Abstract
Introduction:
The burden of disease in Lambert-Eaton myasthenic syndrome (LEMS) patients is unclear. This study focused on the patient’s perspective to obtain patient-reported information on clinical symptoms, burden of illness, impact of LEMS on activities of daily living (ADL), and management of LEMS.
Methods:
Semi-structured, face-to-face interviews with LEMS patients from two specialized centres in Germany between September and December 2010.
Results:
Twelve patients participated; mean age 66.7 ± 9.8 years. First symptoms occurred at age 52.5 ± 14.0 years. Mean time between first symptoms and diagnosis was 4.4 ± 6.2 years. Patients reported neuromuscular, cranial, and autonomic symptoms plus general fatigue. Two-thirds of patients reported 10 or more symptoms. The most frequent symptoms were leg weakness (91.7%) and general fatigue (83.3%). Restrictions in ADL were reported always or often in 75% of patients. Over half of the patients (n = 7) reported poor or very poor health status. Mean EQ-5D utility scores were 0.34 ± 0.35, with little day-to-day variation. Patients visited a number of different clinicians; most had been hospitalized at some point in the course of their disease. The most frequent drug treatments were 3,4-diaminopyridine (3,4-DAP) (83.3%) and pyridostigmine (41.5%). The study has several limitations, including small sample size and the potential influence of recall bias.
Conclusion:
LEMS patients report long individual disease histories. Most patients suffer multiple symptoms which are frequently severe and troublesome, and almost all are restricted in ADL with poor health status. There is high utilization of healthcare resources from diagnosis to ongoing treatment. Physicians should be aware of this rare disease to ensure that patients receive an early diagnosis and prompt and appropriate treatment.
Introduction
Lambert-Eaton myasthenic syndrome (LEMS) is an antibody-mediated autoimmune disorder of neuromuscular transmissionCitation1. The clinical and electrophysiological characteristics of the disease were first described by Eaton and LambertCitation2 in 1957. Cancer is present at diagnosis or subsequently discovered in 50–70% of patients with LEMSCitation3,Citation4. LEMS has an autoimmune basis, regardless of whether cancer is present or not, and ∼25% of LEMS patients have additional autoimmune diseaseCitation4,Citation5. Epidemiological data suggests that LEMS is considerably rarer than myasthenia gravis (MG). Data from The Netherlands reports a calculated prevalence of 2.32 patients per million for LEMS and 106.1 per million for MGCitation6. The German Society for Muscle Patients (DGM) has estimated the prevalence of LEMS at 0.5/100,000Citation7, which equates to ∼400 LEMS patients in Germany.
LEMS is primarily a disease of middle-age and beyond, with an average age of onset of 50 years (range 11–76 years)Citation8. LEMS symptoms are insidious, typically beginning with lower limb muscle weakness and general fatigue, leading to generalized muscle weakness. Patients often have difficulty in rising from a seated position, in climbing stairs, and walking. Autonomic dysfunction such as dry mouth, constipation, poor bladder control, and impotence in men is common in LEMSCitation1. Later in the course of the disease, ocular (double vision, ptosis) and bulbar symptoms (dysphagia, dysarthria) may develop, although less commonly than in MGCitation8.
Few studies have quantified the interval between the onset of symptoms and a valid diagnosis of LEMS. Wirtz et al.Citation9, in a review of 227 cases from 155 publications, found a median interval of 6 months, with a longest delay of 36 years between onset and correct diagnosis. Pellkofer et al.Citation10 examined 25 patients with idiopathic LEMS in Germany, and found a mean duration of 4.4 years between initial symptoms and diagnosis, ranging from 2 months up to 25 years. However, neither study investigated the complete disease history from the onset of symptoms to diagnosis and treatment. Furthermore, little information has been reported using patient relevant outcomes, such as a patient’s health-related quality-of-life (HRQoL), and the burden of illness for LEMS patients and their families is not well understood. Studies have reported HRQoL in MGCitation11 and multiple sclerosisCitation12 using EQ-5D index scores.
