Abstract
Objective:
Although the use of innovative drug delivery systems, like orally disintegrating antipsychotic tablets (ODT), may facilitate medication adherence and help reduce the risk of relapse and hospitalization, no information is available about the comparative cost-effectiveness of standard oral tablets (SOT) vs ODT formulations in the treatment of schizophrenia. This study compared the cost-effectiveness of olanzapine ODT and olanzapine SOT in the usual treatment of outpatients with schizophrenia from a US healthcare perspective. The study also compared olanzapine ODT with risperidone and aripiprazole, two other atypical antipsychotics available in both ODT and SOT formulations.
Methods:
Published medical literature and a clinical expert panel were used to populate a 1-year Monte Carlo Micro-simulation model. The model captures clinical and cost parameters including adherence levels, treatment discontinuation by reason, relapse with and without inpatient hospitalization, quality-adjusted life years (QALYs), treatment-emergent adverse events, healthcare resource utilization, and associated costs. Key outcomes were total annual direct cost per treatment, QALY, and incremental cost-effectiveness (ICER) per 1 QALY gained.
Results:
Based on model projections, olanzapine ODT therapy was more costly ($9808 vs $9533), but more effective in terms of a lower hospitalization rate (15% vs 16%) and better QALYs (0.747 vs 0.733) than olanzapine SOT therapy. Olanzapine ODT was more cost-effective than olanzapine SOT (ICER: $19,643), more cost-effective than risperidone SOT therapy (ICER: $39,966), and dominant (meaning less costly and more effective) than risperidone ODT and aripiprazole in ODT or SOT formulations.
Limitations:
Lack of head-to-head randomized studies comparing the three studied atypical antipsychotics required making input assumptions that need further study.
Conclusions:
This micro-simulation found that the utilization of olanzapine ODT for the treatment of schizophrenia is predicted to be more cost-effective than any other ODT or SOT formulations of the studied atypical antipsychotic medications.
Introduction
Among the greatest challenges in the treatment of schizophrenia is poor patient adherence with antipsychotic medications. Despite the need for long-term maintenance on the medication, more than half of patients are non-adherent with their antipsychotic regimensCitation1. Non-adherence has long been recognized as a potent predictor of relapse and hospitalizationCitation2–4, the costliest component in the treatment of schizophrenia in both economic and personal termsCitation5.
Non-adherence is a complex phenomenon, and successful pharmacotherapy depends on many factors. Although efficacy and tolerability are clearly important, innovative drug delivery systems may enhance adherence and help reduce suboptimal outcomesCitation6–8. Among delivery systems that may facilitate medication adherence are antipsychotics in orally disintegrating tablet (ODT) formulations, currently available for clozapine, olanzapine, risperidone, and aripiprazole. The ODTs disintegrate within seconds of contact with saliva without requiring waterCitation9, mask the taste of the medicationCitation10, and are bioequivalent to comparable dosages of the standard oral tablet (SOT)Citation11.
Although ODTs may be more costly than SOTs, there are no published studies comparing their cost-effectiveness. Prior research has, however, shown that olanzapine ODT is associated with improved patient attitudes toward medicationCitation11,Citation12 and with improved medication adherence in inpatient and outpatient settingsCitation11–13. The most robust data supporting the adherence advantage of olanzapine ODT over its SOT formulation is based on findings of significantly better adherence on olanzapine ODT than olanzapine SOT in the only randomized, double-blind, double-dummy, controlled study to offer a head-to-head comparison of adherence levels on the two formulations in the treatment of schizophreniaCitation13. Moreover, a recent randomized, cross-over, open-label multinational study comparing patient preference of olanzapine ODT vs olanzapine SOT among outpatients with schizophreniaCitation14 found that patients were 2-times more likely to prefer olanzapine in ODT formulation over SOT formulation. Current findings suggest that olanzapine ODT may provide an adherence advantage over its SOT formulationCitation15, which may translate to a reduced risk of relapse and hospitalization, and thus translate to improved cost-effectiveness.
