Abstract
Background:
The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial showed that tolvaptan use improved heart failure (HF) signs and symptoms without serious adverse events.
Objective:
To evaluate the potential cost savings associated with tolvaptan usage among hospitalized hyponatremic HF patients.
Methods:
The Healthcare Cost and Utilization Project (HCUP) 2008 Nationwide Inpatient Sample (NIS) database was used to estimate hospital cost and length of stay (LOS), for diagnosis-related group (DRG) hospitalizations of adult (age ≥18 years) HF patients with complications and comorbidities or major complications and comorbidities. EVEREST trial data for patients with hyponatremia were used to estimate tolvaptan-associated LOS reductions. A cost offset model was constructed to evaluate the impact of tolvaptan on hospital cost and LOS, with univariate and multivariate Monte Carlo sensitivity analyses.
Results:
Tolvaptan use among hyponatremic EVEREST trial HF patients was associated with shorter hospital LOS than placebo patients (9.72 vs 11.44 days, respectively); 688,336 hospitalizations for HF DRGs were identified from the HCUP NIS database, with a mean LOS of 5.4 days and mean total hospital costs of $8415. Using an inpatient tolvaptan treatment duration of 4 days with a wholesale acquisition cost of $250 per day, the cost offset model estimated a LOS reduction among HF hospitalizations of 0.81 days and an estimated total cost saving of $265 per admission. Univariate and multivariate sensitivity analysis demonstrated that cost reduction associated with tolvaptan usage is consistent among variations of model variables.
Conclusions:
The estimated LOS reduction and cost savings projected by the cost offset model suggest a clinical and economic benefit to tolvaptan use in hyponatremic HF patients.
Study Limitations:
The EVEREST trial data may not generalize well to the US population. Clinical trial patient profiles and relative LOS reductions may not be applicable to real-world patient populations.
Introduction
Hyponatremia is common in patients with heart failure and results from the inability to excrete ingested water adequatelyCitation1. Retrospective studies and clinical trials have estimated that 12–27% of acute decompensated heart failure (ADHF) patients develop hyponatremia, with a worsening prognosisCitation2–6. This problem in HF is largely related to the associated fall in effective cardiac output, which stimulates the secretion of three hormones—renin (with a subsequent increase in angiotensin II and aldosterone formation), anti-diuretic hormone (ADH)/vasopressin, and norepinephrineCitation7–9. These neurohormonal changes limit both sodium and water excretion in an attempt to restore perfusion pressure to normal. ADH release directly enhances water reabsorption in the collecting tubulesCitation10. In addition, both low cardiac output and high ADH levels are also potent stimuli to thirst, leading to enhanced water intakeCitation11. ADH release may lead to reductions in serum sodium concentration, the degree of which correlates with the severity of the HFCitation12,Citation13.
This relationship has prognostic importance as this has been associated with increased risk of hemodynamic deterioration, longer hospital stay, and higher rehospitalization and reduction in patient survivalCitation3,Citation6,Citation14–16. The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) study found that hyponatremia was associated with higher 6-month mortality and an increased rate of HF-related rehospitalizationCitation5. The high-dose loop diuretic therapy used to manage the congestion and edema characteristic of CHF can exacerbate existing hyponatremia due to the loss of both free water and electrolytes such as sodium and potassium. As a result, the use of aquaretics, electrolyte sparing agents which promote the excretion of free water, are being considered for the treatment of CHFCitation17.
The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial was a prospective, randomized, double-blind, placebo-controlled clinical trial of 4133 patients to evaluate the short-term and long-term efficacy of tolvaptan when added to standard therapy in patients hospitalized with worsening CHF, reduced left ventricular ejection fraction of ≤40%, with at least two of the following heart failure symptoms: dyspnea, jugular venous distention, or peripheral edemaCitation4,Citation18. Tolvaptan is an orally available vasopressin-2 selective antagonist that promotes free water excretion to correct hyponatremiaCitation17. On the first day of therapy and at discharge, patients receiving tolvaptan had a significantly higher serum sodium concentration from baseline of 3.28 ± 4.11 mEq/L and 2.01 ± 4.55 mEq/L, respectively (p < 0.001)Citation4. The EVEREST trial demonstrated that tolvaptan, combined with standard therapy, improved many HF signs and symptoms without serious adverse events.