The objective of this study was to examine the long-term disease history of LEMS patients from the onset of symptoms to diagnosis and disease progression. The study focused on the patient’s perspective to obtain patient-reported information on clinical symptoms, the burden of illness, LEMS treatments, and the impact of LEMS on activities of daily living (ADL) and HRQoL.
Methods
Study design and patient population
The study was designed as a cross-sectional patient-based interview survey. Between September and December 2010, patients with LEMS diagnosis with or without small cell lung cancer (SCLC) were recruited by physician nomination in two specialized centres for the treatment of neuromuscular disorders in Germany (Charité, Berlin and HANSE Klinikum, Stralsund). A positive ethical review was obtained from the Institutional Review Board at the two study sites before starting the interviews. Interviews were semi-structured and were conducted after obtaining written informed consent from each patient. Face-to-face interviews lasting 1.5–3 h were carried out by trained and experienced staff in the patient’s home. In most cases, spouses participated in the interviews and provided additional information. Two interviewers conducted the interviews and the interviewer was present during the entire interview.
Instruments used for assessment
The interview schedule was developed by IMS Health, taking into consideration disease characteristics and treatment patterns in LEMS. Answers were documented using a standardized protocol (hardcopy form) by the interviewers. The interview consisted of four parts:
Patients’ current situation: demographics, self-assessment of health status, impairment of ADL.
Patients’ situation prior to a diagnosis of LEMS: initial symptoms, journey, and time to explicit diagnosis of LEMS.
LEMS diagnosis: manner of diagnosis, reaction to diagnosis, progression of symptoms post-onset of symptoms, and impairment of ADL.
Post-diagnosis: treatment history, current medication, access to current treatment, assessment of current symptoms, impairment of ADL, and impact of disease (EQ-5D and Work Productivity and Activity Impairment Questionnaire [WPAI] questionnaire).
The presence and severity of clinical symptoms of LEMS were listed on prepared cards, which participants ticked to indicate presence of each symptom together with a symptom score. Patients’ assessments of disease impact were recorded using a 5-point Likert rating scale. Classification of functional impairment used the 5-grade functional scale described by Sharshar et al.Citation13 Grades of impairment were defined as: (1) complete remission, (2) minor symptoms allowing normal activity, (3) moderate symptoms allowing occupational or partial daily activity, (4) major disability requiring discontinuation of occupational activity, and (5) major disability requiring continuous help or mechanical ventilation. In order to gain standardized data, two validated assessment instruments were used: the EQ-5D (version 3L), which has been widely used, including in MGCitation14,Citation15 and WPAICitation16. The EQ-5D is a widely used and validated instrument used to gather information about health status. In the first part of the EQ-5D health status is assessed in five domains (mobility, self-care, usual activity, pain/discomfort, anxiety/depression) with possible responses at three levels (1 = no problems, 2 = some problems, 3 = severe problems). The evaluation of health status utility score within EQ-5D ranges from full health (value of 1) to dead (value of 0). A negative score reflects a state worse than death. In the second part of EQ-5D a visual analogue scale (VAS range 0–100, where 0 is worst imaginable health state and 100 is best imaginable health state) is used to measure the daily health state. The EQ-5D was assessed at the end of the interview and on each day during the 6 days after interview (using patient diaries), to gather more information about day-to-day variability in the assessments made by LEMS patients.
Statistical analyses
Data were entered from the hardcopy interview protocols into a database. All statistical analyses were performed by descriptive statistical methods using SAS® System 9.1 (SAS Inc., Cary, NC). The total numbers and the absolute and relative frequencies were calculated for categorical parameters. Continuous variables were displayed as total number, number of missing values, minimum, median, maximum, mean, and standard deviation. In accordance with the observational design of this survey missing data was not imputed for statistical analysis. EQ-5D utility scores were analysed as described by Greiner et al.Citation17
Single LEMS symptoms were categorized for analysis in limb/trunk symptoms (weakness of legs, weakness of arms, exacerbation of weakness by exercise, hot bath, hot weather, muscle pain or stiffness, difficulty breathing), autonomic symptoms (dry mouth, male impotence, constipation, poor bladder/bowel control, impaired sweating, difficulty in focusing, dizziness), cranial symptoms (double vision, drooping eyelids, slurred speech, difficulty swallowing, difficulty chewing, weak voice), and general symptoms (general fatigue)Citation1,Citation18. All statistical analyses were conducted using SAS® System 9.1 (SAS Inc., Cary, NC).