In a previously published 1-year micro-simulation modelCitation16, we compared the cost-effectiveness of five atypical antipsychotics in SOT formulations (olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole) in the treatment of patients with schizophrenia in usual care settings in the US. That model predicted utilization of olanzapine SOT would improve clinical outcomes and lower total direct healthcare costs better than utilization of comparators, suggesting that olanzapine SOT may be a cost-effective therapeutic option for patients with schizophrenia.
The current study aimed to update and expand our previous cost-effectiveness modelCitation16 by comparing the cost-effectiveness of several ODT and SOT formulations of atypical antipsychotic drugs (olanzapine, risperidone, and aripiprazole). Our primary objective was to compare the cost-effectiveness of olanzapine ODT and olanzapine SOT during the usual treatment of schizophrenia patients from the perspective of third-party payers within the US healthcare system. As a secondary objective, we compared olanzapine ODT with the ODT and SOT formulations of risperidone and aripiprazole, two other frequently used atypical antipsychotics available in both SOT and ODT formulations.
Patients and methods
Model overview
A Monte Carlo Micro-simulation (MCM) model was developed to compare, from the perspective of a public or private third-party healthcare payer in the US, the cost-effectiveness of atypical antipsychotics in SOT and ODT formulations in the usual care of adult patients treated for schizophrenia. The model includes three frequently used atypical antipsychotics (olanzapine, risperidone, and aripiprazole) in their ODT and SOT formulations, thus comprising six treatment cohorts (including three ODT formulations and their respective three SOT formulations). The model simulates the dynamic nature of usual care over a 1-year period, using quarterly cycles and various input parameters which include adherence levels, relapse with and without hospitalization, health state utilities, treatment discontinuation, treatment-emergent adverse events, healthcare resource utilization, suicide risk, and direct healthcare costs, such as medication costs. Results are based on a simulation of 1,000,000 patients. Key clinical outcomes predicted include quality-adjusted life years (QALYs) and psychiatric inpatient hospitalization rates. Costs are expressed in US dollars based on 2010 values. In the US, the ODT formulations cost more than the SOT formulations of the same antipsychotic medication. The model assumes an intent-to-treat approach that attributes all estimated direct medical costs to the initial therapy. Since the current model is an expansion and update of the previous model, further details are available in the publication of the parent studyCitation16, which only included antipsychotics in SOT formulations. The updates implemented in the current evaluation include use of the most current cost and utilization data whenever possible.
Considering this is a cost-effectiveness model rather than an analysis of patient-level data from a pre-existing study, there are no reported p-values. To assess the robustness of the base case findings—which were based on 100,000 simulations per treatment group—numerous sensitivity analyses were conducted (one-way sensitivity analyses and probabilistic sensitivity analyses). These sensitivity analyses enabled us to run so many replications (e.g., probabilistic simulations of 1000 cohorts of 1000 patients for each of the key parameters) that they essentially reflect a much lower p-value than the traditional p < 0.05 reported in patient-level trial data analyses. Generally, p-values are as much dependent on ‘n’—sample size—as they are the characteristics of the underlying distributions around result estimates. When running so many replications the ‘n’ dominates p-value calculations (especially because the implied distributions are normal and relatively narrow).
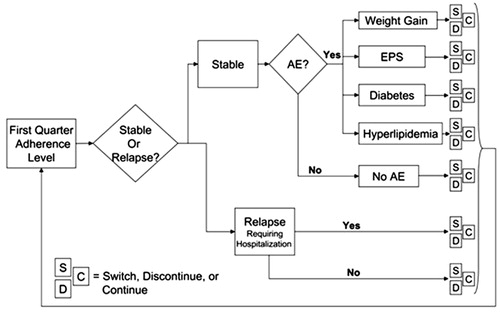
presents a conceptual overview of the micro-simulation model over the first quarter for patients initiated on an antipsychotic medication. Depending on their adherence level, patients may remain stable (no relapse), suffer relapse(s) requiring hospitalization, or relapse(s) not severe enough to warrant psychiatric hospitalization. Patients may also experience treatment-emergent adverse events such as extra-pyramidal symptoms (EPS), clinically significant weight gain (≥7%), diabetes, or hyperlipidemia. Medication discontinuations involve either a switch (S) to another antipsychotic or discontinuing antipsychotic treatment, at least for a while (D). The model takes into account switching patterns and the primary reason for medication discontinuation (poor efficacy, intolerability, patient decision, or other reasons). The patient’s health state at the end of each quarter constitutes the base for the next quarter until the end of four quarters (1 year). If certain adverse events (i.e., diabetes and hyperlipidemia) occur, they are assumed to remain and contribute to treatment costs for the remainder of the study period.
