication. Compared to patients achieving good pain relief without any AE, patients with good pain relief but tolerable AE were assumed to have one extra visit to a general practitioner during the study period. Patients with intolerable AE or poor pain relief were assumed to require one extra visit to a specialist per month (three visits in total)Citation33.
Health outcomes
Effectiveness was measured in terms of the extent of pain relief. As O’Connor et al.Citation33,Citation36 did, ‘good pain relief’ was defined as: (1) patient-reported subjective pain relief of ‘moderate’ or better; or (2) ‘much improved’ or better on the Patient Global Impression of Change (PGIC) scale. For those studies that did not report the PGIC outcome data, the effectiveness was estimated by multiplying the proportion of patients achieving at least 50% pain score reduction by a factor of 1.193. This conversion ratio was proposed in the O’Connor et al.Citation33 study. The cost-utility analysis used the expected number of quality-adjusted life years (QALY) as an end-point.
Data sources
Adherence estimates were derived from a systematic review performed by Saini et al.Citation28. These authors presented information of 20 published studies which explicitly analyzed treatment adherence related to the daily frequency dosing needed. Of these, 15 studies quantified adherence as the proportion of correct number of doses taken (i.e., the proportion of total correct openings). Based on that definition, a once daily dosing scheme had a simple mean adherence of 93% (range 77–100%). Lower values were reported for twice daily (mean 87%, range 74–97%) and thrice daily (mean 80%, range 66–89%) dosing schemes.
Average wholesale prices for medications to governmental healthcare institutions in Mexico were obtained from local and official sourcesCitation37,Citation38. Unit costs of an outpatient consultation in a primary care facility and in a third-level center were obtained from a reference list at the Mexican Institute of Social Security (IMSS)Citation39. All costs were calculated and are expressed in 2010 Mexican pesos (MXN; average exchange rate during 2010 year: 12.64 MXN per 1 US dollar)Citation40.
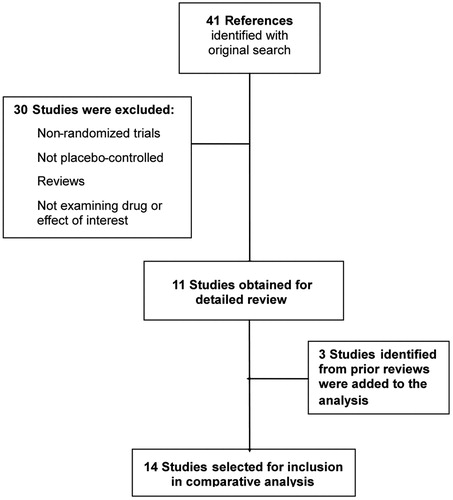
The PubMED/Medline electronic database was searched to identify potentially relevant articles in order to estimate the treatment effectiveness. The following Medical Subject Headings (MeSH) were used in the primary search: ((duloxetine OR pregabalin OR gabapentin) AND diabetic neuropathies). The search was filtered by type of article (limiting to clinical trials) and by language (limiting to English or Spanish publications). This search was supplemented by reviewing the bibliographies of key papers. Inclusion criteria were as follows: (1) randomized and placebo-controlled clinical trials; (2) prospective trial study design; (3) study population comprising adult patients with diabetic neuropathy; and (4) efficacy results reported as either PGIC or proportion of patients achieving at least 50% pain score reduction. Studies enrolling patients with diagnosis other than PDPN (post-herpetic neuralgia, for instance) were excluded. A total of 14 studies were finally selected to the analysis: three from DULCitation22–24, seven from PGBCitation41–47 and four from GBPCitation48–51 (). Study design comprised of cross-over and parallel group trials and period duration ranged from 5–12 weeks. Selected studies were comparable with respect to clinical and demographic characteristics of patients enrolled and the methods used to evaluate efficacy.
The approach followed to estimate the effectiveness of each competing strategy consisted of two steps: First, authors calculated the pooled proportion of patients achieving good pain relief in all the placebo arms in the whole 14 studies. The weighted probability of good pain relief for placebo was 29.2% and this figure can be seen as a reference placebo data or baseline risk (). Second, the risk ratio (RR) of achieving good pain relief in each treatment vs placebo was calculated by pooling the results from the individual trials involving the agent of interest (). A strict intention-to-treat analysis was employed. During the model, these placebo-controlled RR were applied in turn to the placebo reference probability of achieving good pain relief. It is important to mention that only three out of the seven PGB studies analyzed a 300 mg/day dosageCitation42,Citation46,Citation47. Consequently, the placebo-controlled RR of achieving good pain relief with PGB was based on those three references alone. Since none of the GBP studies evaluated a fixed dose of 1800 mg/day the authors decided to include all the four references in the estimation of effectiveness. For DUL estimates, they took into account merely the 60 mg daily dose from the three studies foundCitation22–24.