Despite the increasing knowledge of the link between heart failure and hyponatremia, there are major gaps in the understanding of this relationship including the cost or savings in correcting hyponatremia. Post-hoc analysis determined that the use of tolvaptan was also associated with a decrease in the LOS of CHF patientsCitation19. The primary objective of this study was to evaluate the potential hospital cost savings associated with tolvaptan usage among HF patients with hyponatremia based on the EVEREST trial by constructing a cost-offset model to evaluate the impact of tolvaptan on hospital resource usage among HF patients.
Methods
Model description
An economic cost-offset model was developed to evaluate the potential cost saving associated with the use of tolvaptan in HF patients with hyponatremia based on the outcomes of post-hoc EVEREST trial analyses. The analysis was conducted from the perspective of hospitals in the US. The potential cost reduction due to tolvaptan use was derived from the LOS reduction determined in a post-hoc EVEREST trial analysis and was applied to the associated costs and resource usage of a US study population from the 2008 Nationwide Inpatient Sample (NIS) database consisting of hospitalized adult HF patientsCitation19. Univariate and multivariate Monte Carlo sensitivity analyses were performed as components of the economic model to evaluate the range of the potential cost reduction associated with tolvaptan due to variation in the estimated model parameters.
Model parameter estimate
Resource reduction due to tolvaptan use
A post-hoc analysis was conducted on the patients enrolled in the EVEREST trial to determine the effect of both serum sodium concentration and the use of tolvaptan on the index hospitalization LOSCitation19. After adjustment for treatment as a factor and both geographic region and the interaction between treatment and geographic region as covariates, among patients with serum sodium concentrations <135 mEq/L, those treated with tolvaptan had a mean LOS of 9.72 days in comparison to patients that received a placebo, with a mean LOS of 11.44 days. The relative difference in LOS due to tolvaptan use in the EVEREST trial was considerable (15.0%), but was not statistically significant ().
Table 1. EVEREST Trial—post-hoc analysis of LOSCitation19.
Total hospital costs and LOS associated with HF-related hospitalization
The Healthcare Cost and Utilization Project (HCUP) is a collection of healthcare databases and related software tools/products sponsored by the Agency for Healthcare Research and Quality (AHRQ) to create a national information resource of patient-level healthcare dataCitation20. Part of HCUP, the NIS database is the largest public all-payer inpatient hospital care database of 5–8 million hospital stays from ∼1000 facilities sampled from US community hospitals, defined as short-term, non-Federal, general and other hospitals, excluding hospital units of other institutions (e.g., prisons). The 2008 NIS contains the discharge data from an ∼20% stratified sample of US community hospitals consisting of 1056 hospitals in 42 states, covering ∼90% of all hospital discharges and including persons covered by Medicare, Medicaid, private insurance, and the uninsured. All data are weighted to be representative of the US population. HCUPnet, an on-line HCUP database query system, was used to estimate the mean hospital costs and LOS for diagnosis related group (DRG) hospitalizations of adult (age ≥18 years) HF patients with complications and comorbidities (DRG 292) or major complications and comorbidities (DRG 291) from the 2008 NIS database ().
Table 2. Resource usage of HCUP HF patients.
Incidence of hyponatremia in HF patients
The incidence of hyponatremia among HF patients has been determined by both retrospective analyses as well as through various clinical trials. The results vary based on the study population, study setting, and the delineations of hyponatremia severity. In order to better reflect the real-world HF patient population, the incidence of hyponatremia was limited to the results of retrospective database analyses. The incidence of hyponatremia was determined based on the study by Chen et al.Citation2, where 27 of the 234 patients (11.5%) hospitalized with CHF were diagnosed with severe hyponatremia, defined as a serum sodium concentration of <125 mmol/LCitation2,Citation21–24. The number of hyponatremia admissions among the study population was estimated by applying the 11.5% incidence of hyponatremia among hospitalized HF patients to the number of HF patients identified in the HCUP database query for adult HF-related DRG hospitalizations.
Cost of tolvaptan use
The cost associated with tolvaptan usage per admission in hospitalized HF patients was determined by applying the cost of tolvaptan to the average duration of tolvaptan use during hospitalization. As there are no data available regarding the average duration of tolvaptan use during hospitalization, the duration of tolvaptan use within the cost offset model was estimated to be 4 days. Since the peak increase in serum sodium and urine excretion is achieved within 24 h of the initiation of tolvaptan, and the mean LOS of HF patients is 5–6 days, an average duration of tolvaptan use of 4 days is reasonable. We also tested the cost offset with different durations of tolvaptan usage in the sensitivity analysis. The wholesale acquisition cost of tolvaptan was estimated to be $250 per day.