Sensitivity analyses
We first used sequential bifurcation, a process that iteratively samples inputs and assesses the impact of each input against a pre-determined cost threshold valueCitation17, to determine what variables affecting total treatment costs warrant focus during sensitivity analyses. Our sequential bifurcation tested more than 120 input parameters before selecting the variables for sensitivity analyses. Sensitivity analyses were not conducted on input variables that did not vary between antipsychotic medications, such as the cost of most healthcare resources. We also conducted multivariable probabilistic sensitivity analyses (PSAs) to examine the uncertainty in the model and the stability of the results when varying the input values for adherence rates, relapse rates, and treatment discontinuation rates.
Key clinical and economic input values
Key clinical and economic input values were based on evidence reported in peer-reviewed articles. We used input values derived from a clinical expert panel when information was not available in peer-reviewed articles. Consistent with published comparative dataCitation11–13, we also assumed that each of the three ODT formulations is equal to its SOT counterpart on all clinical input values except for better adherence on ODT.
Adherence levels
Level of medication adherence was based on the annual medication possession ratio, which is the proportion of days with the prescribed antipsychotic medication during the 1-year study period, using patient medical or pharmacy claims recordsCitation18–22. Patients were categorized into one of three adherence levels: fully adherent, partially adherent, or non-adherentCitation23. Citation16, Citation23–25 reports the base case adherence level by medication, along with the data source. Consistent with prior researchCitation20,Citation23,Citation25, adherence levels were categorized as: adherent (MPR ≥ 80%), partially adherent (MPR ≥ 60%, <80%), and non-adherent (MPR < 60%).
Table 1. Adherence rates by medication.
The model also incorporates information about adherence level in subsequent cycles following a relapse in the previous quarterly cycle, because US data indicates that adherence levels change from pre-relapse to post-relapse in the usual treatment of schizophreniaCitation23. reports these baseline assumptions.
Table 2. Adherence rates by adherence level in cycle following relapse.
Relapse rates
The model requires a series of assumptions concerning patient relapse rate by adherence levels. To that end, we used data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)Citation26,Citation27, a large US study sponsored by the National Institute of Mental Health (NIMH). The CATIE schizophrenia study is a randomized, double-blind, 18-month study of antipsychotic therapy in the treatment of schizophrenia, and the only US study to provide comparative data on relapse rates for the studied SOT antipsychotics (olanzapine and risperidone), except for aripiprazole. Citation26–28 presents the base case assumptions for the risk of an initial relapse resulting in an inpatient hospitalization by adherence category for each medication. These were estimated using a 3-step process: first, a baseline relapse rate by adherence level was adopted from a studyCitation20 of Medicaid patients with schizophrenia. Second, relapse rates for each medication group were derived from CATIE, Phase 1, the primary phase of the CATIE schizophrenia trialCitation26,Citation27. Consistent with a prior model comparing the cost-effectiveness of antipsychotics in the treatment of schizophreniaCitation29, we assumed that the rates of relapse for aripiprazole were equivalent to ziprasidoneCitation28 and also assumed a constant proportion of inpatient-to-outpatient rates of relapse by adherence level: 1.00 for fully adherent, 1.13 for partially adherent, and 1.11 for non-adherent for all antipsychotic medications studiedCitation28.
Table 3. Relapse rates requiring and not requiring hospitalization.
The model also requires conditional probabilities to allow for rates of inpatient relapse given a history of inpatient relapse ()Citation30,Citation31 and for multiple relapses within a single quarter ()Citation32. We assumed the same conditional probabilities for all studied antipsychotics. These baseline conditional probabilities resulted in a weighted average number of relapses that was nearly identical to the crude rate of relapse for individuals with a history of one relapse reported in the literatureCitation30,Citation31. Additionally, the model incorporates the risk of attempted and completed suicide ()Citation16,Citation25,Citation33. A suicide attempt is considered a relapse event requiring hospitalization.
