Abstract
Objectives:
The National Institute for Health and Clinical Excellence (NICE) health economic model for assessing the cost-effectiveness of celecoxib plus a proton pump inhibitor (PPI) compared to diclofenac plus PPI in the treatment of osteoarthritis has been updated using new adverse event (AE) risks from the CONDOR trial. In light of this new information, this study aimed to evaluate the incremental cost-effectiveness ratio (ICER) of celecoxib plus PPI compared to diclofenac plus PPI.
Methods:
NICE developed a health economic model as part of their 2008 multiple technology assessment of treatments for osteoarthritis. The model was adapted for this study to update the relative risks of adverse events, using data from the CONDOR trial.
Results:
Using the AE data from the CLASS trial alone, celecoxib plus PPI has an ICER of £9538 per QALY when compared to diclofenac plus PPI. When the AE data from CONDOR alone is used, this ICER decreases to £4773 per QALY. Using the pooled data from both trials, celecoxib plus PPI has an ICER of £9377 per QALY compared to diclofenac plus PPI.
Discussion:
The results suggest that when new AE risks are used, celecoxib plus PPI remains a cost-effective treatment for OA when compared to diclofenac plus PPI. However, this analysis is limited by the short time horizon, and additional AEs that have not been considered.
Introduction
Celecoxib (Celebrex; Pfizer, Kingston upon Thames, UK) is a COX-2 selective inhibitor, with treatment indications that include use in osteoarthritis (OA), as well as rheumatoid arthritis and ankylosing spondylitis. OA is one of the leading causes of pain and disability worldwide. The most common form of arthritis, OA can affect any joint, causing pain, functional limitation, and a reduction in quality-of-lifeCitation2. The pain resulting from OA is treated with either traditional, non-selective Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), or, more recently, with COX-2 selective inhibitors such as celecoxib.
In the UK the National Institute for Health and Clinical Excellence (NICE) produces guidance on public health, health technologies and clinical practiceCitation3. In 2008, NICE produced Clinical Guideline 59 ‘The care and management of osteoarthritis in adults’, recommending appropriate treatment and care for OA, including pharmacological managementCitation2. The clinical- and cost-effectiveness of current treatments were assessed and recommendations provided as to the most appropriate clinical pathways. To support this process, NICE developed a health economic model to assess the cost-effectiveness of COX-2 inhibitors and traditional NSAIDs in the treatment of OA from the perspective of the UK NHS. It was clear from the model that the addition of a proton pump inhibitor (PPI) was cost-effective; as such it was assumed that all NSAIDs and COX-2 inhibitors would be prescribed with a PPI. New data have recently been made available and in this paper the authors set out the impact of its inclusion in the estimation of the cost-effectiveness of celecoxib plus PPI using the NICE model.
The structure and development of the NICE model has been well describedCitation4. It drew upon data from three randomized controlled trials (RCTs): CLASSCitation5 (celecoxib, diclofenac, and ibuprofen), MEDALCitation6 (etoricoxib and diclofenac), and TARGETCitation7,Citation8 (lumiracoxib, naproxen and ibuprofen).
The NICE model analysed the cost-effectiveness of non-selective NSAIDs (diclofenac 100 mg/day, naproxen 750 mg/day, ibuprofen 1200 mg/day), and COX-2 inhibitors (celecoxib 200 mg/day, etoricoxib 60 mg/day), plus paracetamol 3000 mg/day. It was assumed in the model that all treatments were equally effective at controlling OA symptoms, but were differentiated by their individual gastrointestinal (GI) and cardiovascular (CV) risks. The model results demonstrated that treatment with celecoxib plus PPI was cost-effective compared to treatment with diclofenac plus PPI. Sensitivity analysis demonstrated that these results were not sensitive to either the assumed duration of treatment or to the baseline risk of GI events in the population and were relatively insensitive to the baseline risk of CV eventsCitation9. There were, however, considerable uncertainties over the relative rates of adverse events (AEs) from the RCTs.
