Abstract
Objective:
A pharmacoeconomic analysis was undertaken to determine costs, consequences, and cost-effectiveness of a brand of partially hydrolyzed 100%-whey formula manufactured by Nestlé (PHF-W), in the prevention of atopic dermatitis (AD) in ‘at risk’ Danish children compared to extensively hydrolyzed formula (EHF-Whey or Casein).
Methods:
Given the non-significant differences between PHF-W and EHF, the base case analytic approach amounted to a cost-minimization analysis (CMA) reporting the difference in formula acquisition costs over the period of formula consumption for the population of interest. However, sensitivity analyses (SAs) were undertaken to explore applying the nominal efficacy of PHF-W and EHF, thus leading to a cost-effectiveness analysis (CEA). Hence, an economic model based on a 12-month time horizon was developed synthesizing treatment pathways, resource utilization, and costs associated with the treatment of AD in the population of interest. The final economic outcome of the SAs was the incremental cost per avoided case (ICER) defined as the expected cost per avoided case of AD for PHF-W vs EHF, determined from three perspectives: the Ministry of Health (MOH), the family of the subject, and society (SOC).
Results:
In the base case CMA, savings of DKK 9 M, DKK 20 M, and DKK 29 M were generated for PHF-W vs EHF from the MOH, family, and SOC perspectives. In the sensitivity CEA, PHF-W was dominant over EHF-Whey from all perspectives, while EHF-Casein displayed against PHF-W unattractive ICERs of DKK 315,930, DKK 408,407, and DKK 724,337 from the MOH, family, and SOC perspectives. Probabilistic SAs indicated that PHF-W was 86% likely to be dominant over EHF-Whey, whereas EHF-Casein had no likelihood of dominating PHF-W.
Conclusion:
Under a range of assumptions, this analysis demonstrated the attractiveness of PHF-W vs both types of EHF in the prevention of AD among ‘at risk’ Danish infants who are not or cannot be exclusively breastfed.
Introduction
Atopic dermatitis (AD) is one of the most common skin disorders seen in infants and children with an onset during the first 6 months of lifeCitation1,Citation2. The development of AD and other atopic diseases depends on an interaction between genetic factors; environmental exposure to food and inhalant allergens; and non-specific adjuvant factors (e.g., tobacco smoke, air pollution, and infections)Citation3. Hence, allergen avoidance is key in the primary prevention of allergy, as experimental and clinical data indicate that early exposure to dietary allergens may be crucial for the development of allergies such as food allergies and ADCitation4. Furthermore, infants are deemed to be at high risk of developing AD if they have a parent or sibling with a history of allergyCitation3,Citation5,Citation6.
The World Health Organization as well as numerous regional and national guidelines recommend exclusive breastfeeding for the first 6 months of lifeCitation7–12. When the infant cannot be breastfed or breastfeeding duration is shorter than recommended, extensively hydrolyzed infant formulas based on whey (EHF-Whey) or casein proteins (EHF-Casein) are indicated both for treatment and prevention of cow’s milk and food allergy in ‘at risk’ infants in Denmark. Amino acid-based formulas (AAF) are also available for allergy treatment, but at a much higher cost.
One specific brand of 100% whey-based partially hydrolyzed formula, NAN-HA®, manufactured by Nestlé S.A, Switzerland (PHF-W) and branded under NAN-HA 1® in Denmark, has been shown in randomized trials to be as effective as EHF in the prevention of AD, a fact confirmed by two meta-analysesCitation13,Citation14. In addition, partially hydrolyzed formula is associated with lower rates of discontinuation due to a host of factors such as better taste, better texture, and less bitternessCitation4,Citation6,Citation15.
Treatment for AD engenders an important amount of health service resources (be they financial or logistical) and places a significant burden on the child, family, and societyCitation16. A review of the literature did not yield any studies reporting the cost of AD for Denmark. However, a 2006 study of 33 Italian children with AD reported a mean cost of €1254 per year for the familyCitation17; a 1999 study based in Germany estimated the annual cost of AD from the societal perspective to be DM 4827 (€2468)Citation18; and a second German study, based on 91 children and published in 2003, reported annual direct healthcare costs ranging from US$164 in mild cases to US$911 in severe casesCitation19.
The cost-effectiveness of PHF-W in the prevention of AD for ‘at risk’ children has been established in FranceCitation20, but no such economic evaluation has been published for a Danish setting. This has prompted the present pharmacoeconomic analysis in order to determine the costs and consequences of PHF-W vs EHF in the prevention of AD in ‘at risk’ children in Denmark.
