Abstract
Objective:
To evaluate the cost-effectiveness of pregabalin in the treatment of fibromyalgia in a US patient population.
Methods:
A decision-analytic model was developed comparing pregabalin 150 mg twice a day (BID) and pregabalin 225 mg BID to placebo, duloxetine, gabapentin, tramadol, milnacipran, and amitriptyline in patients with severe fibromyalgia (Fibromyalgia Impact Questionnaire score >59; pain score >6.5). The model estimated response rates for all treatments at 12 weeks based on three randomized trials with pregabalin and a systematic review of published randomized controlled trials. Response was categorized as ≥30% improvement in baseline pain score plus global impression of change rating of much improved or very much improved. After 12 weeks of treatment, responders to treatment entered a treatment Markov model in which response was maintained, lost, or treatment discontinued. The cost-effectiveness end-points were cost per responder at 12 weeks and 1 year. Resource use was estimated from published studies and costs were estimated from the societal perspective.
Results:
Over 12 weeks, total cost per patient was $229 higher with pregabalin 150 mg BID than placebo, whereas pregabalin 225 mg BID was $866 less costly than placebo. At 1 year, pregabalin was cost saving and more effective than placebo, duloxetine, tramadol, milnacipran, and gabapentin. Compared with amitriptyline, pregabalin was not cost-effective at both dosages, although when excluding old and methodologically weak studies of clinical effectiveness of amitriptyline, pregabalin 225 mg BID became cost saving and pregabalin 150 mg BID was cost-effective.
Limitations:
Comparisons between pregabalin and other active agents are based on indirect comparisons, not head-to-head trials, and so should be interpreted with caution. Limitations for comparators include an inability to access sub-group data, inconsistency of response definitions, inclusion of older trials, and absence of long-term studies.
Conclusions:
This model found pregabalin to be cost-effective in treating patients with severe fibromyalgia.
Introduction
Fibromyalgia (FM) is a chronic condition typically characterized by widespread pain and stiffness in the soft tissues of the body (muscle, tendons, and ligaments) and tenderness at muscle insertion sites. In a recent focus group, patients with FM expressed living with constant pain that is hard to attribute to a specific place: ‘you hurt all over’Citation1. Other typical FM symptoms include depression, disturbed and unsatisfying sleep, fatigue, cognitive dysfunction, morning stiffness, anxiety, headaches, and an irritable bowel or bladderCitation1. FM is defined, according to the 1990 American College of Rheumatology (ACR) criteria, as chronic widespread pain lasting ≥3 months, and pain in at least 11 of 18 specified tender point sites throughout the body upon digital palpationCitation2. In May 2010, the ACR published new FM diagnostic criteria which exclude tender points and utilize a symptom severity scale, such that a FM diagnosis is based on three criteria: (1) widespread pain and symptom severity above certain cut-off scores; (2) symptoms have been present at a similar level for at least 3 months; and (3) the patient does not have a disorder that would otherwise explain the painCitation3.
The prevalence of FM in the US has been estimated to be 2% (95% confidence interval, 1.4–2.7)Citation4 and age-adjusted incidence in the US has been reported to be 6.88 cases per 1000 person-years for males and 11.28 cases per 1000 person-years for femalesCitation5.
The impact of FM on patients’ daily lives and relationships is profoundCitation1. Patients with FM perform worse on a number of health status domains including physical function, role limitations due to physical and emotional problems, bodily pain, vitality, and general health compared with the general population and patients with rheumatoid arthritis, osteoarthritis, lupus, and myofascial painCitation6. Higher self-reported FM severity was associated with higher pain and sleep disturbance, impact on quality-of-life, and medication useCitation7,Citation8.
In addition, FM causes a substantial healthcare burden. An analysis of a large US medical claims database, PharmetricsCitation9, found that average total healthcare costs over 12 months were ∼3-times higher among patients with FM compared with patients without FM ($9573 [SD $20,135] vs $3291 [SD $13,643], respectively; p < 0.001). A study by White et al.Citation10 found similar total annual healthcare costs to employers for patients with FM (direct medical costs $7286 and pharmacy costs $1630) and osteoarthritis (direct medical costs $8325 and pharmacy costs $1341). However, the annual costs due to lost work days for patients with FM were significantly higher than for patients with osteoarthritis ($2913 vs $2537, respectively; p = 0.0001)Citation10.