Results
Study demographics
A total of 12 patients were interviewed: seven (58.3%) were female and five (41.7%) were male. Mean age was 66.7 ± 9.8 years (range 54–83 years). The patients were from all over Germany; one (8.3%) lived in a large city, four (33.3%) lived in mid-sized towns, one (8.3%) in a small town, and six (50%) in rural locations. The majority of patients lived with their family or with a partner (n = 10, 83.3%) and two (16.7%) patients lived alone. Nine (75%) patients were retired at the time of the interview, one (8.3%) was unemployed, and two (16.7%) worked as freelancers. Almost all patients (n = 11, 91.7%) reported co-morbidities, including five (41.6%) patients who reported other autoimmune diseases and three (25%) patients with a reported history of SCLC. A total of nine (75%) patients were ex-smokers and one patient was a smoker at the time of the interview (). None of the patients were receiving chemotherapy at the time of the interview.
Table 1. Characteristics of the study population.
Disease history
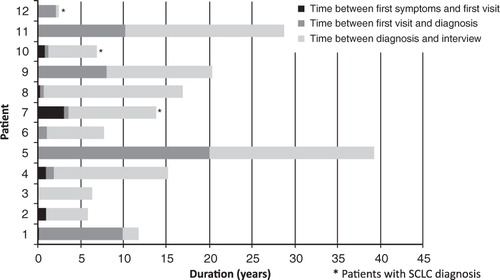
The mean age of the patients when they first became aware of symptoms of the disease was 52.5 ± 14.0 years. The mean time from initial symptoms to first visit to a physician was 0.6 ± 0.9 years (range 0.0–3.0 years), mean time between first visit to a physician and a diagnosis of LEMS was 4.4 ± 6.2 years (median 1 year; range 0.0–20.0 years), and mean time since diagnosis to the day of the interview was 9.6 ± 6.3 years (range 0.2–19.1 years). The mean time between first symptoms and the day of the interview was 15.2 ± 10.9 years. shows the time period between first symptoms and the day of the interview, which ranged between 2.3–39.2 years.
Symptoms of LEMS
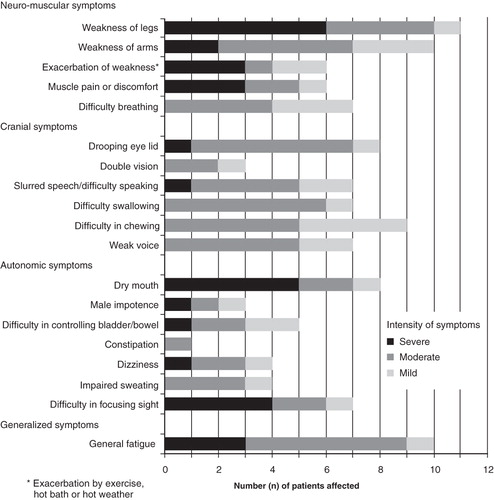
Patients reported multiple neuromuscular, cranial nerve, and autonomic symptoms, plus general fatigue, many of which were rated as severe and troublesome. The most frequently reported symptoms were weakness of the legs and arms, general fatigue, difficulty in chewing, drooping eye lids, and dry mouth (). All but one patient reported having six or more symptoms and two-thirds of patients (n = 8) reported 10 or more symptoms. Most patients reported one or more symptoms from each category of symptoms.
More than 50% of the 11 patients who reported weakness of the legs described it as severe. Similarly, over half of the patients who reported dry mouth and difficulty in focusing described these symptoms as severe. In general, neuromuscular symptoms were most frequently described as severe by the patients.
Patients reported the two most troublesome symptoms as leg weakness (n = 6, 50%) and general fatigue (n = 5, 41.7%).