Methods
Product, disease, and population of interest
The product of interest was PHF-W and the comparators were EHF-Whey and EHF-Casein. All three were assessed for their effectiveness in preventing the disease of interest, AD, the most quantifiable of all allergic manifestations which can be associated with milk consumption. The population of interest was defined as healthy yet ‘at risk’ subjects who were not exclusively breastfed. The term ‘at risk’ refers to children with at least one parent or sibling with a diagnosed history of allergies.
Perspective
The present economic evaluation was undertaken from the perspective of the Danish Public Health System or ‘Ministry of Health’ (MOH), of the family of the child as well as of society as a whole. Specific resources, their utilization and costs were identified for each perspective, with the societal perspective combining the costs of both the MOH and family perspectives.
Type of economic evaluation
In a meta-analysis based on six studies comparing the efficacy of PHF-W vs EHF-Whey and/or EHF-Casein in the prevention of AD in ‘at risk’ childrenCitation4,Citation15,Citation21–25, Szajewska and HorvathCitation13 reported no significant difference in the relative risk (RR) of developing AD symptoms between PHF-W and either EHF preparation. Given that PHF-W and its comparators have a similar efficacy, the economic evaluation which was deemed most appropriate for the base case analysis was a cost-minimization analysis (CMA). In this type of analysis, all costs attributable to PHF-W and its comparators (i.e., the cost of treatment, medical visits, laboratory testing, hospitalization, and all indirect costs) would be equal except for the acquisition cost of the formulas themselves. Hence, the CMA would amount to an analysis of the difference in the acquisition costs of PHF-W vs EHF (Whey and Casein) when these formulas are used in prevention. This approach did not take into consideration the cost of EHF preparations for the treatment of AD, rather its prevention, although these costs were taken into account in the sensitivity analyses (SAs) described below.
Although there was no significant difference in the efficacy of PHF-W and its EHF comparators, there still existed a nominal difference in their efficacy in preventing AD symptoms. The nominal RRs for PHF-W vs both EHF preparations were reported in an extension of the Szajewska and HorvathCitation13 meta-analysis which was published by Iskedjian et al.Citation14. In this latter scenario, if the statistical non-significance would not be taken into account, the outcomes and the costs associated with each infant formula would not be considered equal and would warrant the adoption of a cost-effectiveness analysis (CEA) approach, rather than a CMA.
Hence, a series of CEAs were undertaken as SAs to the base case CMA in order to explore the possible cost-effectiveness of PHF-W vs both EHFs when the nominal differences in their efficacy in prevention of AD symptoms were considered. Each formula was then assigned its nominal efficacy and, in turn, was associated with a specific set of outcomes and costs.
Parameters of the base case CMA
The starting cohort and the acquisition costs for each infant formula were the two key components of the base case CMA. These parameters also applied to the CEAs which were undertaken as SA.
Starting cohort
The starting cohort for the decision-analytic model was based on the population of interest for the present study and was calculated as follows:
(Birth cohort in Denmark) × (1 – Average Exclusive Breastfeeding rate) × (Rate of ‘at risk’ infants)
The number of live births in Denmark in 2010 was obtained from Statistics DenmarkCitation26. The average of the exclusive breastfeeding rates at 1 and 4 months of age was reported for Denmark by Benn et al.Citation27. Three studies provided an approximation of the rate of newborns who were born ‘at risk’ of developing AD (33%)Citation3,Citation5,Citation6. The key components of the starting cohort are presented in .
Table 1. Epidemiological and clinical parameters applied in the base case analysis and in the primary sensitivity analysis.
Cost of infant formula
One specific brand of EHF-Whey (Profylac®, Hørsholm, ALK, Denmark) and one specific brand of EHF-Casein (Nutramigen®, Illinois, Mead Johnson, USA) were selected as the comparators to PHF-W in the present study given that these brands had also been used as comparators in the Szajewska and HorvathCitation13 meta-analysis. The cost of the AAF was derived from Nutramigen AA© (Mead Johnson). The price of the infant formula was obtained from a survey of pharmacies and large-scale retail outlets in Denmark. Infant formulas used by ‘at risk’ infants are reimbursed by the MOH at a rate of 60% in Denmark, for up to 4 months if these formulas are used for prevention and up to 6 months if they are used for treatment (i.e., used after the occurrence of AD symptoms)Citation28. The proportion of formula costs which was not covered by the MOH was assigned to the family of the subject. The impact of modifying the reimbursement rate for infant formulas and the length of such coverage was explored in a set of secondary SAs. The parameters pertaining to the cost of infant formula are presented in .
Table 2. Economic parameters applied in the base case analysis and in the primary sensitivity analysis.
When determining the quantity of infant formula consumed, one important distinction was brought forth: not all subjects who consumed infant formula did so exclusively. Indeed, subjects who were not exclusively breastfed could be either exclusively formula-fed or fed a combination of mother’s milk and infant formula. In order to determine the percentage of infants who are exclusively formula-fed within those who receive formula, the following equation was applied:
[100% − (% ever-breastfed)]/[100% − (% exclusively breastfed)].