Outcomes
Hyponatremia admissions among HCUP HF patients
The number of patients with hyponatremia among the HCUP HF patients was estimated by applying the incidence of hyponatremia among HF patients (11.5%) to the total number of HF-related DRG hospitalizations identified in the 2008 NIS database.
Resource reduction due to tolvaptan usage
The relative difference in LOS of the EVEREST trial patients treated with tolvaptan and placebo was used to determine the per admission reduction in total hospital costs and LOS among the HCUP HF patient population. The estimated reduction in cost and LOS due to tolvaptan use was applied to the mean total hospital costs and LOS per admission from the HCUPnet query results to estimate the potential resource reduction due to tolvaptan use among the HCUP HF patients.
Total cost offset
Cost offsets are the savings associated with the use of tolvaptan after adjustment for the cost of tolvaptan treatment. Total cost offsets were determined per admission as well as for the overall HCUP HF patient population. The total cost offset per admission was determined to be the difference between the EVEREST trial LOS-associated cost reduction per hospitalization and the incremental cost of tolvaptan use per hospitalization. The total cost offset among the HCUP HF patient population was estimated by applying the total cost offset per patient to the estimated number of hyponatremic HCUP HF patients.
Total LOS reduction
The total LOS reduction (days) due to the use of tolvaptan was estimated by applying the calculated EVEREST trial-associated LOS reduction per admission to the estimated number of hyponatremic HCUP HF patients.
Sensitivity analysis
Sensitivity analysis was conducted to determine the impact of variation in the model parameters on the estimated total cost offset and resource reduction. A univariate sensitivity analysis was conducted on the cost offset model to determine the effect of variation in individual model parameters on the total cost offset per admission and the estimated tolvaptan-associated reduction in LOS per admission. For the total cost offset, each of the following variables was allowed to vary by ±20%: hyponatremia incidence rate among hospitalizations for HF with major complications and comorbid conditions, hyponatremia incidence rate among hospitalizations for HF with complications and comorbid conditions, cost per admission for hospitalizations for HF with major complications and comorbid conditions, cost per admission for hospitalizations for HF with complications and comorbid conditions, duration of tolvaptan use, daily cost of tolvaptan, and the relative tolvaptan-associated LOS reduction. For the reduction in LOS per admission, each of the following variables was allowed to vary by ±20%: hyponatremia incidence rate among hospitalizations for HF with major complications and comorbid conditions, hyponatremia incidence rate among hospitalizations for HF with complications and comorbid conditions, LOS per admission for hospitalizations for HF with major complications and comorbid conditions, LOS per admission for hospitalizations for HF with complications and comorbid conditions, and the relative tolvaptan-associated LOS reduction.
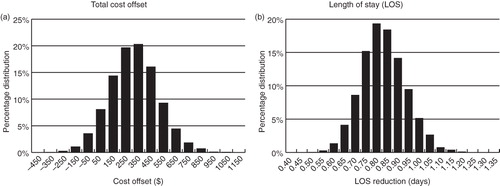
Since variables are often interdependent, a multivariate sensitivity analysis was also conducted where each of the univariate sensitivity analysis variables were varied simultaneously. For each cycle, the value of the corresponding variables was taken randomly from a normal distribution of the variable with a coefficient of variation of 0.20. A Monte-Carlo simulation with 10,000 such iterations was conducted for the multivariate sensitivity analysis.
Results
HCUP HF-related hospitalization resource usage
A total of 688,336 hospitalizations for HF with major complications and comorbid conditions and HF with complications and comorbid conditions were identified from the 2008 HCUP NIS database (). Approximately 50% of the study patients were between the ages of 64–84. The hospital LOS increased with age while the daily cost of hospitalization decreased with age. Hospitalized HF patients had a mean LOS of 5.4 days and mean total hospital costs of $8415.