Impact on ADL, health status, and HRQoL
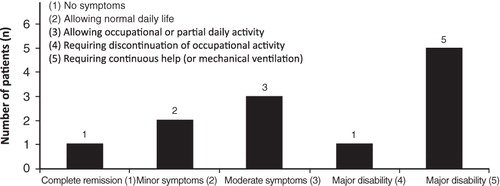
LEMS has a considerable impact on patients’ day-to-day life; nine (75%) patients reported partial or total restrictions in their ADL. The majority of patients experienced some restriction in ADL throughout the course of their disease, with 10 (83.8%) patients reporting restrictions often or always at the time of diagnosis, and nine (75%) reporting restrictions often or always at the time of interview. Half of the patients assessed their physical functioning as ‘major disability’ at the time of the interview ().
At the time of the interview, over half of the patients (n = 7, 58.3%) rated their health status as poor or very poor and only one patient (8.3%) rated his health status as good. Of the 12 patients interviewed, half (n = 6) reported severe problems with their usual activities and half (n = 6) reported severe problems with pain/discomfort on the EQ-5D scale. Two patients reported severe problems with self-care, and one reported severe problems with anxiety/depression. As anticipated, mobility was a problem for all patients (11 patients had some problems with mobility and one had severe problems with mobility) ().
Figure 4. Profile of the EQ-5D scores on the day of interview across domains by patient (1 = no problems, 2 = some problems, 3 = severe problems). Patients 7, 10, and 12 had a diagnosis of SCLC.
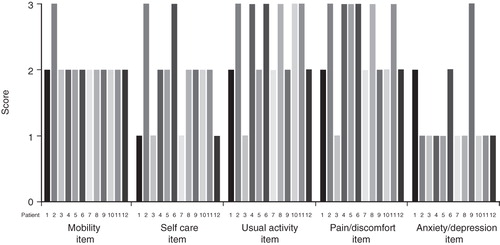
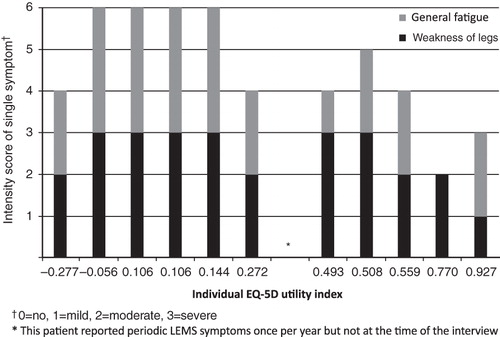
EQ-5D evaluation of health status revealed a mean utility score of 0.34 ± 0.35 on the day of the interview, with a wide range in scores across the patients (range = −0.27–0.93) on the day of the interview. The mean utility score over 7 days was 0.31 ± 0.35 (range = −0.11–0.77) (day of interview plus 6-day follow-up diary), and the within individual patient variability was low for most patients (). Utility scores were lower (indicating greater impact on quality-of-life) in cases where patients reported moderate or severe problems with leg weakness and general fatigue ().
Table 2. Utility index and VAS scores on the day of the interview and the 6-day follow-up period. Patients 7, 10, and 12 had a diagnosis of SCLC.
The EQ-5D VAS assessment of current health status showed a similar profile, with a wide range in scores across patients, ranging from 20–80 mm (mean: 44.5 ± 22.5) on the day of the interview. The mean VAS was 45.6 ± 22.8 (range = 14–80) based on the 7-day assessments. The within patient variability was less for the EQ-5D VAS assessment of current health status than for the utility score.
The WPAI is used to assess the impact of disease on work and productivity. However, of the patients, nine were retired, one was not working, and two worked as freelancers; therefore, the WPAI survey could not be conducted in the majority of the patients and results have not been presented for this end-point.