The rate of infants who were ever-breastfed in Denmark (including those exclusively breastfed) was derived from the Organization of Economic Cooperation and DevelopmentCitation29. In turn, the percentage of infants consuming both formula and breast milk among those infants who were not exclusively breastfed amounted to:
100% − % of exclusively formula-fed infants.
The daily intake of infant formula was determined for each of the first 6 months of life, based on the manufacturer’s instructions for the preparation of PHF-W (4.7 g of formula/30 ml of water with various volumes of water, depending on the age of the infant). These instructions were comparable to those for both EHF brands and for AAF. Exclusively formula-fed infants followed this feeding regimen for the entire 6 months of formula consumption. It was assumed that infants who consumed both formula and breast milk were fed formula 20% of the time in the first month, 50% in the second month, 75% in the third month, and fully formula-fed for the remainder of the first 6 months of life.
Expert panel
Five Danish expert clinicians (SH and AH, two pediatricians with an expertise in nutrition and allergy, BFV, a pediatrician, as well as PS and ML, two family physicians) were presented with questionnaires developed in a similar fashion to those used in a previous publication by Iskedjian et al.Citation20. The answers to these questionnaires were synthesized to determine an average approach to treatment pathways and evaluate the resources utilized in the management of AD symptoms in a Danish setting.
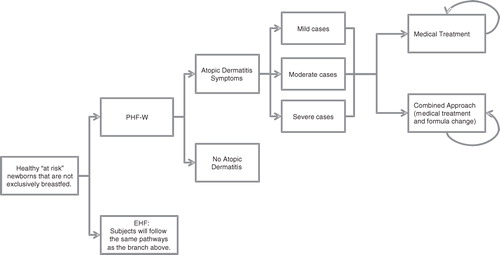
In order to undertake the sensitivity CEAs mentioned in the ‘Type of Economic Evaluation’ section above, a decision-analytic economic model (presented in ) reflecting the evidence-based medical practices associated with the treatment of AD in Denmark was constructed, in line with the input of the expert panel. The remaining sections of the Methods describe this model and the analyses performed based on that model.
Structure of the model
MS Excel® 2003 was used to construct a spreadsheet model applying a series of 3-month cycles, starting with birth. Subjects were assigned to one of two arms receiving either PHF-W or its EHF comparator allowing for a juxtaposition of costs and consequences between the two formulas. PHF-W was first compared to EHF-Whey and then to EHF-Casein.
Subjects within each arm were then divided into two groups: those subjects without AD and those subjects with AD. Three main factors, namely, the age of the subject (which was inherent to the model) as well as the severity (mild, moderate, or severe) and location on the body (face, rest of the body, or both) of the AD manifestation, were used to characterize the cases of AD occurring in the model. The two latter rates were obtained from the expert panel.
At their first medical visit, subjects with AD were presented with an age-specific plan to manage their symptoms. Subjects who were 6 months of age or younger could be treated in one of two ways: a medical treatment approach or an approach combining the medical treatment approach with one or more changes of infant formula. Beyond 6 months of age, it was assumed that infant formulas were no longer consumed. Hence, AD symptoms could only be managed using the medical treatment approach.
The medical treatment approach consisted of up to four lines of a treatment regimen of emollients, topical corticosteroids (Class I, II, III, and IV), and immunosuppressants, assigned to the subject (in accordance with the opinion of the expert panel) based on the severity of AD symptoms, their location on the body, and the age of the subject. In the combined approach to the management of AD symptoms, subjects were also assigned a new infant formula, while being prescribed medications in accordance to the medical treatment approach above. Patients who had been consuming PHF-W, EHF-Whey, or EHF-Casein were assigned EHF-Whey, EHF-Casein, and EHF-Whey, respectively. In the case of response, subjects continued consuming the new milk formula until 6 months of age. If a second change of formula was required, subjects who had started with PHF-W were then assigned EHF-Casein, while the others were assigned AAF. The response rate for each line of treatment in each management approach was supplied by the expert panel. In a series of secondary SAs, the estimated response rates for each line of therapeutic or combined management were reduced by 50% in order to test the effect of these parameters on the results of the sensitivity CEAs.
In addition to treatment, the economic model also took into consideration the resource utilization and costs of medical visits, of laboratory testing and of hospitalization, as well as all the indirect costs associated with these resources (further explained below).
Statistics Denmark published the baseline mortality rates for infants born in Denmark in 2008–2009Citation30. Although AD does not affect mortality rates, these rates were applied at the end of the first and second years of the economic model in order to make it more dynamic.