In 2010, the Lancet published results of CONDOR; a 6-month, double blind, randomized trial comparing treatment with celecoxib and diclofenac plus PPI in patients with osteoarthritis and rheumatoid arthritisCitation10. The aim of CONDOR was to investigate the GI risk of treatment with a NSAID (diclofenac) plus PPI vs a COX-2 selective NSAID (celecoxib) alone, across the entire (upper and lower) gastrointestinal tract. The primary study end-point was a composite of clinically significant upper or lower GI events, and data on cardiovascular events were also reported. CONDOR reported a statistically significant reduction in GI events for celecoxib vs diclofenac plus PPI and non-significant differences in CV events, for the parameters used by NICE.
This study had four aims:
To perform a literature search to assess whether any additional relevant data had been published since the 2008 NICE guidance.
To update the NICE OA model with the results of any identified studies and the CONDOR trial.
To update the modelled cost data with 2011 values.
To enable an updated assessment of the cost-effectiveness of treatment with celecoxib plus PPI compared to diclofenac plus PPI from a UK perspective.
Methods
A literature search was performed based on the search criteria detailed in the CG59 NICE guidance, to identify all relevant trials which could be used to update the model. 30 papers were found, of which only the CONDOR trial publication was considered to be for the treatment of osteoarthritis in the appropriate patient population and include reports of adverse events. Only the CONDOR trial was, therefore, used to inform the update of the model.
The results of the NICE modelling work were mainly driven by AE data from a 6-month analysis from CLASS, a double-blind, randomized controlled trialCitation11. The objective of CLASS was to determine whether celecoxib was associated with a lower incidence of significant upper GI toxic effects and other adverse effects compared with conventional NSAIDs. CLASS demonstrated that celecoxib, at dosages greater than those licenced in the UK, was associated with a lower incidence of symptomatic ulcers and ulcer complications combined, as well as other clinically important toxic effects, compared with NSAIDs at standard dosages.
The primary results from the CONDOR trial were analysed to fit with the classifications given by NICE to the adverse events recorded in CLASS and, thus, were defined as GI symptoms leading to withdrawal, symptomatic ulcers, complicated GI events, MI, stroke, or heart failureCitation10. The CONDOR trial also included a number of non-symptomatic GI end-points that were believed to be surrogate markers for GI damage throughout the entire (upper and lower) GI tract. These surrogate markers included blood loss as measured by a haemoglobin drop of >2 g/dl from a defined site, or presumed small bowel blood loss. Patients were removed from the trial if they met a pre-defined end-point. As the modelling approach developed by NICE did not consider these surrogate end-points, they have been excluded from this analysis. More patients in the diclofenac plus PPI arm than the celecoxib arm had a significant decrease in haemoglobin (77 vs 15 patients). These patients discontinued from the trial, but may have later developed more serious GI events. The true burden of the adverse events that would be seen in clinical practice is therefore potentially under-estimated. This under-estimation of GI adverse events may be greater in the diclofenac plus PPI arm, since more patients were removed from the trial prior to the potential development of GI AEs in the model. Therefore, the relative risks of each GI AE used in the model for celecoxib compared to diclofenac from CONDOR may be over-estimated, leading to a conservative estimate for the cost-effectiveness of celecoxib compared to diclofenac plus PPI.
Adverse events associated with NSAID and COX-2 inhibitor use are assumed to be dose-related in the NICE model. A key concern in the original NICE modelling work was that the dose levels used in the supporting trials were higher than those used in clinical practice. Doses in these trials were generally high for NSAIDs (but within licensed dose range), whilst they were far above licensed levels for some of the COX-2 inhibitors consideredCitation9 (TARGET: lumiracoxib 400 mg, naproxen 1000 mg, ibuprofen 2400 mg; CLASS: celecoxib 800 mg, diclofenac 150 mg, ibuprofen 2400 mg; MEDAL: etoricoxib 73 mg, diclofenac 150 mg; EDGE: etoricoxib 90 mg, diclofenac 150 mg). The observed doses were therefore adjusted to Average Daily Quantities (ADQs), a measure of prescribing volume based upon prescribing behaviour in EnglandCitation12. The ADQ’s for celecoxib and diclofenac were reported to be 200 mg and 100 mg, respectively. CONDOR used the maximum licensed daily doses of celecoxib (400mg per day), and diclofenac (150mg per day). The NICE model also makes an adjustment for the population baseline risk of GI events. The AE rates reported in were therefore adjusted to reflect the ADQ doses for celecoxib and diclofenac using the same methodology described by NICECitation10, where it was assumed that the adverse event rate reduction has a relative risk of half the dose reduction. For example, if the dose of each drug was reduced by 50%, then the adverse event rate reduction for each drug would be 25%. The adjusted numbers of events are reported in . Dose-adjusted adverse event rates were then converted to relative risks as required by the model.