Table 1. Pooled analysis of achieving good pain relief in placebo arms.
Table 2. Risk ratios of achieving good pain relief with active treatment.
A similar approach was followed to estimate the probabilities of any AE and withdrawal owing to intolerable AE. Data for reference placebo was obtained from the placebo arms in controlled clinical trials of PGB and GBP included in the NICE guidelineCitation20. Risk ratios for PGB and GBP were extracted from the same reference. Pooled analysis of the 60 mg branches in the DUL trialsCitation22–24 were used to estimate RR for that drug.
Utilities associated with different pain states were derived from published literature. Doth et al.Citation11 performed a systematic review on the burden of neuropathic pain and reported that three full-text articles have assessed health utilities by pain severity in diabetic neuropathyCitation12–14. Mean utility values were significantly lower in patients suffering severe pain (0.20–0.25) than in those experiencing mild pain (0.59–0.70)Citation11. Simple average values for mild, moderate, and severe pain from data reported in the three original studiesCitation12–14 were calculated. In a cross-sectional survey of 255 with PDPN performed in the US, more patients reported ‘moderate’ pain (45% of the sample) than ‘severe’ pain (26%)Citation12. Since the target population in the present model is composed by patients with moderate-to-severe pain, a weighted average of the mean value of the moderate (0.48) and severe (0.22) pain states was estimated, yielding a baseline utility of 0.38, which was used for the outcome ‘poor pain relief’. Following O’Connor et al.Citation33, it was assumed that outcome ‘good pain relief’ corresponds to improving from the baseline pain state to mild pain, with the associated utility of 0.64 (equal to the simple average of the mean values reported in the three studies)Citation12–14. A disutility (loss of utility) of 5% was applied to patients experiencing tolerable AE; that figure for intolerable AE was 10%. This assumption has been made in prior cost-effectiveness studiesCitation33,Citation35. When AE were present, the overall health-state utility was estimated by multiplying the pain state by the AE disutility factor adjustment (0.95 and 0.90 for tolerable and intolerable AE, respectively)Citation33.
Statistical analysis
Expected cost and effectiveness (utilities) were calculated for a hypothetical cohort of 4000 patients divided into four groups of 1000 patients each, assigned to any of the four competitive strategies. After the base-case results were completed, a sensitivity analysis was conducted to assess the potential impact of uncertainty in parameter values. The authors varied parameters through the range of published and plausible values (). When possible, ranges were derived from the 95% confidence intervals. Costs were allowed to vary by 10%. Disutility weights associated with AE were assumed to vary by 0.025 (tolerable AE) and by 0.05 (intolerable AE). One-way analysis was performed to all parameters and a tornado diagram was generated to summarize the results of the deterministic sensitivity analysis. Potential threshold values were also explored.
Table 3. Model parameters values: base-case, ranges, and distributions.
Probabilistic sensitivity analysis was based on a second-order Monte Carlo simulation with 1000 repetitions. Parametric distributions were assigned to each parameter following the recommendations stated by Briggs et al.Citation52. Therefore, the authors selected a beta distribution for binomial outcomes (probability of adherence) as well as for utility values related to pain severity, a gamma distribution for costs, and a logNormal distribution to fit risk ratios. The distributions for the probability of treatment adherence, the pain-states utilities and the costs were approximated with the mean (base-case value) and a standard deviation equal to 10% of the mean value. A uniform distribution was assigned to those parameters derived from a lower-quality evidence source. When it was necessary, calculation of confidence intervals and risk ratios was carried out in Stata version 9 (StataCorp LP, College Station, TX). All cost-effectiveness (utility) analyses were performed using TreeAge software (TreeAge Pro Suite 2009, TreeAge Software, Inc., Williamstown, MA).
Results
Base-case analysis
shows the expected and incremental costs and benefits for the competing strategies. Generic gabapentin was the least costly alternative, with an average cost per patient of MXN 3 070 followed by DUL, PGB, and brand-GBP. The most-effective treatment was DUL, with gains of 23 and 64 in the number of patients achieving GPR per each 1000 treated in comparison with PGB and the versions of GBP, respectively. As a consequence, DUL was also the treatment associated with the most number of QALY.