Parameters unique to the sensitivity CEAs
The epidemiological, clinical, and economic inputs for the SAs are presented in Tables and .
Calculation of the incremental cost-effectiveness ratios
The final outcome of the sensitivity CEAs were incremental cost-effectiveness ratios (ICERs) detailing the expected cost per avoided case of AD by adopting PHF-W rather than either EHF preparation. The ICER in this study is the difference in costs between PHF-W and EHF preparations divided by the negative value of the difference in the number of cases between PHF-W and EHF preparations, as presented in the following simplified mathematical formulation:
The number of cases of AD attributable to each infant formula was determined by applying the nominal RRs for PHF-W vs EHF-Whey and PHF-W vs EHF-Casein. This approach using avoided cases rather than occurring cases was warranted as this economic evaluation explored the prevention of AD when PHF-W was consumed. A similar approach has previously been adopted in the analysis of other preventative interventions such as vaccinesCitation31.
The intermediate economic outcomes associated with each infant formula were the aggregated costs applicable to the MOH perspective (i.e., the reimbursed costs of formula, medical visits, medications, and laboratory tests), the perspective of the family (i.e., the non-covered portion of formula costs, travel costs, as well as such indirect costs as time loss and productivity loss), and the societal perspective (all of the above-mentioned costs).
Time horizon
In the primary SA, a time horizon of 12 months was adopted as it represented the time during which most cases of AD first occur while extending beyond the period of milk consumption. Secondary SAs were carried out by applying a time horizon of 6 months (the period of milk consumption) or of 3 years, at which point most AD symptoms have either dissipated or have evolved into broader allergic manifestations such as rhinitis or asthma.
Resource utilization and costs
According to the expert panel, all first-line medical visits were with a family physician (general practitioner). Subsequently, 50% of subjects with moderate AD and all subjects with severe AD were immediately referred to a specialist (a pediatrician, a dermatologist, or an allergist). Of those subjects who had not been referred to a specialist, failure of second-line treatment prompted such a referral. For subjects who did not respond to treatment, all subsequent medical visits occurred every time a treatment was revised or a change of formula was assigned. In Denmark, medical visits are fully reimbursed, but the cost of medical visits varies according to the specialty of the consulted physician and the number of medical visitsCitation32,Citation33.
Pharmacies in Denmark were surveyed to obtain the cost of emollients and the brand with the lowest price was selected. In accordance with the recommendations of the expert panel, all subjects used 2000 g of emollient creams for a period of 2 months at every occurrence of AD symptoms, as per the quantity per month (1000 g) reported in a study by Beattie and Lewis-JonesCitation34.
Depending on the age of the subject as well as the severity and location of AD symptoms, the expert panel recommended the use of one or a combination of corticosteroid creams (of very low, low, moderate, or high potency) and immunosuppressants (details of the regimen are available upon request). The Danish Medicines Agency provided the cost of the medicationsCitation35 and the reimbursement rate for these medications for each range of medication costsCitation36.
According to the expert panel, subjects with mild symptoms of AD were not administered any laboratory tests. However, 50% of subjects with moderate AD and all subjects with severe AD were given a Prick Test and an Oral Provocation Test. In addition, 10% of all moderate or severe cases were eligible for a Specific IgE Test and 10% of severe cases were also subject to the Food Eviction Test. Furthermore, according to the expert panel, 1% of all severe cases are hospitalized for a period of 2 days. The cost of the laboratory testing and hospitalization are fully reimbursed in Denmark; these costs were obtained from a survey of a local medical clinic and hospital, respectively.
When analyzing the family perspective and the societal perspective, indirect costs due to leisure time and/or productivity loss were included in the model. The calculation of time lost was based on the population rate of participation in the workforce in Denmark (published by the Statistics Denmark for 2010)Citation37 as well as the average gross hourly wage and weekly hours of work published for 2007 and 2008, respectively, by the International Labour OrganizationCitation38,Citation39. This calculation yielded a daily number of hours worked of 7.27. A total of 4 hours loss was granted to the family for physician visits and laboratory testing (including travel to and from the medical office). Two full days were assumed to be needed for childcare after the initial medical visit while, as per expert opinion, time lost for applying topical cream to the skin of the affected subject was taken into account at a rate of 10 min per application. It was assumed that all topical interventions (such as emollients and corticosteroids) were applied once daily.
The cost of travel to and from the physician’s office, for an assumed distance of 10 km, was established by using an average of the cost of public transportation (bus and metro), the cost of using a taxi, and the cost of operating a personal car in the city of Aarhus, Denmark. The cost of operating a personal car was approximated by using the per kilometer rate for a taxi (i.e., excluding service charges and the additional fare for waiting in traffic).