Table 1. Numbers of adverse events observed in CONDOR.
The main issue in adapting the NICE model to assess the results from CONDOR is that the trial compared celecoxib to diclofenac plus PPI, whereas the NICE model is structured to assess the CLASS trial which compared celecoxib to diclofenac alone. The NICE model estimates the effect of PPIs by applying relative risks to adjust the event rates for GI AEs when a PPI is addedCitation13. The relative risks of AEs with a PPI, when added to NSAID, are 0.43 for GI symptoms, 0.37 for symptomatic ulcer, and 0.46 for complicated GI event. These relative risks were used to adjust the event rates for GI AEs for diclofenac plus PPI in CONDOR, to estimate the event rates if diclofenac were used without a PPI (). The relative risks for AEs from CLASS and adjusted CONDOR rates were then pooled and used in the base-case. Meta analysis, assuming a fixed effects model, was used to combine the CONDOR data with the existing CLASS data to gain a pooled relative risk for each event (). Fixed effects meta-analysis assumes that the true effect is the same in all studies, unlike random effects meta-analysis which allows the true effect to vary across studies. Although the random effects assumption is typically considered to be more realistic, a large number of studies are required to obtain a reliable estimate of the distribution of possible trials. Since only two studies are included in this analysis a fixed effects model was used.
Table 2. Relative risks of adverse events based on estimated dose adjusted treatment effects.a
When CONDOR is not adjusted to remove the effect of PPI from diclofenac, but the dosage of celecoxib and diclofenac is adjusted, the incidence of GI symptoms with celecoxib alone is similar to that with diclofenac plus PPI. The risk of symptomatic ulcers and complicated GI events is lower for celecoxib alone than for diclofenac plus PPI. This challenges the view that celecoxib necessarily needs to be prescribed with a PPI, so analysis has been performed to assess the cost-effectiveness of celecoxib without PPI compared to diclofenac plus PPI.
A sensitivity analysis to check the internal validity of the analysis was performed in which the diclofenac AE rates from the CLASS trial adjusted to estimate event rate for diclofenac plus PPI, which was pooled with the CONDOR event rates for diclofenac plus PPI.
All NHS costs were updated to the recent 2010/11 UK NHS reference costsCitation14 (). Drug costs were updated to 2011 UK prices using the British National FormularyCitation15. Costs for the management of CV events were inflated to 2011 costs, using the PSSRU Hospital & Community Health Services Pay & Prices indexCitation16.
Table 3. Estimated costs for the management of adverse events.a
A 3-month time horizon was used in the base case. For time horizons over 1 year, costs and benefits were discounted at an annual rate of 3.5%, in line with NICE guidanceCitation18.
Sensitivity analyses were performed which included varying the treatment period from 3 months to 24 months, the age of patients from 55 to 65, removing CV benefits and an analysis which examined the effect of using only the CONDOR results. A probabilistic sensitivity analysis (PSA) was also performed, sampling 1000 parameter sets from the distributions around parameters in the model to generate a probability distribution of calculated cost-effectiveness ratios, reflecting the combined uncertainty in the underlying parameters of the model. The 95% confidence intervals for the event relative risks given in were used in the PSA. Distributions for all other parameters were the same as in the original NICE analysis.