In June 2007, Lyrica (pregabalin) became the first US Food and Drug Administration (FDA)-approved drug for managing FM. Two other drugs were later approved for the treatment of FM, Cymbalta (duloxetine hydrochloride) in June 2008 and Savella (milnacipran) in 2009. (Lyrica is a registered trademark of Pfizer Inc, New York, NY, Cymbalta is a registered trademark of Eli Lilly and Company, Indianapolis, IN, and Savella is a registered trademark of Forest Laboratories, Inc., New York, NY.) Recent meta-analyses comparing these medications on symptom reduction (including pain, fatigue, sleep disturbance, depressed mood, reduced health-related quality-of-life) and safety demonstrated that on most efficacy end-points, each drug was superior to placebo, but exceptions occurred for duloxetine on fatigue, milnacipran on sleep disturbance and pregabalin on depressed moodCitation11,Citation12. While not indicated for FM, older agents including amitriptyline, gabapentin, and tramadol have been recommended for FM management in treatment guidelinesCitation13,Citation14. Meta-analyses have been conducted on gabapentin and amitriptyline, but the results are limited by the number and quality of clinical studiesCitation15–17.
Pregabalin is a novel α2δ ligand that belongs to the anti-convulsant class of drugs. Pregabalin acts by binding to the α2δ sub-unit of voltage-gated presynaptic calcium channels, which results in reduction of calcium flow through the channels. This decreased calcium flux subsequently inhibits presynaptic release of neurotransmitters, including glutamate and substance P, which have been implicated in the pathogenesis of abnormal pain processing seen in FMCitation18. The efficacy and safety of pregabalin in FM has been demonstrated in five placebo-controlled, randomized clinical trialsCitation19–23 and a recent Cochrane reviewCitation24.
The present study aimed to investigate the cost-effectiveness of pregabalin in the treatment of FM compared with placebo, duloxetine, milnacipran, gabapentin, tramadol, and amitriptyline from the US perspective.
Patients and methods
Patients
The economic analysis considered patient profiles based on participants in the pregabalin randomized FM trial program. Three studiesCitation19,Citation22,Citation23 enrolled adults from North America, Europe, Asia, and Australia meeting the ACR criteria for FMCitation4. Participants were required to have an average pain score of ≥4 on an 11-point numeric rating scale (NRS; 0 = no pain to 10 = worst possible pain) during the baseline assessment period. Patients with FM with evidence of inflammatory or rheumatic diseases, other pain conditions, or ongoing severe psychiatric or medical illness that could interfere with assessment were excluded from these studies. Further details on the design of each study are described elsewhereCitation19,Citation22,Citation23. This cost-effectiveness analysis used data for individuals who met the criteria for study entry and had severe FM (visual analog scale pain score >6.5 and Fibromyalgia Impact Questionnaire [FIQ] score >59 at baseline)Citation25,Citation26.
Model structure and comparators
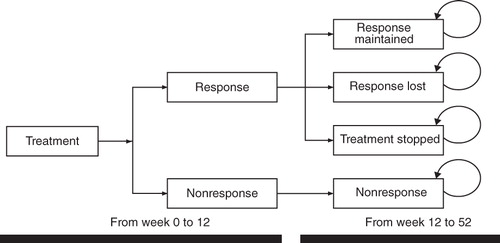
A cost-effectiveness model to compare costs and number of responders with pregabalin compared with current treatment alternatives for FM was built. The model () was based on a decision tree, with treatment nodes corresponding to treatment response outcomes for a cohort of people during an initial 12-week period, after which response to treatment was assessed. Costs were assigned to respective healthcare resource use and outcomes. At 12 weeks, upon achieving a response or not, patients were entered into the second phase of the model that evaluated FM costs and outcomes over the medium term. The second phase was a Markov model built using Microsoft Excel, which included three possible outcomes: (1) responders maintain a therapeutic response; (2) patients remain on treatment but lose response; or (3) patients discontinue treatment. Non-responders remained such after week 12. In the base-case, the model time frame was 1 year, with Markov cycles of 28 days. lists comparators included in the model, which reflects licensed dosages or dosages used in clinical trials.
Table 1. Comparators included in the model.