Discounting
Costs beyond 1 year were discounted, but outcomes were considered with or without discounting as the discounting of outcomes is still controversialCitation40. Discount rates of 0, 3, and 5% were applied as per the recommendations by Alban et al.Citation41.
Probabilistic sensitivity analyses
A set of 10,000 Monte Carlo simulations was undertaken to provide a broad evaluation of use of PHF-W vs both EHF with the context of a CEA. This probabilistic SA allowed the simultaneous variation of key parameter values in a random fashion according to ranges and types of distribution, thus covering a wide breadth of possibilities within the CEA analysis. Monte Carlo ICERs were obtained by dividing the average incremental costs by the average avoided cases which were obtained as a result of the simulations while median ICERs were identified by using the ICERs generated from each Monte Carlo simulation, thus accounting for the incremental costs and outcomes of each simulation.
Results
Cost minimization analysis—Base case analysis
For a birth cohort of 63,411 newborns in Denmark in 2009, the starting cohort entering the model had 4544 ‘at risk’ newborns consuming infant formula. presents the results of the base case analysis from three perspectives (MOH, family, and society) when comparing subjects who consumed PHF-W to those who consumed EHF-Whey or EHF-Casein for prevention. The formula acquisition costs for PHF-W, EHF-Whey, and EHF-Casein were DKK 10,639,893, DKK 39,583,835, and DKK 39,491,205, respectively. Hence, the base case CMA yielded savings of DKK 28,943,942 for PHF-W vs EHF-Whey and savings of DKK 28,851,312 when PHF-W was compared to EHF-Casein, including savings from the MOH perspective of DKK 9,442,695 and DKK 9,411,420, respectively. Therefore, from all three perspectives, PHF-W was dominant over both EHF preparations.
Table 3. Results of the base case analysis presented from the perspective of the Ministry of Health, of the family of the subject, and of society as a whole.
Cost effectiveness analysis—Primary and secondary sensitivity analyses
PHF-W vs EHF-Whey
In the primary SA wherein the nominal efficacy of PHF-W and EHF-Whey were taken into account (see ), the expected numbers of cases attributed to PHF-W and EHF-Whey were 453 and 728, respectively, yielding a total of 274 avoided cases of AD by selecting PHF-W over EHF-Whey. The total direct and indirect costs associated with PHF-W and EHF-Whey were DKK 16,460,337 and DKK 48,856,577, respectively, yielding savings with PHF-W. From all three perspectives, the highest cost was attributable to formula. The expected incremental costs per avoided case of AD (i.e., the expected ICERs) were −DKK 35,502, −DKK 82,565, and −DKK 118,067 from the MOH, family, and societal perspectives, respectively. These negative ICER values and the fact that more cases were avoided by using PHF-W rather than EHF-Whey indicate dominance of PHF-W over EHF-Whey from all three perspectives. The findings of the secondary SAs which were undertaken by varying some parameters of the primary SA are presented in . PHF-W was again dominant over EHF-Whey in all scenarios except one: when applying the upper bound of the 95% CI of the RR of developing AD, a scenario with low probability.
Table 4. Results of the primary sensitivity analysis comparing PHF-W to EHF-Whey, presented from the perspective of the Ministry of Health, of the family of the subject, and of society as a whole.
Table 5. Results of the secondary sensitivity analyses comparing PHF-W to EHF-Whey, presented from the perspective of the Danish Ministry of Health, of the family of the subject, and of society as a whole.
EHF-Casein vs PHF-W
Given that the nominal efficacy of EHF-Casein is better than that of PHF-W, the CEA comparing these two formulas was geared towards evaluating the incremental cost of EHF-Casein, not PHF-W; this analysis effectively became an analysis of EHF-Casein vs PHF-W. Hence, the formula presented in the Methods was reversed to show the incremental cost of EHF-Casein, which had a higher acquisition cost, for each additional expected avoided case of AD.
The results of the primary CEA are presented in . The expected number of avoided cases by selecting EHF-Casein over PHF-W was 26. The total costs associated with EHF-Casein were DKK 44,982,191 and the highest cost driver was the cost of formula. The expected ICERs for EHF-Casein vs PHF-W were DKK 365.585, DKK 746,073, and DKK 1,111,658 from the MOH, family, and societal perspectives, respectively. These ICERs convey an unattractive cost-effectiveness for EHF-Casein vs PHF-W, an outcome which was confirmed in the secondary SAs (particularly the SA where the time horizon was limited to 6 months), presented in . The only secondary SA which yielded a negative ICER (displaying dominance for PHF-W) was the SA applying the lower bound of the 95% CI of the RR of developing AD, where the advantage of PHF-W over EHF-Casein was greatly increased.
Table 6. Results of the primary sensitivity analysis comparing EHF-Casein to PHF-W, presented from the perspective of the Ministry of Health, of the family of the subject, and of society as a whole.