Cost offset model
Based on the estimated 15% relative reduction in LOS demonstrated by the post-hoc analysis of EVEREST trial patients, the tolvaptan-mediated LOS reduction among HCUP HF hospitalizations was estimated to be 0.81 days (). The tolvaptan-mediated reduction in total hospital costs among HCUP HF patients was $1265.21 per admission (). With the tolvaptan-mediated LOS reduction, the average LOS for HF admission would be 4.59 days. Using an inpatient tolvaptan treatment duration of 4 days with a daily wholesale acquisition cost of $250, as published by the drug manufacturer, the cost of tolvaptan treatment was estimated to be $1000 per hospitalization. After taking into account the cost of tolvaptan treatment, the use of tolvaptan to treat HCUP HF patients with hyponatremia results in an estimated cost savings of ∼$265 (). The cost neutral breakeven mean duration of tolvaptan inpatient therapy was 5.06 days. Incidence of hyponatremia among HF patients was approximated at 11.5% based on the results from a prior studyCitation2. This incidence was used to estimate that 79,159 HCUP HF patients are putatively hyponatremic. Consequently, the use of tolvaptan to treat hyponatremic HF patients would result in a total cost offset of approximately $21 million () among the HCUP population. The total reduction in hospital LOS among hyponatremic HF patients treated with tolvaptan would be 63,917 days among the HCUP population ().
Table 3. Resource reduction due to tolvaptan usage (per admission).
Table 4. Overall resource reduction due to tolvaptan usage.
Sensitivity analysis
Univariate and multivariate sensitivity analysis demonstrated consistent cost reduction associated with tolvaptan usage. The univariate sensitivity analysis indicated that, based on the single variable worst case scenario, the estimated total cost offset would be $12.17 and, under optimal conditions, could be as high as $518.25 (). Similarly, univariate sensitivity analysis of the tolvaptan-mediated LOS reduction ranged from 0.65–0.97 days (). Multivariate sensitivity analysis also supported the validity of the model. The Monte Carlo simulation showed the 95% confidence interval for the total cost offset reduction to be −$108.44 to $659.90. The mean total cost offset was $265.73 ± $193.89, with a median of $264.32, a minimum of −$562.38, and a maximum of $1052.67 (). Multivariate sensitivity analysis of the LOS reduction revealed the 95% confidence interval for the LOS reduction to be 0.615–1.02 days. The mean LOS reduction was 0.807 ± 0.103 days, with a median of 0.802 days, a minimum of 0.470 days, and a maximum of 1.22 days ().
Figure 1. Univariate sensitivity analysis. Individual model parameters were allowed to vary ±20%. (a) Total cost offset reduction, (b) LOS reduction.
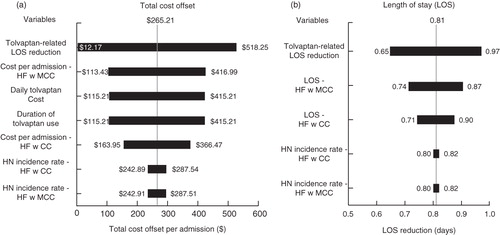
Figure 2. Multivariate sensitivity analysis. Each variable used for the cost offset or LOS univariate sensitivity analysis was allowed to vary simultaneously for 10,000 cycles, with the value of the corresponding variables taken randomly from a normal distribution with a coefficient of variation of 0.20 for each cycle. (a) Total cost offset, (b) LOS reduction.
Discussion
In May 2009, the FDA approved the use of tolvaptan for the treatment of clinically significant hypervolemic and euvolemic hyponatremia including patients with HF, cirrhosis, and syndrome of inappropriate anti-diuretic hormone secretion (SIADH). In this analysis, tolvaptan-mediated total hospital costs among the HCUP HF patients was reduced by $1265.21 per admission from tolvaptan treatment. The cost offset model constructed in the study determined that the use of tolvaptan for 4 days resulted in an estimated mean $265 cost savings per admission among HF patients. In addition, HF patients treated with tolvaptan were projected to have a 0.8 day reduction in their hospital LOS. The 10,000 cycles of Monte Carlo simulations showed that the 95% confidence interval for the total cost offset reduction was −$108.44 to $659.90, with 91.8% of the cycles having a net cost saving (offset >$0). The Monte Carlo simulation was designed to mimic more closely the real-world settings, where all model variables can vary simultaneously. It indicates that in the vast majority of cases tolvaptan usage may result in a net cost saving for hospitals.