Effectiveness data
Efficacy estimates for pregabalin and placebo in the management of FM during the initial 12 weeks of treatment were derived from the data of three large multi-center, double-blind, clinical trialsCitation19,Citation22,Citation23, in which patients were randomized to one of the two indicated dosages of pregabalin (150 mg twice a day [BID] or 225 mg BID) or placebo (a higher dose [300 mg BID] was evaluated in these clinical studies, but was not included in the present analysis since it exceeds the recommended therapeutic dose range for treating FM). Two further studies of pregabalinCitation20,Citation21 were not used in the analysis because data from these trials were of different design and not compatible with the model structure.
Data from trials were used to inform the response rate with pregabalin treatment in the model. The proportion of responders in the trials, a supplemental measure of the primary efficacy end-point, was defined as a ≥30% reduction in mean daily pain diary score between baseline and week 12. A co-primary study end-point was the Patient Global Impression of Change (PGIC) scoreCitation27. The PGIC is based on the validated Clinical Global Impression of Change scale and evaluates patient self-reported change after treatment on a 7-point Likert scale that ranges from 1 (very much improved) to 7 (very much worse). A pre-defined secondary end-point measured improvement of global FM symptoms after treatment using the FIQCitation25,Citation26. The FIQ is a 21-item self-report questionnaire designed to capture the global impact of FM on patients’ lives by evaluating difficulty performing large muscle tasks, number of days patients missed work or ‘felt good’, difficulty of FM interference with work, and severity of symptoms of pain, fatigue, non-restorative sleep, stiffness, anxiety, and depression over the past week. The global FIQ score varies from 0 (no impact) to 100 (worst possible impact).
In the model, response was considered an improvement of ≥30% in the pain NRS plus a score of much improved or very much improved on the PGIC. In the base-case, the model focused on a sub-group of patients with severe FM. Baseline pain score and global severity of FM symptoms as measured by the FIQ were used as proxy measures for FM disease severity. Bennett et al.Citation26 recently demonstrated that FIQ total scores of <39 corresponded to mild symptom severity, scores ≥39 and ≤59 to moderate symptom severity, and scores of >59 to severe symptom severity. Therefore, patients with a pain score of >6.5 and a FIQ score of >59 were defined as having severe FM at baseline.
Therapeutic response rates of other comparators were obtained from a systematic review and indirect comparison of the effectiveness of each treatmentCitation12. Briefly, a systematic search for randomized controlled trials of amitriptyline, duloxetine, tramadol, gabapentin, and milnacipran was conducted to assess the efficacy of these drugs in patients with FM, fibrositis, myofascial pain, or muscular rheumatism. The databases searched were MEDLINE (1950 to August 13, 2009), EMBASE (1980 to August 13, 2009), and the Cochrane Library (1995 to August 13, 2009) using the Ovid access platform. From the 679 hits initially retrieved, 23Citation19–22,Citation28–46 met the inclusion criteria for the review parameters in this cost-effectiveness study. In addition, the data from a recent trial of pregabalin were availableCitation23. Methods and results of the systematic review are available on request from the authors.
A wide range of clinical outcome measures have been utilized in published FM treatment trials to quantify therapeutic responseCitation19–22,Citation28–33,Citation35–41,Citation43–46. For modeling purposes, it was not possible to identify a single disease measure used consistently for all comparators. Therefore, the analyses only included studies that reported the proportion of patients who had responded to treatment compared with a placebo armCitation19,Citation22,Citation23,Citation28–30,Citation33,Citation35–39,Citation41,Citation43–46 to allow indirect comparisons between active treatments. These studies are summarized in . Relative rates of response for each treatment compared with placebo were calculated and pooled using meta-analytic methods implemented in Stata 9.0. shows the results of the meta-analysis and efficacy of pregabalin using different definitions of response. The probability of losing treatment response and of discontinuing treatment were estimated from participants in three randomized, placebo-controlled pregabalin FM trialsCitation19,Citation22,Citation23 limited to individuals with severe FM who lost response during the course of the studies (12 in 215).
Table 2. Studies of effectiveness of treatment for FM, including at least one active treatment compared with placebo.
Table 3. Pooled relative risk of response compared with placebo, active treatments.