Table 7. Results of the secondary sensitivity analyses comparing EHF-Casein to PHF-W, presented from the perspective of the Danish Ministry of Health, of the family of the subject and of society as a whole.
Cost effectiveness analysis—Probabilistic sensitivity analyses
PHF-W vs EHF-Whey
The parameter distribution and variation applied in the probabilistic SAs comparing PHF-W, EHF-Whey, and EHF-Casein are displayed in , along with the results of the probabilistic SA for PHF-W vs EHF-Whey. The obtained average and median Monte Carlo ICERs confirmed the cost-effectiveness of PHF-W over EHF-Whey with an 86% probability of showing dominance.
Table 8. Parameter distributions and variations in the probabilistic sensitivity analyses and presentations of the results of the probabilistic sensitivity analysis for PHF-W vs EHF-Whey.
EHF-Casein vs PHF-W
As in the primary SA, the probabilistic SA comparing EHF-Casein to PHF-W used EHF-Casein as the basis for the analysis, given its higher nominal efficacy. EHF-Casein was associated with a 76% likelihood of causing less cases of AD than PHF-W but with average Monte Carlo ICERs of DKK 315,930, DKK 408,407, and DKK 724,337, from the MOH, family, and societal perspectives, respectively. This probabilistic SA did not yield any probability of EHF-Casein being dominant over PHF-W, but the opposite, with PHF-W causing less cases of AD and costing less than EHF-Casein, was true in 24% of observed cases.
Discussion
To our knowledge, this is the first published economic evaluation of PHF-W in the prevention of AD in Danish ‘at risk’ children. This study differs in some aspects from a similar study published for FranceCitation20: the French MOH does not reimburse EHF for prevention, thus prompting a CEA comparing PHF-W to the most commonly used infant formula, standard cow’s milk-based formula. In the present analysis, EHF-Whey and EHF-Casein were selected as the main comparators to PHF-W because their use is currently reimbursed at a rate of 60% by the Danish MOH when used in the target population of the present study, namely ‘at risk’ infants who are not exclusively breastfed.
The present base case analysis which applied equal efficacy to PHF-W and its EHF comparators (as the difference in the nominal efficacy rate of each of these formulas did not reach the level of statistical significance)Citation13,Citation14 amounted to a CMA with PHF-W displaying significant savings when compared to both EHF preparations. These savings were comparable, as the acquisition cost of each EHF preparation was almost equal. For all three formulas, the acquisition costs were higher from the family’s perspective than from the Danish MOH perspective given that the 60% reimbursement rate for infant formulas in ‘at risk’ infants only applies for a period of 4 months. This entailed that the family covered 40% of formula acquisition costs for the first 4 months and 100% of these costs for the last 2 months of formula consumption. If the observed savings for PHF-W vs either EHF preparation are converted into savings per child in the starting cohort, savings of ∼DKK 2000, DKK 4300, and DKK 6300 were observed from the MOH, family, and societal perspectives, respectively.
A series of SAs were undertaken to explore a scenario wherein the nominal efficacy of PHF-W and its comparators were applied, thus requiring a CEA approach. In the primary comparison to EHF-Whey, PHF-W displayed dominance from all perspectives. These findings were confirmed by a set of secondary SAs in which PHF-W was always dominant over EHF-Whey except for the scenario which applied the upper bound of the 95% CI of the RR of developing AD, a low-probability scenario wherein the advantage of PHF-W over EHF-Whey in prevention was greatly diminished. The probabilistic SA yielded an 86% probability of PHF-W being dominant over EHF-Whey.
For the comparison of PHF-W and EHF-Casein when nominal efficacy was taken into account, the SAs used EHF-Casein as the basis for the analyses as its nominal efficacy was, very moderately, better than that of PHF-W. The observed expected ICERs for the primary and secondary SAs did not point to EHF-Casein as being an attractive alternative to PHF-W when used in prevention. The expected ICERS of DKK 365,582 for the MOH and DKK 1,111,658 for society would indicate that the cost of preventing one case of AD would be much higher when selecting EHF-Casein over PHF-W. This can be explained by the fact that their nominal efficacies are quite similar, whereas the acquisition cost of EHF-Casein is much greater than that of PHF-W. The probabilistic SA based on the comparison of these two formulas pointed to a similar conclusion while also dispelling the possibility of EHF-Casein showing dominance over PHF-W.
Limitations
The main limitation of the CMA consists of the fact that it was based on the lack of statistical significant differences in efficacy. However, in order to address any uncertainty arising from that approach, a full CEA model was built and various analyses performed. Those analyses confirmed the dominance of PHF-W over EHF-Whey while they failed to show any attractive cost-effectiveness for WHF-Casein.