Heart failure (HF) is a growing problem in the US, presenting a substantial burden on the healthcare system. The prevalence of HF in the US was estimated to be 5.8 million in 2006, with the development of 670,000 new cases in patients ≥45 years of age. Correspondingly, HF-related healthcare resource usage has also greatly increased. During the 25 year period between 1979 and 2004, HF-related hospitalizations in the US tripled from 1,274,000 to 3,860,000, 80% of which involved patients over the age of 65 yearsCitation25. The direct and indirect costs of HF in 2010 are estimated to be $39.2 billion; the majority of the costs are expected to be related to hospitalizationCitation26. Several studies have established that the presence of hyponatremia in patients hospitalized with HF results in longer hospitalizations in comparison to normonatremic patientsCitation19,Citation21,Citation27. In a retrospective analysis of 4323 patients with CHF, in addition to a hyponatremia-related increased LOS, the total median cost of admissions for patients with moderate-to-severe hyponatremia was $19,698, $1775 higher than that of normonatremic CHF patientsCitation21. Another retrospective study determined that a diagnosis of severe hyponatremia among CHF patients resulted in mean hospital costs that were $1917 higher than that of normonatremic CHF patients. These studies indicate that the presence of hyponatremia in HF patients results in greater total hospital costs.
One component of the total hospital costs is the cost associated with laboratory testing. Additional laboratory tests are recommended with the use of tolvaptan to monitor changes in serum electrolytes and volume. Testing performed every 6–8 h over a period of several days may accrue to represent a significant amount. The HCUP NIS database contains the total charges for each hospital discharge which also include the cost of laboratory testing. Since the intensity of monitoring sodium levels is also a function of the severity of hyponatremia present, a portion of the additional testing costs are incorporated within the total charges reported in the database. However, any further testing that may be ordered due to the use of tolvaptan will not be included in these charges and could impact the cost offset determined using the current model.
The incidence of hyponatremia among HF patients varies between studies. To some extent, the inconsistencies are inherent to the study design or to the study population being investigated. In particular, the incidence of hyponatremia determined during the use of claims databases may be inaccurate. A study was conducted to determine the inclusivity and accuracy of the identification of hospitalized patients with hyponatremia from an administrative claims database using ICD-9 codingCitation28. Of those subjects with serum sodium laboratory results that could be used to assess the presence of hyponatremia, only one third had an appropriate ICD-9 code recorded. The results of the study suggest that the incidence of hyponatremia among hospitalized patients is greatly under-estimated. A similar study assessing the sensitivity of ICD-9 codes for the determination of the outpatient incidence of hyponatremia also found that <30% of hyponatremia cases were identifiableCitation29. The patient discharge data collected within the HCUP NIS database precludes its use for the calculation of the incidence of hyponatremia as it is not possible to identify those patients with newly onset disease from those with a chronic condition. The 11.5% hyponatremia incidence rate used in this study to determine the number of hyponatremic HF patients within the HCUP HF patient population was one of the more conservative incidence rates, chosen to more accurately reflect the low incidence of hyponatremia among the patients enrolled in the EVEREST trial (10.7%)Citation19. Other clinical trials have reported incidence rates of hyponatremia among HF patients as high as 27%Citation5,Citation6,Citation14. Therefore, it is very probable that the cost offset model under-estimates the proportion of hyponatremic HF patients. Consequently, the overall total cost offset for the hyponatremic HF patients within the 2008 HCUP NIS database would be greater than the currently estimated $21 million.
As with most economic models, the analysis was based on multiple data sources and was reliant on certain analytical assumptions, with the results dependent upon the relative accuracy of the assumptions made and the generalizability of clinical trial and database analysis results. The constraints imposed by the availability of data to determine model parameters led to certain limitations to the cost offset model constructed in the study. For example, there are little real-world data available with respect to tolvaptan usage. The real-world duration of tolvaptan use is not well defined and currently there are no data available regarding the prescribing practice associated with tolvaptan. As a result, the average duration of tolvaptan usage among HF patients in the US was estimated to be 4 days. Given that the estimated average LOS among HF admissions without tolvaptan usage is 5.4 days and that the LOS with tolvaptan usage is estimated to be 4.59 days, an estimate of 4 days for the duration of tolvaptan therapy is reasonable. However, the actual duration of tolvaptan usage may be longer and, consequently, the cost-offset will be less. The cost break-even duration of tolvaptan usage as estimated from the model is 5.06 days. Furthermore, the study population and its associated costs and resource usage was identified using a retrospective database analysis of the 2008 HCUP NIS database. The NIS is a database consisting of all-payer inpatient care and contains the clinical and resource use for hospital discharges. The study assumes that the NIS data, collected from a 20% stratified sample of US community hospitals, is representative of the entire US population. While the HCUP database is large and national, it still may not represent all types of hospitals, and certain patient populations may not be accurately represented.