The long-term likelihood of maintaining a therapeutic response was taken from a long-term safety study by Florian et al.Citation47, a 1-year open-label extension of the double-blind randomized pregabalin FM treatment trial by Arnold et al.Citation19 In this study, a responder was defined as a patient whose mean pain score had decreased by ≥30% from baseline. The total dropout rate at 12 months of 36% (78/215) from this study was used to calculate the probability of discontinuing treatment in the post 12-week Markov phaseCitation47. This response loss and dropout rate were applied to placebo and all other comparators in the Markov phase of the model.
In the systematic review, no studies that reported loss of response over time with duloxetine, gabapentin, tramadol, or amitriptyline were found. One 60-week safety and efficacy study of duloxetine in patients with FM provided information on long-term dropout rates from duloxetine over 52 weeksCitation48. However, a probability for loss of response could not be derived from this publication, and these data were only used in the sensitivity analysis. Long-term withdrawal data were not available for the other comparators and, consequently, the same long-term transition probabilities for pregabalin were applied to all arms of the model. All transition probabilities applied in the Markov model were obtained from event rates applying the formula:
where p is the transition probability, r is the rate observed in the study, t is the duration of the model cycle, and z is the total period of observation in the original study from which the rate was takenCitation49.
Resource use, unit costs, and health utility data
Daily drug costs, utilities, and annual healthcare and societal costs applied in the model are shown in . Therefore, drug costs were based on effective drug consumption and reimbursement data to reflect the true ‘opportunity costs’Citation49 of the treatments considered in the model. Treatment costs for each comparator and strength were calculated based on Medicaid drug utilization dataCitation50 using total volumes and reimbursement data () obtained by matching National Drug Codes using labeler, product, and packaging codes for each drugCitation51. Unit costs per dose were calculated using specific National Drug Codes for branded products and the weighted average of costs and volumes for generics.
Table 4. Costs, annual resource use, and utility estimates for the model.
Resource use other than drug costs was obtained from a cross-sectional observational study of the burden of FM illness in the USCitation52. In this study, the FIQ was administered to patients with FM and data were collected on resource use and days work missed. Costing algorithms were developed to estimate healthcare direct costs from the payer perspective from health-related utilization data priced at Medicare payment rates. For indirect costs, costing algorithms were developed to estimate patient and caregiver lost productivity. Generalized linear regression models with gamma distributions were used to estimate the relationship between FIQ score and annualized direct and indirect costs.
Resource use data were not available from the pregabalin clinical trialsCitation19,Citation22,Citation23. Direct and indirect costs were estimated for each patient using that patient’s FIQ score and the costing algorithms derived from Goldenberg et al.Citation52 Costs over the initial 12-week period assumed a linear change in FIQ over the 12-week period. Costs after week 12 were assumed to be maintained in patients who remained in response but to increase to the average level estimated for non-responders in patients where response was lost.
Sensitivity analysis
Univariate and probabilistic sensitivity analyses were performed to explore the robustness of results, to identify model drivers, and to test model assumptions. A sub-group sensitivity analysis was conducted to determine whether baseline characteristics were associated with better cost-effectiveness.
Results
The base-case analysis investigated the cost-effectiveness of pregabalin in patients with severe FM (FIQ score >59 and pain diary score >6.5) at baseline compared with placebo. The proportion of patients with severe FM at baseline was 59%, 60%, and 57% for placebo, pregabalin 150 mg BID, and pregabalin 225 mg BID, respectively. The end-point definition of response at week 12 was a ≥30% improvement in pain diary score and improvement rating of 1 (very much improved) or 2 (much improved) on the PGIC. Patient-level data from 682 patients were eligible for inclusion in this analysis.
At week 12, 23% of patients treated with placebo had a ≥30% improvement in pain compared with 39% of patients receiving pregabalin 150 mg BID and 45% of patients receiving pregabalin 225 mg BIDCitation19,Citation22,Citation23. Twenty-five per cent of patients in the placebo arm scored either a 1 or 2 on the PGIC compared with 39% and 43% for pregabalin 150 mg BID and 225 mg BID at week 12, respectively. The response rate at week 12 was 16% for placebo, 32% for pregabalin 150 mg BID, and 33% for pregabalin 225 mg BID. The proportion that reported severe FM at the end of the treatment period was 41% (placebo), 36% (pregabalin 150 mg BID), and 31% (pregabalin 225 mg BID). Efficacy was also assessed as proportion of days in response, which over 12 weeks of treatment resulted in an incremental response of 14 days for both pregabalin doses relative to placebo.
reports incremental cost and outcomes. After the first 12 weeks of treatment, direct costs of pregabalin were higher than placebo. When indirect costs were taken into account, pregabalin 225 mg BID was cost-saving. In the long-term analysis, pregabalin was less costly than placebo both for direct and indirect costs. Pregabalin generated about twice as many days of response than placebo. Consequently, pregabalin was cost-saving.