Healthcare utilization
A number of healthcare professionals in Primary and Secondary care, together with family caregivers, were involved in the management and care of the LEMS patients. Half of the patients (n = 6, 50%) initially visited their General Practitioner at the onset of symptoms, four (33.3%) attended an office-based specialist, and two (16.7%) visited a hospital specialist. Ten of the patients (83.3%) had visited a hospital specialist prior to a formal diagnosis of LEMS. All 12 of the patients eventually received a diagnosis of LEMS in a hospital-based setting, after blood tests and nerve stimulation tests. Eleven of the patients received their diagnosis from a neurologist and one from an orthopaedic surgeon. A wide range of alternative diagnoses were frequently considered prior to the final diagnosis of LEMS. The following conditions were considered prior to a confirmed diagnosis of LEMS: MG (n = 3), diabetic neuropathy (n = 2), rheumatic disease (n = 1), multiple sclerosis (n = 1), post-menopausal symptoms (n = 1), vertebrae disease (n = 1), Parkinson’s disease (n = 1), venous disease in the legs (n = 1), myocardial infarction (n = 1), Lyme disease (n = 1), infection (n = 1), age-related visual disturbance (n = 1), psychosomatic causes (n = 1), and limb-girdle muscular dystrophy (n = 1).
A number of different clinicians were involved in the care of LEMS patients; two-thirds (n = 8) saw their General Practitioners, half (n = 6) saw office-based specialists (neurologist, oncologist, or radiologist) and three-quarters (n = 9) saw hospital specialists (neurologist) for their day-to-day care. Less than half of the patients (n = 5, 41.7%) had received physiotherapy. Half of the patients (n = 6) were cared for informally by their families.
More than half of the patients were hospitalized prior to their diagnosis of LEMS (n = 7, 58.3%) and almost all (n = 11, 91.7%) were hospitalized post-diagnosis.
Drug treatments used for the initial treatment of LEMS were pyridostigmine (n = 5, 41.6%) and 3,4-diaminopyridine (3,4-DAP) (n = 4, 33.3%). Three (25%) patients did not receive any drug treatment immediately after diagnosis; reasons for not initiating treatment and the period of delay prior to initiation were not identified in the study. The use of 3,4-DAP and immunosuppressive treatments increased over time, and, at the time of interview, 3,4-DAP was the most frequently used drug (n = 10; 83.3%), with five (41.6%) patients receiving pyridostigmine, four (33.3%) receiving corticosteroids, and three (25%) receiving immunoglobulin. Five of the 10 patients receiving 3,4-DAP at the time of interview were taking 3,4-DAP as monotherapy and the other five patients as combination therapy (). Most patients (n = 7/10, 70%) received 3,4-DAP at a dose of 60–80 mg per day, irrespective of whether they received treatment as monotherapy or as combination therapy.
Table 3. Combinations of drug treatment used in current treatment of LEMS.
Discussion
The authors report the first qualitative study of LEMS patients, following the patient journey from the onset of symptoms to confirmed disease and beyond. This study is the first to report detailed descriptive data on LEMS patients in Germany. It reveals that LEMS has a considerable impact on the patient, their families, and the healthcare system.
LEMS is considered to be a disease that develops in middle age. In our population of 12 LEMS patients the first symptoms of the disease emerged in middle age with a mean age of 52.5 ± 14.0 years. The gender and the age at onset of LEMS-disease symptoms observed in our population are comparable with results previously described for other LEMS study populationsCitation4,Citation8,Citation18,Citation19.
Around 50–70% of patients with LEMS have co-existing malignancy, most commonly SCLC. However, patients with SCLC were under-represented in this study since only 25% had SCLC. Previous work comparing clinical features in LEMS patients with and without cancer has found that patients with co-existing malignancy receive an earlier diagnosis of their LEMS, are older at diagnosis, and have less concomitant autoimmune disease than those without co-existing malignancyCitation9.
Around 25% of patients with LEMS have concomitant autoimmune disease. In this study, 42% of patients had concomitant autoimmune disease: two had MG, two had thyroid disease, and one had rheumatoid arthritis. It is important to consider that many of these co-morbidities may cause similar symptoms to LEMS, particularly fatigue and difficulty with mobility. Patient age plays a part in co-morbidities, the mean age at the time of the survey was 66.7 ± 9.8 years, and patients of this age would be expected to have co-morbid disease. Indeed, rates of thyroid disease, rheumatoid arthritis, and type 2 diabetes all increase with age. However, concomitant MG and LEMS is extremely rare, and the rate seen in this study is not representative of the general LEMS population, this may stem from recall bias on the part of the patients.