Table 4. Outcomes from the base-case analysis (per 1000 patients).
Branded gabapentin was dominated (i.e., it was more costly and at least equal of effective than comparators) by all the other three interventions. Both DUL and PGB were more effective and more costly than gen-GBP, warranting an incremental cost-effectiveness analysis (). Compared to gen-GBP, the cost per additional patient with good pain relief and the cost per additional QALY were much lower for DUL than with PGB. Indeed, DUL dominates (i.e., is more effective and less costly than) PGB. The cost per additional QALY gained when DUL is used instead of gen-GBP is MXN$ 102 433.
Sensitivity analysis
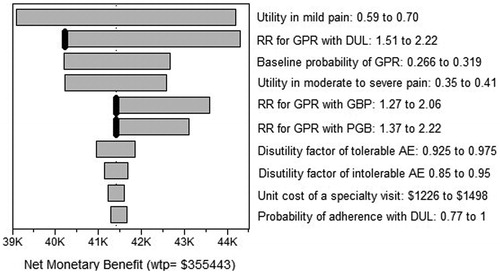
displays the tornado diagram for the net monetary benefit (NMB) using a willingness to pay (WTP) for an additional QALY equal to 3-times the gross domestic product (GDP) per capita in MexicoCitation53. Instead of making a direct comparison between DUL and either PGB or any of the GBP formulations, the tornado plot shows the sensitivity analysis in terms of the effect of each variable on the NMB. This kind of analysis allows describing uncertainty when several competing alternatives are simultaneously evaluated. For simplicity, only the 10 parameters that influenced the most on the NMB are presented. Even though there are other variables that may generate more uncertainty in the magnitude of the NMB, the RR of achieving good pain relief with each of the active drugs relative to placebo were the only parameters with threshold values: 1.699 for DUL, 1.770 for gen-GBP, and 1.983 for PGB.
Figure 3. The tornado diagram: Painful diabetic peripheral neuropathy. The WTP for an additional QALY is set equal to 3 × GDP per capita in Mexico. RR: Risk ratio; GPR: Good pain relief; DUL: Duloxetine; GBP: Gabapentin; PGB: Pregabalin; AE: Adverse events; K denotes a thousand; WTP:Willingness to pay; GDP: Gross domestic product.
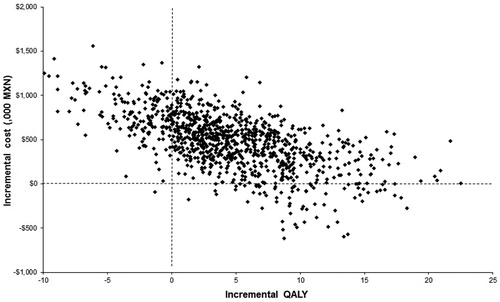
An incremental cost-effectiveness scatter plot of DUL vs gen-GBP is depicted in . This is the most relevant comparison since in the base-case both brand-GBP and PGB were dominated by at least one of these two more preferred alternatives. As can be seen, most of the simulations are located on quadrant I, which means that DUL is more effective and more costly than its comparator. For a threshold value of 3-times the GDP per capita in Mexico (MXN$ 355 443) per QALY gained, DUL is a cost-effective intervention when compared to gen-GBP in ∼64% of the simulations. In addition, DUL was dominant (both less costly and more effective than gen-GBP) in 7.3% of the simulations.
Figure 4. Incremental cost-effectiveness scatter plot (DUL vs. gen-GBP). MXN: Mexican pesos; DUL:Duloxetine; gen-GBP: Generic gabapentin; QALY: Quality adjusted life year. Data per 1000 patients.
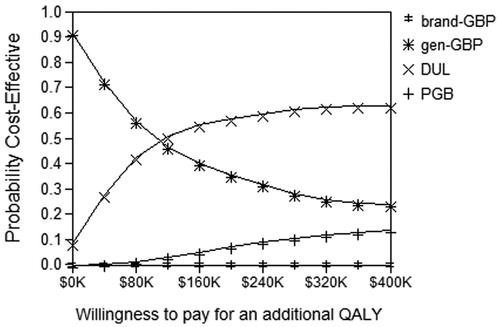
displays the acceptability curves. When the WTP for an additional QALY is set at the value of one GDP per capita, DUL and gen-GBP had practically the same chance of being cost-effective. For a most commonly used threshold of 3 GDP per capita, DUL had the highest probability of being cost-effective (61%), followed by gen-GBP (25%) and PGB (14%).