Table 5. Base-case results.
Further analyses compared pregabalin with duloxetine, milnacipran, amitriptyline, gabapentin, and tramadol (). Pregabalin was cost-saving compared with duloxetine, gabapentin, milnacipran, and tramadol, but was not cost-effective compared with amitriptyline.
Table 6. Pregabalin vs comparators in patients with severe fibromyalgia and severe pain over 1 year.
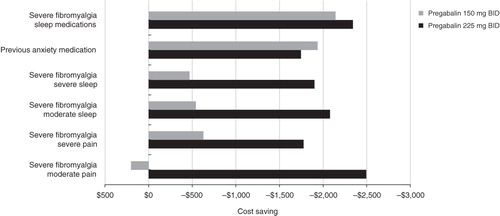
To identify patient sub-groups with higher cost-effectiveness of pregabalin relative to placebo, an analysis using baseline patient characteristics was conducted. Patients with severe FM, use of sleep or anxiety medications, or sleep problems at baseline had an incremental cost-saving effect above the overall patient population (). Sleep problems were defined as moderate (3.5–6.5) or severe (>6.5) based on baseline scores on the 11-point (0–10) NRS scale that evaluated rate of pain interference with sleep.
Figure 2. Incremental cost-effectiveness of pregabalin vs placebo in patient sub-groups. BID, twice a day.
Sensitivity analysis
A series of sensitivity analyses were conducted to assess the sensitivity of the model to changes in parameter estimates and model assumptions. The model was robust to most parameter changes, maintaining that pregabalin was less costly and more effective at both dosages ().
Table 7. Results of the one-way sensitivity analysis on key inputs: pregabalin 150 mg BID vs placebo at 1 year.
Pregabalin 150 mg BID and 225 mg BID were not cost-effective compared with amitriptyline in the base-case. In the initial meta-analysis, the relative risk of response for amitriptyline was 2.18 compared with placebo. However, the initial meta-analysis reported significant heterogeneity (). Two amitriptyline studies with low sample sizes (<50) and unclear methods of blinding likely accounted for the heterogeneityCitation37,Citation39. A sensitivity analysis was carried out to assess the impact of excluding these two studies. The revised meta-analysis did not report significant heterogeneity, and the relative risk of response decreased to 1.63. The revised response ratio generated a cost per responder day of $11 for pregabalin 150 mg BID, while pregabalin 225 mg BID was cost-saving. The cost-effectiveness results were robust to changes in the baseline severity of FM symptoms in the patient population. In particular, the cost-effectiveness results were unchanged when restricting the analysis to patients with FIQ scores >70 (the traditional definition of severe FMCitation26).
Discussion
The cost-effectiveness of pregabalin compared with a range of other drugs recommended in guidelines or approved by the FDA to treat FM was assessed. The results indicate that pregabalin was cost-saving to most other treatment options and placebo in ∼58% of the patient population typically participating in FM clinical trials—patients with severe FM as determined by high baseline pain diary and FIQ scores. In this model, pregabalin 225 mg BID was consistently more cost-effective than pregabalin 150 mg BID. This was due to the equivalence in price between the two doses and slightly superior efficacy of the 225 mg BID dosage. Pregabalin 225 mg BID was also cost-saving when compared against placebo, duloxetine, tramadol, milnacipran, and gabapentin if direct and indirect costs were taken into account. However, when compared with amitriptyline, pregabalin was not cost-effective. Finally, the univariate sensitivity analysis suggested the probability of response after 12 weeks of treatment is the most important driver of the model for patients with severe FM.
To the authors’ knowledge, this is the first study to estimate the cost-effectiveness of FM treatments in the US. Another study conducted in the UK found that pregabalin was cost-effective to the UK National Health Service where effectiveness was measured with quality-adjusted life years (QALY) based on a patient’s utility gain from treatment response. The incremental cost-effectiveness ratio (ICER) in that study was £23,166/QALY for pregabalin 150 mg BID and £22,533/QALY for pregabalin 225 mg BID compared with placeboCitation53. In the severe FM population, pregabalin 225 mg BID was also cost-effective compared with duloxetine 60 mg (ICER = £19,224/QALY) and 120 mg (ICER = £14,096/QALY) and against gabapentin (ICER = £35,737/QALY).