With regard to uncertainties arising from the CEA model, several SAs, including probabilistic Monte Carlo simulations, were carried out. These SAs confirmed the robustness of the CEA model and the direction of the analyses.
As for generalizability of results, while the economic model was created and populated with an ‘average’ approach synthesized on input from various clinical practitioners and experts in the area, although there may be differences in individual practices and between various geographic areas, the probabilistic SAs have covered a wide array of results, all leading towards superiority of PHF-W.
Conclusions
Under a certain range of assumptions and using both a CMA and CEA approach, the present analysis has established the attractiveness of NAN HA 1®, a specific brand of 100% whey-based partially hydrolyzed formula, in the prevention of AD in infants and very young children in Denmark. NAN HA 1® demonstrated dominance over EHF-Whey and EHF-Casein from all perspectives in the base case CMA. In a series of SAs, a CEA approach confirmed dominance of PHF-W over EHF-Whey, while it failed to establish attractive cost-effectiveness ratios for EHF-Casein, effectively confirming PHF-W to be the alternative of choice in the prevention of AD in Denmark.
Transparency
Declaration of funding
This study was funded by the Nestlé Nutrition Institute (NNI, Vevey, Switzerland).
Declaration of financial/other relationships
Professor Dr Ferdinand Haschke, Dr Jenny van Odijk, and Dr Jörg Spieldenner are employed by NNI; Michael Iskedjian, Bechara Farah, and Jade Berbari are employed by PharmIdeas.
Acknowledgments
The authors would like to acknowledge Dr Pia Ehlers, Dr Peter Stokvad, Dr Mette Lystrom, and Dr Birgitte Frederiksen Videbaek for their clinical input; these experts received honoraria for their services. Further clinical input was provided by Dr Susan Halken and Dr Arne Høst. We would also like to acknowledge Dr Patrick Detzel (of NNI) for his analytical and editorial input.
References
- Mateos M. Guía de tratamiento de la dermatitis atópica en el niño. Documento de consenso. Grupo de expertos. Barcelona, Spain: ERGON, 2006
- Spergel J, Paller A. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003;112:S118-27
- Halken S. Allergy Review Series VI: the immunology of fetuses and infants. The lessons of noninterventional and interventional prospective studies on the development of atopic disease during childhood. Allergy 2000;55:793-802
- von Berg A, Koletzko S, Filipiak-Pittroff B, et al. The effect of hydrolyzed cow's milk formula for allergy prevention in the first year of life: The German Infant Nutritional Intervention Study, a randomized double-blind trial. J Allergy Clin Immunol 2003;111:533-40
- Bergmann R, Edenharter G, Bergmann K, et al. Atopic dermatitis in early infancy predicts allergic airway disease at 5 years. Clin Exp Allergy 1998;28:965-70
- Exl B-M. A review of recent developments in the use of moderately hydrolyzed whey formulae in infant nutrition. Nutr Res 2001;21:355-79
- Lasarte Velillas J. Recomendaciones para la lactancia materna. Comité de Lactancia Materna de la Asociación Española de Pediatría 2011;1-6 http://www.aeped.es/sites/default/files/lacmatespanol.pdf [Last Accessed: December 23, 2011]
- Host A, Koletzko B, Dreborg S, et al. Dietary products used in infants for treatment and prevention of food allergy. Joint statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child 1999;81:80-4
- Host A, Halken S, Muraro A, et al. Dietary prevention of allergic diseases in infants and small children. Amendment to previous published articles in Pediatric Allergy and Immunology 2004, by an expert group set up by the Section on Pediatrics, European Academy of Allergology and Clinical Immunology. Pediatr Allergy Immunol 2008;19:1-4
- Société Française de Dermatologie. Prise en charge de la dermatite atopique de l'enfant. Texte des recommandations. Ann Dermatol Vénéréol 2005;132:1S9-1S18
- World Health Organization and United Nations Children's Fund. Global strategy for infant and young child feeding. Geneva: NLM Classification: WS 120, 2003
- World Health Organization. World Health Organization's recommendations on breasfeeding. http://www.who.int/topics/breastfeeding/en. Accessed February 9, 2010
- Szajewska H, Horvath A. Meta-analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Curr Med Res Opin 2010;26:423-37
- Iskedjian M, Szajewska H, Spieldenner J, et al. Extension of a meta-analysis of the evidence for a partially hydrolyzed 100%-whey formula in the prevention of atopic dermatitis: brief research report. Curr Med Res Opin 2010;26:2599-606