Discussion
In this study, the cost and effectiveness of four different alternatives commonly used in the first-line setting to manage pain in adults with PDPN were evaluated. Under the perspective of the public healthcare institutions in Mexico, the present analysis showed that DUL provides good value for money. This investigation suggests that DUL is associated with a higher probability of achieving good pain relief when compared to either PGB or GBP. This is consistent with the recommendations on sequential management of PDPN stated by NICE, where DUL is described as the preferred option for first-line treatmentCitation20. Other authors have previously pointed out that DUL has better outcomes than PGB and GBPCitation4,Citation33.
PGB was shown to be dominant over brand-GBP in the treatment of neuropathic pain associated with PDPN or post-herpetic neuralgia in CanadaCitation54. In the O’Connor et al.Citation33 study, DUL dominated both brand-GBP and PGB. Beard et al.Citation4 proposed that there is a strong case for the use of first-line DUL, prior to anticonvulsant therapy. The present analysis seems to confirm all these conclusions. In the base-case of this economic evaluation, DUL therapy yielded 23 and 64 additional patients with good pain relief (per 1000) compared to PGB and GBP, respectively. These differences represent increments of 4.6% and 13.7% for DUL in each case. In addition, DUL was less costly, with savings reaching MXN$ 1 009 836 (22%) and MXN$ 1 741 971 (33%) per 1000 patients in comparison to PGB and brand-GBP, respectively. Compared with gen-GBP, DUL was projected to be more effective and more costly. For situations like that, decisions had to be made on the basis of the incremental cost effectiveness ratioCitation30. There is a kind of consensus that interventions that cost less than the local GDP per capita to get an extra QALY are highly cost-effective and, traditionally, the threshold value for cost-effectiveness has been set at 3 local GDP per capita per QALY gainedCitation55–57. In the present study, the mean cost per additional QALY obtained with DUL in comparison to gen-GBP was MXN$ 102 433. This figure is slightly lower than the amount projected as the GDP per capita for 2010 year in Mexico (MXN 118 481)Citation53. Therefore, DUL should be considered a highly cost-effective intervention to treat PDPN in adults in Mexico.
To the authors’ knowledge, this is the second economic evaluation that included a generic formulation of GBP into the analysis and the first one that aimed to compare DUL vs gen-GBP in PDPN. Rodríguez et al.Citation55 found that PGB is more effective and more costly than gen-GBP in the treatment of PDPN or post-herpetic neuralgia in Spain. The authors reported an incremental cost per QALY gained of €20 535 and concluded that PGB is cost-effective, since this estimate is around the value of one GDP per capita in Spain. A different conclusion was drawn from the present study given that the mean cost per QALY gained with PGB in comparison to gen-GBP is 4.4-times the GDP per capita in Mexico. This could be a consequence of the differences in the GDP per capita between Mexico and Spain (Mexican value is less than a third of the Spanish)Citation55.
The results derived in the present model were quite sensitive to the estimates of the efficacy in the competitive treatments. Maintaining all other things constant, if the RR of achieving good pain relief with DUL drops to less than 1.669 or the correspondent RR with GBP is higher than 1.77, then gen-GBP would emerge as the preferred option. In the same way, if the RR of achieving good pain relief with PGB is higher than 1.983, this treatment would displace DUL as the more cost-effective therapy. Besides these three variables, the model was robust to changes in the parameters. Letting all the parameters vary at the same time during the probabilistic sensitivity analysis showed that DUL possess the highest probability (61%) of being classified as the more cost-effective intervention. Generic GBP and PGB would earn this honor in 25% and 14% of the cases, respectively.
When evaluating several competing alternatives it is desirable that evidence comes from clinical trials that included all the treatments of interest in the same study. Nevertheless, this is often not possible. In the absence of direct comparisons obtained from head-to-head clinical trials, an indirect comparison may be performedCitation58,Citation59. One of the most reliable and frequently applied methods of indirect comparison consists of using a common shared comparatorCitation60–62. Therefore, efficacy and safety data adjusted by placebo were used during the analysis. It appears that O’Connor et al.Citation33 did not perform any kind of indirect comparison but they used the pooled proportions of good pain relief for each treatment without adjusting by a common comparator. On the other hand, Beard et al.Citation4 used placebo-adjusted RR but they had limited information at that moment.