Most of the patients reported multiple neuromuscular, cranial, and autonomic symptoms as well as general fatigue. Two-thirds of the patients reported 10 or more symptoms. The patients rated many of the symptoms, particularly leg weakness and fatigue, as severe and troublesome. These results are consistent with results from a population of 25 German LEMS patients who also described a wide range of symptoms, including paresis (mainly proximal and in the legs) in all patients as well as autonomic dysfunction (dry mouth) and other symptoms such as muscle stiffness or painCitation10. In our study population the most frequently reported symptoms were leg weakness and general fatigue, which is consistent with the findings described by Wirtz et al.Citation9 from an analysis of 227 case studies reported in 155 publications. However, one patient in our study had not experienced symptoms for at least 1 year and, prior to participation in the survey, acute symptoms were only evident once a year, necessitating hospital admission for 2 weeks; it may be that symptomatic control is particularly good in this patient, or this may demonstrate that there is wide variation in the symptoms of LEMS.
The mean EQ-5D utility score was 0.34 ± 0.35 on the day of the interview, which is comparable to patients hospitalized with an exacerbation of asthma (0.33)Citation20 or patients with severe multiple sclerosisCitation12. The mean EQ-5D utility score in patients with LEMS is worse than patients prior to lung transplantation (0.5)Citation21, patients with multiple sclerosis (no disability to moderate disability) (0.76)Citation12, and patients with ischaemic heart disease (0.45 for severe disease to 0.80 for mild disease) or heart failure (0.51 for severe disease to 0.78 for mild disease)Citation22. Utility scores reported by this group of LEMS patients are significantly lower than those reported for patients with MG (0.89)Citation11.
The EQ-5D utility score of eight of the patients in the present study was below 0.5, by way of comparison patients with severe heart failure report a EQ-5D utility score of 0.5Citation22. The utility score for two patients on the day of interview and for four patients during the 7-day observation period was below zero (i.e. a state worse than death).
More than one-half of the patients (n = 7) reported poor or very poor health status, and restrictions in the ADL were reported to occur always or often in 75% of patients (58.3% and 16.6%, respectively). Indeed, many patients reported major problems with usual activities and pain/discomfort. Self-care, mobility, and anxiety/depression resulted in some problems for the majority of patients. The study found that the utility scores tended to be lower in patients who reported some or major problems with leg weakness and general fatigue. The impact of LEMS on health status and restrictions in ADL has a considerable influence on HRQoL, and this data suggests that patients with LEMS have a poor HRQoL.
Previous work has shown that German patients with MG differ significantly from the normal population in terms of physical functioning, vitality, and mental healthCitation23. However, there is little data available on the HRQoL of LEMS or MG patients. There is a need for more research into the health status of patients suffering from severe neurological conditions to improve our understanding of the impact of symptoms on ADL and on HRQoL.
Despite the wide range in utility scores across the population interviewed, there was little day-to-day variation within patients during the 6-day follow-up compared with the score on the interview day, suggesting a consistent impact on health status.
Patients generally sought medical advice within a few months of their initial symptoms, although there was considerable variation in the type of clinician approached and subsequent time to diagnosis. There was a delay of a number of years (mean 4.4 ± 6.2 years) before a correct diagnosis was made with a wide range (0.0–20.0 years) between the first symptoms and diagnosis. Retrospective recollection of symptoms is subject to recall bias and may be unreliable. However, the data presented in this paper is consistent with findings in other LEMS populationsCitation4,Citation10,Citation18 and is not unusual for a diagnosis of a rare disease. Indeed, a survey conducted by the European Organization for Rare Diseases (EURORDIS) in 2006 into the diagnosis of eight rare diseases (Crohn’s disease, cystic fibrosis, Duchenne muscular dystrophy, Ehlers-Danlos syndrome, Marfan syndrome, Prader Willi syndrome, tuberous sclerosis, and fragile X syndrome) revealed that 25% of patients had to wait between 5–30 years from early symptoms to confirmatory diagnosis of their diseaseCitation24.