The cost savings demonstrated in this study were derived from a relative LOS reduction of less than 1 day (0.81 days), which may not translate into a significant real-world reduction in costs. Many hospitals are paid a fixed amount per discharge based on DRG, regardless of the LOS. For these hospitals, even a partial day LOS reduction can translate into a real benefit, resulting in a lower actual cost per admission due to the shorter LOS, while receiving the same fixed payment per DRG. However, for cases in which the payment to hospitals corresponds to the number of hospital days a patient was admitted (e.g., payment on a per diem basis), a reduction in LOS may also reduce the final payment received by the hospital for the admission. After accounting for the change in revenue, the full financial benefits of a LOS reduction may not be as great as demonstrated in this model. In addition, the level of resource intensity may vary based on the date of service during the hospitalization (e.g., diagnostic vs treatment period). Furthermore, the setting in which treatment is received may impact the overall cost savings. A LOS reduction in general ward settings may not be associated with the same cost level as that estimated from the HCUP database. However, given that hyponatremia patients are also often treated in the ICU settings, the overall reduction of LOS among hyponatremic HF patients can lead to beneficial cost savings to hospitals as demonstrated in this cost evaluation. Although the LOS reduction was less than a day, it remained consistent despite variation in treatment setting and differences in region and the standard therapies used to treat hyponatremia since the EVEREST study was an international, multi-center study in which patients received the standard of care for hyponatremia and either tolvaptan or a placeboCitation3.
The EVEREST study was a global endeavor and included 359 centers in North America, South America, and Europe. In the current study, the LOS and hospital direct costs were derived from US population data from the NIS database. The data from the global EVEREST trial may not generalize well to the NIS US population data. Geographic differences in healthcare resource usage and treatment standards may also influence study results. For example, in the post-hoc analysis of EVEREST trial patients, the unadjusted difference in LOS between patients with serum sodium concentrations <135 mEq/L treated with tolvaptan or placebo was 2.11 days for the all qualified patients. When the sub-set of US patients was examined, the difference in LOS was 1.39 days. The possibility remains that the global results from the EVEREST trial may not be applicable to US population data and it may not be accurate to assume that the relative LOS reduction determined for the EVEREST trial patients is representative of the LOS reduction as a result of tolvaptan use among the US hospitalized patient population.
Finally, the real-world patient profile may differ from that of clinical trials. This is most clearly demonstrated in the proportion of hyponatremic patients that were enrolled in HF-related clinical trials. While the OPTIMIZE-HF, OPTIME-CHF, ESCAPE, and EVEREST clinical trials all recruited patients with systolic dysfunction, the proportion of hyponatremic patients was 19.7%, 27.2%, 23.8%, and 10.7%, respectivelyCitation5,Citation6,Citation14,Citation19. Although the clinical trials were designed to investigate patients with systolic dysfunction, the specific patient selection criteria used during recruitment resulted in different patient populations. The real-world patient population, which includes the patients that are typically excluded from clinical trials, may be substantially different from those examined in the clinical trials. As a result, it is not clear whether or not the relative LOS reduction seen in the clinical trials will also be present in the real-world patient population.
Conclusions
Based on the results of the EVEREST trial, tolvaptan use was associated with a shorter hospital LOS than placebo among hyponatremic HF patients. The cost offset model demonstrated that, despite the incremental cost of tolvaptan treatment, tolvaptan is associated with an estimated mean hospital cost reduction of $265 per admission in the US. The estimated cost savings projected by the cost offset model suggest a clinical and economic benefit of tolvaptan use in hyponatremic HF patients.
Transparency
Declaration of funding
This research was supported by Otsuka America Pharmaceutical, Inc., Princeton, NJ.
Declaration of financial/other relationships
J.D. and J.C. are consultants for and have received honoraria from Otsuka. J.L. is an employee of Novosys Health, which has received research funds from Otsuka America Pharmaceutical. R.C. is an employee of Otsuka America Pharmaceutical, Inc. S.K. was an employee of Otsuka America Pharmaceutical, Inc. at the time of the study.