Another strength of the present study consists of using the most recent published evidence of the effect of medication dosing frequency on adherenceCitation28. Since the analysis was performed in an intention-to-treat basis, the authors believe that the proportion of patients achieving good pain relief in each treatment already reflect the differences in the frequency the medications have to been taken. Consequently, adherence rates were incorporated only for the calculation of the expected cost and not for the outcomes.
In contrast to other studies, information derived from clinical trials that enrolled patients presenting a diagnosis other than PDPN was excluded, even if they were conformed by a mixed population. Thus, the results of the present analysis are only applicable to those patients suffering PDPN.
This study has several limitations. In first place, since none of the clinical trials of GBP investigated the 1800 mg/day dosing scheme, it was necessary to pool efficacy data derived from different doses. A weighted mean daily dose from the six arms included in the four clinical trials of GPB evaluated in the present analysis yields 1980 mg/day, which is close to the average dose of interestCitation48–51. By the other way, the efficacy analysis focused on the typical daily dosage of PGB (300 mg), which can be achieved by giving either 100 mg thrice or 150 mg twice a day. In the National Formulary of Drugs in MexicoCitation63, pregabalin is available only in 75 and 150 mg. Hence, to accomplish the 300 mg/day dosage, the usual scheme consists of administering a 150 mg capsule twice a day. It is important to note that two out of the three studies of PGB evaluated 300 mg/day dosage had a thrice schemeCitation46,Citation47, meanwhile the other had a twice a day schemeCitation42. Freeman et al.Citation64 found in a systematic review that only the PGB 600 mg/day dosage showed efficacy when administered twice a day. The US Food and Drug Administration (FDA) approved the pregabalin dosage of 300 mg/day administered in three divided dosesCitation33. In Mexico, the recommended dosage for this anticonvulsant ranges from 150–600 mg/day, divided into two or three dosesCitation26.
Pain relief was based on categorical scales such as PGIC, which was the most common instrument applied across the trials in which results were reported as proportions. However, two out the 10 studies used to estimate efficacy (both of PGBCitation42,Citation47) did not provide enough information, and in those cases good pain relief rates were calculated indirectly by using a conversion ratioCitation33. Therefore, results for PGB should be taken with some caution. Other questionnaires such as The Brief Pain Inventory (BPI) contains several 11-point numeric rating scales that ranges from 0 (no pain) to 10 (worst possible pain)Citation65. Results from these numerical scales were reported only in an aggregated level (e.g., mean pain scores), with no patient-level data available. Therefore, these kinds of scales were not considered an option, given the need to count with efficacy measures reported as proportions to be included into the decision tree. Since there are a wide range of instruments to assess pain relief, as well as diverse type of variables (continuous, categorical, etc.) to be analyzed, it is possible that comparisons of efficacy among competing alternatives look different when distinct measures are used.
One can reasonably argue that a longer timeframe would better represent the chronic nature of PDPN, but, following O’Connor et al.Citation33, the authors decided to kept the time horizon of the model at 3 months to be in line with the duration of the clinical trials of DUL and to reflect the usual time to evaluate a first-line therapy for this condition. Adopting a short timeframe avoids the need to make assumptions about titration schemes, the composition of the sequential treatments and sustainability (duration) of the effects (i.e., duration of pain control). Studies that have explored these assumptions in longer timeframes agree that DUL is the more cost-effective optionCitation31. Beard et al.Citation4 suggest that giving DUL as a first or second-line (after a TCA) instead of using first a TCA and then an anticonvulsant result in a dominant scenario favorable to DUL sequences. Fox-Rushby et al.Citation20,Citation31 analyzed a life-time horizon and also found that first-line DUL is the preferred alternative to treat PDPN.
There is scarce information regarding health-related quality-of-life issues in the Mexican population, so it is not infrequent to gather utility values from international published literature. It is noteworthy that pain-state utility values derived through different regions of the world are very consistent with each otherCitation11.
Conclusion
The present study suggests that duloxetine, when administered as 60 mg once daily to the first-line treatment of PDPN in adults in Mexico, provide both additional benefits and overall reductions in health-related cost in comparison with PGB in doses of 300 mg/day and branded gabapentin in doses of 1800 mg/day. This economic evaluation also suggests that giving duloxetine 60 mg once daily rather than generic gabapentin is a highly cost-effective intervention to the first-line management of PDPN in adults in Mexico.