Table 4. Adjusted relapse rates given a history of relapse.
Table 5. Probability of multiple relapses within a single quarter.
Table 6. Probability of suicide event given adherence level.
Treatment-emergent adverse events
The model requires assumptions about the likelihood of patients experiencing four types of treatment-emergent adverse events that are relevant to the studied antipsychotics: EPS, clinically significant weight gain (≥7% weight gain from baseline weight), diabetes, and hyperlipidemia. Citation26,Citation30,Citation34–36 reports baseline assumptions concerning these treatment-emergent adverse events by medication and data source.
Table 7. Treatment emergent adverse event values.
Treatment discontinuation rates
Consistent with results of the primary phase of the CATIE schizophrenia trial, Phase 1Citation26, patients were assumed to have discontinued medications due to lack of efficacy, medication intolerability, patient decision, or other reasons. Annual discontinuation rates were also based on Phase 1 of the CATIE trialCitation26 (except for aripiprazole, which was not included in CATIE) and are reported in Citation26,Citation35. Following medication discontinuation, patients could switch to another antipsychotic medication. presents baseline assumptions concerning the medication switch patterns by reason for switching. The new medication to which a patient was switched depended on the reason for the switch and the medication from which the patient was being switched. Patients who were treated with a given ODT or SOT formulation and required a switch to another antipsychotic were assumed to have switched to another medication in the same formulation.
Table 8. Treatment discontinuation rates.
Table 9. Treatment switch patterns by reason for switching.
Utility and quality-adjusted life years
Disease-specific utility values for eight schizophrenia disease states were reported by Lenert et al.Citation37 using the Positive and Negative Syndrome Scale, a standard measure of symptom severity in schizophrenia research. presents the baseline utility values assigned to each of the nine possible combinations of three adherence levels by three relapse statuses in the model. A panel of 12 schizophrenia experts was surveyed to determine which of the eight health statesCitation37 that best match the utility of a schizophrenia patient in each of the model’s nine possible adherence/relapse outcomes. Each average survey response was assigned the published utility valueCitation37.
Table 10. Utility values for health states and disutility multipliers for treatment-emergent adverse events.
also reports baseline assumptions concerning disutility among patients experiencing treatment-emergent adverse events. The disutility multipliers for EPS and clinically significant weight gain were derived from Lenert et al.Citation37. Since there are no known peer-reviewed articles reporting utility information for patients with schizophrenia experiencing diabetes or hyperlipidemia, we assumed that utilities among patients experiencing diabetes or hyperlipidemia were equal to utilities of patients experiencing EPS. This assumption was based on the highest, thus most conservative, estimate of adverse event disutility in the Lenert utility study among model’s disutility values.
Medication costs
Medication cost is often related to daily dose levels. To use comparable medication doses for the treatment of schizophrenia patients, we used daily dose levels reported in published, randomized, controlled, schizophrenia studiesCitation26,Citation38,Citation39. Citation26,Citation38–41 reports baseline model assumptions concerning dosing and cost for each medication, reflected by 2010 net wholesale price (NWP)Citation40, showing that, in the US, antipsychotics in ODT formulations cost more than their SOT counterparts.
Table 11. Economic input parameters; medication costs.
Health services resource utilization
Resource utilization assumptions for nine types of healthcare services across five patient outcomes and their data sources are reported in Citation29,Citation32. The length of stay during psychiatric inpatient hospitalization for schizophrenia is derived from the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient SampleCitation29. All other baseline utilization assumptions made were consistent with prior US cost-effectiveness researchCitation16,Citation32.
Table 12. Economic input parameters: health service resource utilization.
Health service resource costs
The baseline costs of each health service resource are reported in . All unit costs were inflated to reflect the value of 2010 US dollars using the medical services component of the consumer price indexCitation42.
Table 13. Economic input parameters: unit costs of health service resources.