Results
A deterministic approach was used to assess the cost effectiveness at the mean parameter values results using the pooled CLASS and CONDOR AE results. The analysis demonstrated that, over a patient’s lifetime, treatment with celecoxib plus PPI when compared to treatment with diclofenac plus PPI was associated, with an increase in discounted costs of £56 per patient, and a gain in Quality Adjusted Life Years (QALYs) of 0.006 per patient, resulting in an ICER of £9377. When the AE data from CONDOR alone is used, the QALY gain is increased to 0.010, and the marginal cost is £49 per patient, resulting in an ICER of £4773. When AE data from CLASS was adjusted to obtain an event rate for diclofenac plus PPI and pooled with the CONDOR data, the resulting ICER was £8507. If the AE data is used from CONDOR alone, and with no dose adjustment, such that celecoxib and diclofenac are both used at their maximum daily dose (which may be used in clinical practice), celecoxib compared to diclofenac plus PPI is associated with an increase in costs of £48 and an increase in QALYs of 0.004, resulting in an ICER of £12,736. In the same scenario, celecoxib plus PPI compared to diclofenac plus PPI is associated with an increase in costs of £44 and an increase in QALYs of 0.013, resulting in an ICER of £3340. However, these results are heavily influenced by the high dosing of both celecoxib and diclofenac. Results including ICERs are shown in .
Table 4. Costs, QALYs and ICERs for the trial data.
Sensitivity analysis varying the treatment period from 3 months to 24 months had minimal impact on the ICER. Increasing the population age from 55 to 65 lowered the ICER, however equalizing CV relative risks had an effect of increasing the ICER ().
Table 5. Results of sensitivity analyses.
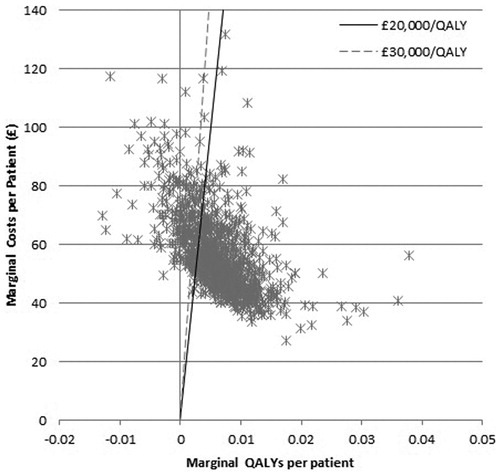
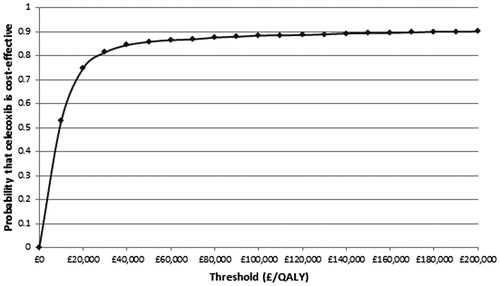
PSA was performed and the results plotted on a cost-effectiveness plane with lines representing thresholds of £20,000 and £30,000. (). Points to the right of a threshold line indicated that celecoxib plus PPI was cost-effective compared to diclofenac plus PPI at that threshold. Points to the left of the vertical line indicated that celecoxib was dominated by diclofenac. Points between the vertical line and a threshold line indicated that celecoxib was not cost-effective compared to diclofenac. It can be seen that many of the points were clustered around the centre of the thresholds, with some results present on either side. The majority of samplings showed celecoxib plus PPI to be cost-effective and celecoxib plus PPI was dominated by diclofenac plus PPI on only 8% of occasions. These results were then plotted on a cost effectiveness acceptability curve (), which showed that celecoxib plus PPI is the cost-effective option on 75% of occasions at a willingness to pay threshold of £20,000 per QALY, and on 83% of occasions at a threshold of £30,000 per QALY.
Discussion
In the original NICE model, the differences in total costs and total QALY gain between celecoxib plus PPI and diclofenac plus PPI were very smallCitation4. These small differences are unchanged by the update to the model performed here, to include data from the CONDOR trial. Using the data from CONDOR alone decreased the ICER comparing celecoxib plus PPI with diclofenac plus PPI reported in the original NICE model. Pooling the data from CONDOR and CLASS reported an ICER that was below a £20,000 threshold and, therefore, treatment with celecoxib can be considered a cost-effective treatment option for patients with OA.