Overall, the cost-effectiveness results reflect an improvement in quality-of-life consistent with other studies that used clinical data from pregabalin trials, particularly in patients with severe FM. A study by Moore et al.Citation54 found that gains in QALYs with pregabalin were more substantial in patients with more severe FM; with a QALY gain ranging from 0.026 QALYs in individuals with marginal pain improvement (between 9–15% reduction from baseline) to 0.114 QALYs gained in individuals with substantial pain reduction (≥50% reduction from baseline). In a meta-analysis that compared pregabalin, duloxetine, and milnacipran, Häuser et al.Citation11 found that pregabalin was superior to milnacipran in reducing pain and sleep disturbances and was superior to duloxetine in reducing fatigue.
Studies have shown that the cost of illness associated with FM is substantialCitation9. This is reflected in the high cost per patient estimated here and in the recent cost of illness studyCitation52, where a strong relationship was found between FIQ scores and direct medical costs and between the days patients missed work in the FIQ and indirect medical costs. Consequently, the results of this analysis show that, by reducing the severity of symptoms through pharmacologic treatments, substantial savings can be made in healthcare costs and in the burden to society. Consequently, pregabalin is found to be cost saving in this analysis among patients with severe FM, as indicated by high FIQ scores and severe pain.
The model provides a suitable economic evaluation of pregabalin in the US. All patients from the studies by Arnold et al.Citation19 and Mease et al.Citation22 were recruited from the US population, while the majority of patients from the study by Pauer et al.Citation23 were European. The analysis was informed by data from these studies; therefore, it is to a large extent representative of patients treated in the US healthcare setting. Patients recruited into the pregabalin randomized trials were already receiving a number of treatments that were not providing symptomatic relief. Since patients with FM enrolled in the analyzed pregabalin trials were required to have baseline pain NRS scores ≥4 and 60% of patients had a FIQ score in the severe range (>59), this suggests pregabalin is effective in treating refractory patients with severe pain and severe global FM symptoms.
A well known limitation of randomized control trials is that they impose a certain schedule and pattern of disease management which would be quite unrepresentative of typical patient behavior and associated healthcare resource utilization. Real world data studies, on the other hand, have been commonly recognized as the best source for resource use data. To that effect, the Goldenberg et al.Citation52 study was used as a real world data study which provides much more accurate data on typical healthcare resource use of US fibromyalgia patients. Comparing the demographics of the patients in the Goldenberg et al.Citation52 study and the ones in the pregabalin, milnacipran, and duloxetine studiesCitation19,Citation21,Citation43,Citation45, similar characteristics were observed. Moreover, the distribution of patients in mild, moderate, and severe ranges of FM, a key driver of cost differentials, was very similar among the Goldenberg et al.Citation52 and pregabalin studiesCitation26. Therefore, there appears to be high compatibility between the different data sources in terms of their population characteristics for the purpose of their healthcare resource use assessment.
It is important to acknowledge the limitations of this analysis. One limitation relates to the method of sub-group analysis. Although the relative risk of response for pregabalin by specific sub-groups derived directly from clinical trial data was incorporated, sub-group relative risk data for the other comparators were not available. As a result, the effectiveness of pregabalin is not being directly compared against the effectiveness of the comparators in patients with severe FM. Furthermore, the definition of response is not consistent across all trials, which introduces an additional element of uncertainty into the analysis. In addition, this model does not account for safety-related detriments or potential limitations for drug use due to contraindications, warnings/precautions, or drug interactions known to be common in patients prescribed amitriptyline for painful neuropathic disordersCitation9,Citation55.
A major limitation of the model relates to the absence of reliable data to inform key characteristics of the comparator treatments. The data for amitriptyline and tramadol were based on old trials, with possibly different inclusion/exclusion criteria, that were not designed to meet the current regulatory authorities’ criteria for therapeutic response. Analysis of data from these older studies produced extremely high response ratios that were probably falsely elevated. In addition, the reliability of these results is questionable given the poor quality of these studies. Furthermore, there is an absence of long-term studies of response and dropout for the comparators to inform the transition probabilities in the Markov stage of the model. An assumption was made that the long-term withdrawal rate for all comparators was equal to that of pregabalin, which is believed to be conservative.