The European Federation of Neurological Societies issued guidance on autoimmune neuromuscular transmission disorders in 2010Citation2Citation5. The guidelines recommend 3,4-DAP as a first-line treatment in symptomatic LEMS. The guidelines suggest that an additional therapeutic effect may be obtained if 3,4-DAP is given in combination with pyridostigmine. If symptomatic treatment is insufficient, the guidelines recommend immunosuppressive therapy. Although the majority of patients questioned received 3,4-DAP (n = 10, 83.3%) later in the course of their disease (five as monotherapy and five in combination with other treatments), only four (33.3%) received 3,4-DAP as initial treatment. Patients in this study received a range of combination treatments, and there does not appear to be a standard treatment pathway for patients with LEMS. A similar pattern of drug treatment has previously been described in a population of 25 German LEMS patients, in whom 3,4-DAP and pyridostigmine were the most frequently used treatmentsCitation10.
Given the similarity of the patient profile to those in other publicationsCitation9,Citation10, the authors consider that the population interviewed was broadly representative of the wider LEMS population, with the exception of co-morbid LEMS and MG, despite being a small sample recruited from two specialist physicians. The present sample has a lower rate of SCLC (25%) compared with other previously described LEMS populations (50–70%) and a higher rate of concomitant autoimmune disease (42% vs ∼25% in previously described populations). However, it should be remembered that LEMS is a rare orphan disease, and recruitment of a large cohort of patients is extremely difficult.
However, it is important to consider the potential impact of recall bias, since patients recalled information about their disease over a considerable time period. Recall bias has been described as being a factor which may influence quantitative evaluation of patient-reported symptomsCitation26,Citation27. However, the interviewers considered that the patients were well informed about their disease, the treatments received, and co-morbidities. The information provided by the patients was not followed up with a cross-reference to hospital notes which could have been an option in the research design of this study.
Conclusion
In summary, many LEMS patients have a long disease history, each reporting an individual journey. Diagnosis is frequently delayed, and the utilization of the healthcare system from diagnosis to ongoing treatment is relatively high, related to the complexity of LEMS and the limited opportunities for diagnosis and treatment. In Germany physicians from different specialties are involved in the management of patients with LEMS, as well as other medical care providers and family caregivers. Although the most frequently reported symptoms are the typical symptoms (leg weakness and general fatigue), most patients show multiple neuromuscular, cranial, and autonomic symptoms. Symptoms were often described as severe and troublesome, resulting in restrictions on ADL and a poor health status. The low mean EQ-5D utility score reported by LEMS patients demonstrates that the impact on health status is considerable and comparable to patients with severe multiple sclerosis or patients hospitalized for asthma. The impact of LEMS on health status and restrictions in ADL has a considerable negative influence on HRQoL. HRQoL in patients with LEMS is considerably impaired; indeed two of the patients in this study rated their utility score on the day of interview as less than zero—which reflects a state worse than death.
It is important that physicians are aware of this rare disease to ensure that patients with LEMS receive an early diagnosis and prompt and appropriate treatment to relieve their clinical symptoms and improve the restrictions on ADL, overall health status, and HRQoL.
Transparency
Declaration of funding
This survey was funded by BioMarin Europe Limited, UK. Editorial support was provided by Tricia Dixon, JB Medical Ltd, UK.
Declaration of financial/other relationships
JPS and LH have disclosed that they were paid consultants for BioMarin regarding the planning of this study. AEW and RG have disclosed that they are employees of BioMarin Europe Limited, UK. RS, VC, and CP have disclosed that they are employees of IMS Health, a company that received payment for their involvement in this project.