The results of the CONDOR trial demonstrate that treatment with celecoxib reduces the risk of upper and lower GI events, compared with treatment with diclofenac and PPI, and has no statistically significant impact on CV events. The celecoxib risk of GI symptoms in CONDOR was lower than the baseline population risk of GI symptoms utilized in the NICE model and, therefore, when adjusting for the baseline risk of event, along with the adjustment made for the dose, the risk of GI symptoms therefore increases, compared to the value reported in CONDOR (). The same effect is also seen for the rate of GI symptoms reported for diclofenac plus PPI in CONDOR. The relative risks of ulcers and complicated GI events remain much lower for celecoxib than diclofenac plus PPI, which are much more costly and have a greater utility decrement than GI symptoms, which drives the advantage for celecoxib. These risks have been adjusted using a fairly crude methodology which assumed a simple, proportional linear relationship between dose and risk of AE. It is suggested that this relationship is more complex and, were a study investigating this performed, it may produce adjustments that are more favourable to celecoxib. When the CONDOR and CLASS results were pooled, however, then celecoxib is shown to reduce the risk of all GI events compared to diclofenac.
The results from CONDOR provide evidence that when celecoxib (without a PPI) and diclofenac plus a PPI are given at maximum NSAID dosages, celecoxib alone is cost-effective when compared to diclofenac plus a PPI. This result questions whether the addition of a PPI is necessary to treatment celecoxib. However, when using the CONDOR unadjusted results and treatment with celecoxib plus a PPI is compared to celecoxib alone, there is a decrease in costs of £5, and an increase in QALY gain of 0.01. As such, the addition of a PPI to celecoxib dominates treatment with celecoxib alone. This is consistent with the NICE modelling results, and is supported by clinical evidenceCitation19.
It has been noted elsewhereCitation4 that the main driver of cost-effectiveness in the NICE model is the risk of stroke estimated from CLASS, which reports a low relative risk favouring celecoxib (after dose adjustment). These risks are based on very small numbers of events from a trial that was not powered to demonstrate a difference in this event. The instability of these data is demonstrated by the inclusion of data from CONDOR, to the CLASS data, in which only three stroke events are added to each arm, but then increases the relative risk of stroke from 0.38 for celecoxib vs diclofenac to a value of 0.51 in the pooled analysis. The validity of NICE using ‘anecdotal CV’ data from trials that were not designed and under-powered to study such outcomes has been questionedCitation20. Given that there are currently no statistically significant data differentiating the effect of treatment with celecoxib and diclofenac on CV events, probably the most appropriate sensitivity analysis is that examining the effect of only applying the pooled efficacy data to GI events and assuming a relative risk of 1 for CV events. In this scenario celecoxib remains the most cost-effective option with an ICER well below a £20,000 threshold.
The main limitations of this analysis are the short time horizon used, and the dose-adjustment. The analysis of CLASS and CONDOR was for only a 6-month period, whereas to fully assess the impact of treatment of OA requires modelling over a longer time horizon. The model and cost-effectiveness analysis use a 3-month time horizon in the base case and so does not extrapolate beyond the trial horizon. As discussed earlier, the dose adjustment method used in the NICE modelling may not be appropriate in this update, whereby the risks of some AEs were lower in patients receiving treatment than in the general population. The results from the CONDOR trial without using the dose adjustment are influenced by the much higher drug costs for both celecoxib and diclofenac compared to normal usage. These, however, are limitations of the NICE model which has been accepted for guidelines development in the UK, and is not a limitation of this study.
The non-symptomatic end-points reported in CONDOR have not been considered in this analysis. The approach for the inclusion of the CONDOR results was consistent with the NICE modelling, but presents conservative results for celecoxib.
Conclusion
The inclusion of the CONDOR results in the NICE model ensured that all available trial data were used and thus the updated model was the most robust analysis available to assess the cost-effectiveness of OA treatments. The inclusion of a PPI in the diclofenac arm of CONDOR caused complications in pooling the trials results, but the small differences in the results from the two pooling methods demonstrate internal validity in the results.