Transparency
Declaration of funding
Funding for this study was provided by Eli Lilly y Compañía de México, S.A. de C.V.
Declaration of financial/other relationship
Authors Jocelyn Ramírez-Gámez, Héctor Dueñas, and Rosa María Galindo-Suárez are or were employees of Eli Lilly y Compañía de México, S.A. de C.V. when this study was conducted. Author Fernando Carlos has received funds for health economics research from Eli Lilly y Compañía de México, S.A. de C.V. and Pfizer, S.A. de C.V. over the past 12 months. Author Elisa Ramos has no conflict of interest to report.
Acknowledgments
No assistance in the preparation of this article is to be declared. This study was presented in poster format at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 3rd Latin American Conference; Mexico City, Mexico; September 8–10, 2011.
References
- O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009;27:95-112
- IASP. Task force ont: classification of chronic pain, 2nd edn. Seattle, WA: IASP Press, 1994
- Hartemann A, Attal N, Bouhassira D, et al. Painful diabetic neuropathy: diagnosis and management. Diabetes Metab 2011;37(5):377-88
- Beard SM, McCrink L, Le TK, et al. Cost effectiveness of duloxetine in the treatment of diabetic peripheral neuropathic pain in the UK. Curr Med Res Opin 2008;24:385-99
- Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med 2008;9:660-74
- Ziegler D. Painful diabetic neuropathy: treatment and future aspects. Diabetes Metab Res Rev 2008;24:52-7
- Hartsfield CL, Korner EJ, Ellis JL, et al. Painful diabetic peripheral neuropathy in a managed care setting: patient identification, prevalence estimates, and pharmacy utilization patterns. Popul Health Manag 2008;11:317-28
- Barrett AM, Lucero MA, Le T, et al. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med 2007;8:50-62
- Jensen TS, Backonja MM, Hernández Jiménez S, et al. New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc Dis Res 2006;3:108-19
- Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206-13
- Doth AH, Hansson PT, Jensen MP, et al. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain 2010;149:338-44
- Gore M, Brandenburg NA, Dukes E, et al. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005;30:374-85
- Currie CJ, Poole CD, Woehl A, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia 2006;49:2272-80
- Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications 2006;20:26-33
- Gore M, Brandenburg NA, Hoffman DL, et al. Burden of illness in painful diabetic peripheral neuropathy: the patients' perspectives. J Pain 2006;7:892-900
- Ritzwoller DP, Ellis JL, Korner EJ, et al. Comorbidities, healthcare service utilization and costs for patients identified with painful DPN in a managed-care setting. Curr Med Res Opin 2009;25:1319-28
- Dworkin RH, Malone DC, Panarites CJ, et al. Impact of postherpetic neuralgia and painful diabetic peripheral neuropathy on health care costs. J Pain 2010;11:360-8
- Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85(3 Suppl):S3-14
- Bril V, England JD, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy–report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve 2011;43:910-7
- Centre for Clinical Practice at NICE. NICE clinical guideline 96. Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings of neuropathic pain in adults in non-specialist settings. National Institute for Health and Clinical Excellence, London 2010
- Quilici S, Chancellor J, Löthgren M, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol 2009;9:6
- Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005;116:109-18
- Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6:346-56
- Wernicke JF, Pritchett YL, D'Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67:1411-20
- Kajdasz DK, Iyengar S, Desaiah D, et al. Duloxetine for the management of diabetic peripheral neuropathic pain: evidence-based findings from post hoc analysis of three multicenter, randomized, double-blind, placebo-controlled, parallel-group studies. Clin Ther 2007;29:2536-46
- Dictionary of Pharmaceutical Specialties DEF-55 PLM 2009. Editorial Thompson Mexico, Mexico City, Mexico 2009
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23:1296-310
- Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care 2009;15:22-33
- Kobelt G. Health economics: an introduction to economic evaluation. 2nd edn. London, UK: Office for Health Economics, 2002;1:9-24
- Drummond MF, Sculpher MJ, Torrance G, et al. Methods for the economic evaluation of health care programmes. 3rd Edn. UK: Oxford University Press, 2005