- von Berg A, Filipiak-Pittroff B, Krämer U, et al. Preventative effect of hydrolyzed formulas persists until age 6 years: long-term results from the German Infant Nutritional Intervention Study (GINI). J Allergy Clin Immunol 2008;121:1442-7
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 2006;60:984-92
- Ricci G, Bendandi B, Pagliara L, et al. Atopic dermatitis in Italian children: evaluation of its economic impact. J Pediatr Health Care 2006;20:312-5
- Gieler U, Hohmann M, Niemeier V, et al. Cost evaluation in atopic eczema. J Dermatol Treat 1999;10:S15-S20
- Weinmann S, Kamtsiuris P, Wickman H, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol 2003;14:18-26
- Iskedjian M, Dupont C, Kanny G, et al. Economic evaluation of a 100% whey-based, partially hydrolyzed formula in the prevention of atopic dermatitis among French children. Curr Med Res Opin 2010;26:2607-26
- Halken S, Hansen K, Jacobsen H, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol 2000;11:149-61
- Nentwich I, Michkova E, Nevoral J, et al. Cow's milk-specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy 2001;56:1144-56
- Porch M, Shahane A, Leiva L, et al. Influence of breast milk, soy or two hydrolyzed formulas on the developent of allergic manifestations in infants at risk. Nutr Res 1998;18:1413-24
- von Berg A, Koletzko S, Filipiak-Pittroff B, et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three-year results of the German Infant Nutritional Intervention Study. J Allergy Clin Immunol 2007;119:718-25
- Willems R, Duchateau J, Magrez P, et al. Influence of hypoallergenic milk formula on the incidence of early allergic manifestations in infants predisposed to atopic disease. Ann Allergy 1993;71:147-50
- Statistics Denmark. Number of live births in Denmark in 2010. HISB8: total number of live births - summary vital statistics by new increases/stock and time. http://www.statbank.dk/statbank5a/default.asp?w=1280. Accessed April 26, 2011
- Benn C, Wohlfahrt J, Aaby P, et al. Breastfeeding and risk of atopic dermatitis, by parental history of allergy, during the first 18 months of life. Am J Epidemiol 2004;160:217-23
- Astma-Allergi Danmark. Reimbursement rate of infant formula for treatment and prevention in Denmark. http://dinhverdag.astma-allergi.dk/oekonomi/saerligetilskud/merudgiftertilboern/hvadkandusoegeom?doAsUserId=WQ99xGdlbJk%253D. Accessed April 26, 2011
- Organization for Economic Cooperation and Development ECD. Breastfeeding rates. Organization for Economic Cooperation and Development. Social Policy Division 2010; 1–3. http://www.oecd.org/dataoecd/30/56/43136964.pdf [Last accessed: December 23, 2011]
- Statistics Denmark. Mortality rates in Denmark. HISB8: life table (2 years tables) by age, sex and life table. http://www.statbank.dk/statbank5a/SelectVarVal/Define.asp?Maintable=HISB8&PLanguage=1. Accessed: April 26, 2011]
- Iskedjian M, De Serres G, Einarson TR et al. Economic impact of the intruduction of an accellular pertussis vaccine in Canada: a 6-year analysis. Vaccine 2010;28:714-23
- General practitioner fees in Denmark in 2010. Almen lægehjælp, takstmappe. Report no.: t.55.10.1. http://okportal.dk/. Accessed April 26, 2011
- Specialist fees in Denmark in 2010. Speciallægehjælp, takstmappe. Report no.: t.55.10.1. http://okportal.dk/. Accessed April 26, 2011
- Beattie PE, Lewis-Jones MS. A pilot study on the use of wet wraps in infants with moderate atopic eczema. Clin Exp Dermatol 2004;29:348-53
- Danish Medicines Agency. Cost of medications in Denmark. http://medicinpriser.dk/. Accessed April 26, 2011
- Danish Medicines Agency. Reimbursement rate for medications in Denmark. 2011. http://laegemiddelstyrelsen.dk/en/topics/statistics,-prices-and-reimbursement/reimbursement-of-medicines/reimbursement-thresholds.aspx. Accessed April 26, 2011
- Statistics Denmark. Labour force survey in Denmark in 2010. http://www.dst.dk/homeuk/Statistics/focus_on/focus_on_show.aspx?sci=1364. Accessed April 26, 2011
- International Labour Organization. Wages by economic activity in Denmark in 2007. Table 5A. http://laborsta.ilo.org. Accessed April 11, 2011
- International Labour Organization. Hours of work per week by economic activity in Denmark in 2008. Table B6. http://laborsta.ilo.org. Accessed April 11, 2011
- Drummond M, Sculpher M, Torrance G, et al. Methods for the economic evaluation of health care programmes. 3rd edn. Oxford, UK: Oxford University Press, 2006
- Alban A, Gyldmark M, Pedersen AV, et al. The Danish approach to standards for economic evaluation methodologies. PharmacoEconomics 1997;12:627-36