This study provides strong support that treatment with celecoxib is cost-effective when compared to diclofenac plus a PPI using data from CONDOR alone, CLASS alone, and both methods of pooling the results. Importantly the update of the NICE model does not alter the conclusions reached in the original NICE guidance, and celecoxib remains a cost-effective treatment for the symptomatic management of OA in the UK.
The results from the CONDOR trial also demonstrate that, at maximum dosage, celecoxib can be considered a cost-effective treatment for the symptomatic management of OA in the UK when compared to diclofenac plus PPI.
Transparency
Declaration of funding
This paper was funded by Pfizer, Kingston upon Thames, UK. The publication of study results was not contingent on the sponsor’s approval or censorship of the manuscript.
Declaration of financial/other relationships
N.B. and B.W. have disclosed that they are employees of BresMed Health Solutions, a company that received funding to prepare this paper. R.A. has disclosed that he is an associate director of BresMed Health Solutions. None of the authors received individual payments for their involvement in the model and manuscript development.
Acknowledgements
The authors would like to thank Pfizer for giving their full permission to use their clinical data. The authors would also like to thank the University of Sheffield for giving their full permission to use their economic model.
References
- Celebrex Summary of Product Characteristics. The electronic Medicines Compendium (eMC). Surrey, UK: Datapharm Communications Ltd, 2011. http://www.medicines.org.uk/EMC/medicine/14534/SPC/Celebrex+100mg+%26+200mg+Capsules/. Accessed July 12, 2011
- National Collaborating Centre for Chronic Conditions. Osteoarthritis: national clinical guidelines for care and management in adults. London, UK: Royal College of Physicians, 2008. http://www.nice.org.uk/nicemedia/live/11926/39720/39720.pdf. Accessed January 27, 2011
- National Institute for Health and Clinical Excellence (NICE). 2011. http://www.nice.com. Accessed January 27, 2011
- Latimer N, Lord J, Grant RL, et al. National Institute for Health and Clinical Excellence Osteoarthritis Guideline Development Group. Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ 2009;339:b2538
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247–55
- Cannon CP, Curtis SP, FitzGerald GA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Longterm (MEDAL) programme: a randomised comparison. Lancet 2006;368:1771–81
- Kirshner H, Ruland S, Verheugt FW, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004;364:675–84
- Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364:665–74
- National Collaborating Centre for Chronic Conditions. Appendix D, Osteoarthritis: national clinical guidelines for care and management in adults. London: Royal College of Physicians, 2008. http://www.rcplondon.ac.uk/. Accessed January 27, 2011
- Chan FKL, Lanas A, Scheiman J, et al. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 2010;376:173–79
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247–55
- Average Daily Quantities. Leeds, UK: The Information Centre for Health and Social Care, 2011. Available at: http://www.ic.nhs.uk/services/prescribing-support-unit-psu/using-the-service/reference/measures/volume-measures/average-daily-quantities-adq. Accessed July 12, 2011
- Latimer N, Lord J, O’Mahony, et al. Value of information in the osteoarthritis setting: cost effectiveness of COX-2 selective inhibitors, traditional NSAIDs and proton pump inhibitors. Pharmacoeconomics 2011;29:225–37
- NHS Reference Costs 2009–2010. London, UK: Department of Health, 2011. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123459. Accessed April 1, 2011
- British National Formulary (BNF), 2010. http://www.bnf.org. Accessed April 1, 2011
- Curtis L. Unit costs of health and social care. Kent, UK: Personal Social Services Research Unit (PSSRU), 2010. http://www.pssru.ac.uk/uc/uc2010contents.htm. Accessed April 1, 2011
- Hypertension: management of hypertension in adults in primary care: partial update. London, UK: National Institute for Health and Clinical Excellence (NICE), 2006. Available at: www.nice.org.uk/nicemedia/pdf/CG034NICEguideline.pdf. Accessed April 1, 2011
- Guide to the methods of technology appraisal. London, UK: National Institute for Health and Clinical Excellence (NICE), 2008. Available at: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf. Accessed July 12, 2011
- Chan FKL, Wong VWS, Suen BY, et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet 2007;369:1621–26
- Ray WA. Cardiovascular safety of NSAIDs. BMJ 2011;342:c6618