- Fox-Rushby JA, Griffith GL, Ross JR, et al. The clinical and cost-effectiveness of different treatment pathways for neuropathic pain [NP]. NIHR Health Technology Assessment (HTA) programme, ref. 05/30/03. 2011. In press
- Wu EQ, Birnbaum HG, Mareva MN, et al. Cost-effectiveness of duloxetine versus routine treatment for U.S. patients with diabetic peripheral neuropathic pain. J Pain 2006;7:399-407
- O’Connor AB, Noyes K, Holloway RG. A cost-utility comparison of four first-line medications in painful diabetic neuropathy. Pharmacoeconomics 2008;26:1045-64
- Consejo de Salubridad General. Guía para la conducción de estudios de evaluación económica para la actualización del Cuadro Básico de Insumos del Sector Salud en México. México, DF, Agosto 2008. Available at: http://www.csg.salud.gob.mx/descargas/pdfs/cuadro_basico/GUxA_EVAL_ECON25082008_2_ech.pdf
- Cepeda MS, Farrar JT. Economic evaluation of oral treatments for neuropathic pain. J Pain 2006;7:119-28
- O’Connor AB, Noyes K, Holloway RG. A cost-effectiveness comparison of desipramine, gabapentin, and pregabalin for treating postherpetic neuralgia. J Am Geriatr Soc 2007;55:1176-84
- Portal de transparencia del Instituto Mexicano del Seguro Social. Available at: transparencia.imss.gob.mx. Accessed January 27, 2011
- Portal del sistema electrónico de compras gubernamentales. Available at: www.compranet.gob.mx. Accessed January 31, 2011
- Listado de costos unitarios por nivel de atención en el Instituto Mexicano del Seguro Social. Diario Oficial de la Federación. Martes 18 de mayo de 2010
- Banco de México website. Available at: www.banxico.org.mx/indicadores/fix.html. Accessed February 7, 2011
- Arezzo JC, Rosenstock J, Lamoreaux L, et al. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol 2008;8:33
- Tölle T, Freynhagen R, Versavel M, et al. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain 2008;12:203-13
- US Food and Drug Administration, Department of Health and Human Services, Center for Drug Evaluation and Research. Lyrica (pregabalin) capsules. Company: Pfizer Global Research & Development. Application no.: 021446. Approval date: 12/30/2004 [online]. Available at: www.fda.gov/cder/foi/nda/2004/021446_LyricaTOC.htm. Accessed March 5, 2007
- Freynhagen R, Strojek K, Griesing T, et al. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005;115:254-63
- Richter RW, Portenoy R, Sharma U, et al. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 2005;6:253-60
- Lesser H, Sharma U, LaMoreaux L, et al. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 2004;63:2104-10
- Rosenstock J, Tuchman M, LaMoreaux L, et al. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004;110:628-38
- Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther 2003;25:81-104
- Gorson KC, Schott C, Herman R, et al. Gabapentin in the treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial. J Neurol Neurosurg Psychiatry 1999;66:251-2
- Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J Clin Neuromuscul Dis 2001;3:53-62
- Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998;280:1831-6
- Briggs A, Sculpher M, Claxton K. Modelling methods for health economic evaluation. New York: Oxford University Press, 2006
- International Monetary Fund. World Economic Outlook Database. IMF, Washington D.C., USA. October 2010
- Tarride JE, Gordon A, Vera-Llonch M, et al. Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective. Clin Ther 2006;28:1922-34
- Rodríguez MJ, Díaz S, Vera-Llonch M, et al. Cost-effectiveness analysis of pregabalin versus gabapentin in the management of neuropathic pain due to diabetic polyneuropathy or post-herpetic neuralgia. Curr Med Res Opin 2007;23:2585-96
- Zhao FL, Yue M, Yang H, et al. Willingness to pay per quality-adjusted life year: is one threshold enough for decision-making?: results from a study in patients with chronic prostatitis. Med Care 2011;49:267-72
- WHO Commission on Macroeconomics Health. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization, 2001
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91
- Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9:1-134
- Song F, Altman DG, Glenny AM, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326:472
- Song F, Harvey I, Lilford R. Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J Clin Epidemiol 2008;61:455-63
- Edwards SJ, Clarke MJ, Wordsworth S, et al. Indirect comparisons of treatments based on systematic reviews of randomised controlled trials. Int J Clin Pract 2009;63:841-54
- General Health Council. Health Sector’s Formulary and Catalogue of Medication 2010. http://www.csg.salud.gob.mx/. Accessed August 29, 2011
- Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care 2008;31:1448-54
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38