Acknowledgments
The authors would like to acknowledge Tina Wright from Otsuka America Pharmaceutical, Inc. for her critical review of the manuscript. No other assistance in the preparation of this article is to be declared. Various aspects of the study results have been presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 16th Annual International Meeting in Baltimore, MD on May 21st to May 25th, 2011 and presented at the American College of Chest Physicians (ACCP) 2011 Conference as an Oral Presentation.
References
- Haley H, Ploth DW. Dyshomeostasis of serum sodium concentration in congestive heart failure. Am J Med Sci 2010;340:42–7
- Chen MC, Chang HW, Cheng CI, et al. Risk stratification of in-hospital mortality in patients hospitalized for chronic congestive heart failure secondary to non-ischemic cardiomyopathy. Cardiology 2003;100:136–42
- Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 2004;291:1963-71
- Gheorghiade M, Konstam MA, Burnett JC Jr., et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332-43
- Gheorghiade M, Rossi JS, Cotts W, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med 2007;167:1998-2005
- Klein L, O'Connor CM, Leimberger JD, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 2005;111:2454-60
- Triposkiadis F, Karayannis G, Giamouzis G, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009;54:1747-62
- Francis GS, Goldsmith SR, Levine TB, et al. The neurohumoral axis in congestive heart failure. Ann Intern Med 1984;101:370-7
- Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol 2005;95:2B-7B
- Lee CR, Watkins ML, Patterson JH, et al. Vasopressin: a new target for the treatment of heart failure. Am Heart J 2003;146:9-18
- Sica DA. Hyponatremia and heart failure–treatment considerations. Congest Heart Fail 2006;12:55-60
- Jao GT, Chiong JR. Hyponatremia in acute decompensated heart failure: mechanisms, prognosis, and treatment options. Clin Cardiol 2010;33:666-71
- Sica DA. Sodium and water retention in heart failure and diuretic therapy: basic mechanisms. Cleve Clin J Med 2006;73(2 Suppl):S2-7; discussion S30–3
- Gheorghiade M, Abraham WT, Albert NM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J 2007;28:980-8
- Adrogue HJ. Consequences of inadequate management of hyponatremia. Am J Nephrol 2005;25:240-9
- Lee WH, Packer M. Prognostic importance of serum sodium concentration and its modification by converting-enzyme inhibition in patients with severe chronic heart failure. Circulation 1986;73:257-67
- Ferguson-Myrthil N. Novel agents for the treatment of hyponatremia: a review of conivaptan and tolvaptan. Cardiol Rev 2010;18:313-21
- Konstam MA, Gheorghiade M, Burnett JC, Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319-31
- Cyr PL, Slawsky KA, Olchanski N, et al. Effect of serum sodium concentration and tolvaptan treatment on length of hospitalization in patients with heart failure. Am J Health Syst Pharm 2011;68:328-33
- (HCUP), HCaUP, HCUP Nationwide Inpatient Sample (NIS). 2007–2009. Rockville, MD: Agency for Healthcare Research and Quality
- Callahan MA, Do HT, Caplan DW, et al. Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study. Postgrad Med 2009;121:186-91
- Krumholz HM, Chen YT, Bradford WD, et al. Variations in and correlates of length of stay in academic hospitals among patients with heart failure resulting from systolic dysfunction. Am J Manag Care 1999;5:715-23
- Mohammed AA, van Kimmenade RR, Richards M, et al. Hyponatremia, natriuretic peptides, and outcomes in acutely decompensated heart failure: results from the International Collaborative of NT-proBNP Study. Circ Heart Fail 2010;3:354-61
- Panciroli C, Galloni G, Oddone A, et al. Prognostic value of hyponatremia in patients with severe chronic heart failure. Angiology 1990;41:631-8
- Fang J, Mensah GA, Croft JB, et al. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol 2008;52:428-34
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 2010;121:e46-e215
- Shorr AF, Tabak YP, Johannes RS, et al. Burden of sodium abnormalities in patients hospitalized for heart failure. Congest Heart Fail 2011;17:1-7
- Movig KL, Leufkens HG, Lenderink AW, et al. Validity of hospital discharge International Classification of Diseases (ICD) codes for identifying patients with hyponatremia. J Clin Epidemiol 2003;56:530-5
- Shea AM, Curtis LH, Szczech LA, et al. Sensitivity of International Classification of Diseases codes for hyponatremia among commercially insured outpatients in the United States. BMC Nephrol 2008;9:5