References
- Seneviratne U, De Silva R. Lambert-Eaton myasthenic syndrome. Postgrad Med J 1999;75:516-20
- Eaton LM, Lambert EH. Electromyography and electric stimulation of nerves in diseases of motor unit; observations on myasthenic syndrome associated with malignant tumors. J Am Med Assoc 1957;163:1117-24
- Stickler DE, Huff JS, Kleinschmidt P. Lambert-Eaton Myasthenic Syndrome. 2010. http://emedicine.medscape.com/article/792803. [Last accessed 19 January 2012]
- O'Neill JH, Murray NM, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome. A review of 50 cases. Brain 1988;111:577-96
- Wirtz PW, Bradshaw J, Wintzen AR, et al. Associated autoimmune diseases in patients with the Lambert-Eaton myasthenic syndrome and their families. J Neurol 2004;251:1255-9
- Wirtz PW, Nijnuis MG, Sotodeh M, et al; Dutch Myasthenia Study Group. The epidemiology of myasthenia gravis, Lambert-Eaton myasthenic syndrome and their associated tumours in the northern part of the province of South Holland. J Neurol 2003;250:698-701
- Plaghi OW, Das J-P. Lambert-Eaton-Myasthenie Syndrom. Freiburg: Deutsche Gesellschaft für Muskelkranke e.V. DGM, 2011
- Wirtz PW, Sotodeh M, Nijnuis M, et al. Difference in distribution of muscle weakness between myasthenia gravis and the Lambert-Eaton myasthenic syndrome. J Neurol Neurosurg Psychiatry 2002;73:766-8
- Wirtz PW, Smallegange TM, Wintzen AR, et al. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg 2002;104:359-63
- Pellkofer HL, Armbruster L, Linke R, et al. Managing non-paraneoplastic Lambert-Eaton myasthenic syndrome: clinical characteristics in 25 German patients. J Neuroimmunol 2009;217:90-4
- Winter Y, Schepelmann K, Spottke AE, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol 2010;257:1473-81
- Kobelt G, Lindgren P, Smala A, et al. The German Cost of MS Study Group. Costs and quality of life of multiple sclerosis: an observational study in Germany. Eur J Health Econ 2001;2:60-8
- Sharshar T, Chevret S, Mazighi M, et al. Validity and reliability of two muscle strength scores commonly used as endpoints in assessing treatment of myasthenia gravis. J Neurol 2000;247:286-90
- Sieb JP, Kraner S, Köhler W, et al. Myasthenia gravis und myasthene Syndrome. Deutsches Ärzteblatt 2011;97(Heft 51–52):A3496-500
- Winter Y, Schepelmann K, Spottke AE, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol 2010;257:1473-81
- Work productivity and activity impairment questionnaire. http://www.reillyassociates.net/WPAI-SHP-PSO-German__Germany__V1.2.doc. [Last accessed 19 January 2012]
- Greiner W, Claes C, Busschbach JJ, et al. Validating the EQ-5D with time trade off for the German population. Eur J Health Econ 2005;6:124-30
- Titulaer MJ, Wirtz PW, Kuks JB, et al. The Lambert-Eaton myasthenic syndrome 1988-2008: a clinical picture in 97 patients. J Neuroimmunol 2008;201–202:153-8
- Maddison P, Lang B, Mills K, et al. Long term outcome in Lambert-Eaton myasthenic syndrome without lung cancer. J Neurol Neurosurg Psychiatry 2001;70:212-7
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7
- Groen H, van der Bij W, Koëter GH, et al. Cost-effectiveness of lung transplantation in relation to type of end-stage pulmonary disease. Am J Transplant 2004;4:1155-62
- Dyer MT, Goldsmith KA, Sharples LS, et al. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010;8:13
- Twork S, Wiesmeth S, Klewer J, et al. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual Life Outcomes 2010;8:129
- Survey of the delay in diagnosis for 8 rare diseases in Europe (EURORDISCARE 2). http://www.eurordis.org/sites/default/files/publications/Fact_Sheet_Eurordiscare2.pdf. [Last accessed 19 January 2012]
- Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2010;17:893-902
- Lurie F, Kistner RL. In prospective study using Specific Quality of Life & Outcomes Response-Venous (SQOR-V) questionnaire the recall bias had the same magnitude as the minimally important difference. Qual Life Res 2011;20:1589-93
- McPhail S, Beller E, Haines T. Physical function and health-related quality of life of older adults undergoing hospital rehabilitation: how strong is the association? J Am Geriatr 2010;58:2435-7