Conclusions
This Markov model simulation assessed the effectiveness of commonly used FM treatments, and found pregabalin to be a cost-effective treatment option relative to duloxetine, milnacipran, gabapentin, and tramadol, but not amitriptyline. The results of this analysis suggest that treatment with pregabalin can result in a greater number of days with pain response relative to other treatment options, thus possibly leading to reduced healthcare and societal costs associated with FM.
Transparency
Declaration of funding
This study was funded by Pfizer Inc. Editorial support for development of this manuscript was provided by UBC Scientific Solutions and was funded by Pfizer Inc.
Declaration of financial/other relationships
AC and GZ are full-time employees of Pfizer Inc. AL is a full-time employee of IMS and served as a paid consultant to Pfizer Inc during the conduct of this study and the development of this manuscript. EC and CB received a consultancy fee from Pfizer Inc for contributing to this work. CB serves as a consultant for Forest Pharmaceuticals, Eli Lilly and Company, and Pfizer Inc, and has received research funding from Pfizer Inc.
Acknowledgments
The authors would like to thank Zahava Gabriel for conducting the systematic literature review of fibromyalgia treatments; Steve Lister, Javier Rejas, Sophie Marbaix, Sally Thompson, and Christin Prutz for their review, comments, and suggestions on the model, and Robert Sanchez and Joanna Lui for their technical contributions to the study. Editorial support was provided by Diane Hoffman, PhD, of UBC Scientific Solutions and was funded by Pfizer Inc. JME peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
References
- Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns 2008;73:114-20
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160-72
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600-10
- Wolfe F, Ross K, Anderson J, et al. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995;38:19-28
- Weir PT, Harlan GA, Nkoy FL, et al. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th revision codes. J Clin Rheumatol 2006;12:124-8
- Hoffman DL, Dukes EM. The health status burden of people with fibromyalgia: a review of studies that assessed health status with the SF-36 or the SF-12. Int J Clin Pract 2008;62:115-26
- Chandran A, Goldenberg D, Schaefer C, et al. Can fibromyalgia severity help us to better understand the patient: results from a cross-sectional survey in the United States? Journal of Pain 2010;11:S4
- Silverman S, Sadosky A, Evans C, et al. Toward characterization and definition of fibromyalgia severity. BMC Musculoskelet Disord 2010;11:66
- Berger A, Dukes E, Martin S, et al. Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int J Clin Pract 2007;61:1498-508
- White LA, Birnbaum HG, Kaltenboeck A, et al. Employees with fibromyalgia: medical comorbidity, healthcare costs, and work loss. J Occup Environ Med 2008;50:13-24
- Häuser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain 2010;11:505-21
- Choy E, Marshall D, Gabriel ZL, et al. A systematic review and mixed treatment comparison of the efficacy of pharmacological treatments for fibromyalgia. Semin Arthritis Rheum 2011;41:335-45
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004;292:2388-95
- Carville SF, Arendt-Nielsen S, Bliddal H, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 2008;67:536-41
- O'Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med 2000;15:659-66
- Hauser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin–a meta-analysis of randomized controlled trials. Pain 2009;145:69-81
- Tzellos TG, Toulis KA, Goulis DG, et al. Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta–analysis. J Clin Pharm Ther 2010;35:639-56
- Kim L, Lipton S, Deodhar A. Pregabalin for fibromyalgia: some relief but no cure. Cleve Clin J Med 2009;76:255-61
- Arnold LM, Russell IJ, Diri EW, et al. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain 2008;9:792-805
- Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:1264-73
- Crofford LJ, Mease PJ, Simpson SL, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalin. Pain 2008;136:419-31
- Mease PJ, Russell IJ, Arnold LM, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol 2008;35:502-14
- Pauer L, Winkelmann A, Arsenault P, et al. An International, randomized, double-blind, placebo-controlled, phase iii trial of pregabalin monotherapy in treatment of patients with fibromyalgia. J Rheumatol 2011;38:2643-52
- Moore RA, Straube S, Wiffen PJ, et al. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev 2009;CD007076
- Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol 1991;18:728-33
- Bennett RM, Bushmakin AG, Cappelleri JC, et al. Minimal clinically important difference in the Fibromyalgia Impact Questionnaire. J Rheumatol 2009;36:1304-11
- Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: Alcohol, Drug Abuse, and Mental Health Administration, Public Health Service, US Department of Health, Education, and Welfare 1976
- Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 2004;50:2974-84
- Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005;119:5-15
- Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum 2007;56:1336-44
- Arnold LM, Crofford LJ, Martin SA, et al. The effect of anxiety and depression on improvements in pain in a randomized, controlled trial of pregabalin for treatment of fibromyalgia. Pain Med 2007;8:633-8
- Bennett RM, Schein J, Kosinski MR, et al. Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Rheum 2005;53:519-27
- Bennett RM, Kamin M, Karim R, et al. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med 2003;114:537-45
- Biasi G, Manca S, Manganelli S, et al. Tramadol in the fibromyalgia syndrome: a controlled clinical trial versus placebo. Int J Clin Pharmacol Res 1998;18:13-9
- Carette S, Bell MJ, Reynolds WJ, et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia. A randomized, double-blind clinical trial. Arthritis Rheum 1994;37:32-40
- Carette S, McCain GA, Bell DA, et al. Evaluation of amitriptyline in primary fibrositis. A double-blind, placebo-controlled study. Arthritis Rheum 1986;29:655-9
- Carette S, Oakson G, Guimont C, et al. Sleep electroencephalography and the clinical response to amitriptyline in patients with fibromyalgia. Arthritis Rheum 1995;38:1211-7
- Gendreau RM, Thorn MD, Gendreau JF, et al. Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol 2005;32:1975-85
- Ginsberg F, Mancaux A, Joos E, et al. A randomized placebo-controlled trial of sustained release amitriptyline in primary fibromyalgia. J Musculoskeletal Pain 1996;4:37-47
- Goldenberg D, Mayskiy M, Mossey C, et al. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum 1996;39:1852-9
- Hannonen P, Malminiemi K, Yli-Kerttula U, et al. A randomized, double-blind, placebo-controlled study of moclobemide and amitriptyline in the treatment of fibromyalgia in females without psychiatric disorder. Br J Rheumatol 1998;37:1279-86
- Heymann RE, Helfenstein M, Feldman D. A double-blind, randomized, controlled study of amitriptyline, nortriptyline and placebo in patients with fibromyalgia. An analysis of outcome measures. Clin Exp Rheumatol 2001;19:697-702
- Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 2008;136:432-44
- Russell IJ, Kamin M, Bennett RM, et al. Efficacy of tramadol in treatment of pain in fibromyalgia. J Clin Rheumatol 2000;6:250-7
- Clauw DJ, Mease P, Palmer RH, et al. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther 2008;30:1988-2004
- Mease PJ, Clauw DJ, Gendreau RM, et al. The efficacy and safety of milnacipran for treatment of fibromyalgia. A randomized, double-blind, placebo-controlled trial. J Rheumatol 2009;36:398-409
- Florian H, Young JP, Jr. Haig G. Efficacy and safety of pregabalin as long-term treatment of pain associated with fibromyalgia: a 1-year, open-label study [abstract]. Arthritis Rheum 2007;56(9 Suppl):S602
- Chappell AS, Littlejohn G, Kajdasz D, et al. A 1-yr safety and efficacy study of duloxetine in patients with fibromyalgia [abstract]. Ann Rheum Dis 2008;67(II Suppl):253
- Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996
- Medicaid. Drug utilization data. Baltimore, MD: Centers for Medicare & Medicaid Services/US Department of Health & Human Services, 2010
- National drug code directory. Rockville, MD: US Food and Drug Administration/US Department of Health & Human Services, 2010
- Goldenberg D, Gerwin R, McNett M, et al. The economic burden of mild, moderate and severe fibromyalgia: results from a cross-sectional survey among working-age adults in the United States. J Manag Care Pharm 2011;9:71
- Choy E, Richards S, Bowrin K, et al. Cost effectiveness of pregabalin in the treatment of fibromyalgia from a UK perspective. Curr Med Res Opin 2010;26:965-75
- Moore RA, Straube S, Paine J, et al. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149:360-4
- Gore M, Dukes E, Rowbotham D, et al. Prevalence of contraindicated medical conditions and use of precluded medications in patients with painful neuropathic disorders prescribed amitriptyline. Pain Pract 2006;6:265-72