Cost of adverse events
The model captures the direct healthcare cost associated with treating four types of treatment-emergent adverse events. Input values and their data sources are presented in Citation42–45. The model assumes that all patients have undergone metabolic monitoring according to published expert consensus guidelinesCitation44, which include lab costs for fasting glucose level or hemoglobin A1c at the time of initiation, 4 months after starting, and at 12 months. However, we assumed patients with clinically significant weight gain would incur the cost of undergoing metabolic monitoring every 4 months.
Table 14. Economic input parameters: costs of adverse events.
Model outcome measures
Clinical outcomes
The model estimates three key clinical outcomes: the proportion of patients experiencing outpatient relapse, those experiencing inpatient relapse, and those without an inpatient or outpatient relapse (i.e., stable).
Economic outcomes
The model also reports mean total direct healthcare costs for the following outcomes: cost of stable patients, cost of outpatient relapse, cost of inpatient relapse, and cost of adverse events. Finally, the model reports the total annual antipsychotic medication cost by medication group.
Cost-effectiveness information
The major cost-effectiveness outcome is cost per 1 QALY gained for each medication. The model also calculates incremental cost-effectiveness ratios (ICERs) as the difference in costs divided by the difference in the appropriate measure of effectiveness.
Results
Clinical outcomes
presents base case results for the key clinical outcomes. Overall, olanzapine ODT was the most effective option reflecting the lowest outpatient relapse rate (14%), lowest inpatient relapse rate (15%), and highest percentage of stable patients (not relapsed) during the study period (72%). Olanzapine SOT was the second most effective medication across these clinical outcomes. Olanzapine ODT yielded the fewest mean inpatient relapses per patient () and the highest QALY (). Results also indicate that each of the three antipsychotics in ODT formulations (olanzapine, risperidone, and aripiprazole) outperformed their respective SOT formulations.
Figure 2. Base case clinical outcomes: Relapse rates. ARIP, aripiprazole; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine; RIS, risperidone.
![Figure 2. Base case clinical outcomes: Relapse rates. ARIP, aripiprazole; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine; RIS, risperidone.](/cms/asset/74a5f3ad-316b-42dd-bb55-ae99052bcc9a/ijme_a_662923_f0002_b.jpg)
Economic outcomes
presents the base case overall direct healthcare cost for each treatment group. The model predicted that the mean total annual costs associated with risperidone SOT—the only atypical antipsychotic available generically in the US—were the lowest ($8881), with olanzapine SOT having the second lowest estimated total direct medical cost ($9533) followed by olanzapine ODT ($9808). also presents the mean annual direct costs for selected estimated cost components (i.e., outpatient, inpatient, adverse events, and medication). These results indicate that the mean annual cost varied by selected cost component. For example, olanzapine ODT and olanzapine SOT had the highest annual medication acquisition cost ($4007 and $3566, respectively), while risperidone SOT (generic cost) had the lowest ($463). In addition, olanzapine ODT and SOT had the highest mean annual total cost of treating relapse-free (stable) patients, $1621 and $1607, respectively. On the other hand, the model estimated that olanzapine ODT had the lowest annual mean cost of treating relapses in either inpatient ($3376) or outpatient ($432) settings, and olanzapine SOT had the second lowest cost for both types of relapses ($3541 and $449, respectively).
Cost-effectiveness
Cost-effectiveness results for the base case () show that, compared with olanzapine SOT therapy, olanzapine ODT therapy was more costly ($9808 vs $9533) but more effective in terms of better QALYs (0.747 vs 0.733) and a lower hospitalization rate (15% vs 16%) (). Direct pairwise comparisons () of olanzapine ODT and other therapies show that olanzapine ODT was cost-effective compared with olanzapine SOT (ICER: $19,643) and risperidone SOT therapy (ICER: $39,966), and dominant (less costly and more effective) compared with risperidone ODT and aripiprazole in ODT or SOT formulations.
Table 15. Base case cost-effectiveness results.
Table 16. Base case relapse rates.
shows the base case relapse rates and mean number of inpatient relapses per person per treatment group, indicating that the cost-effectiveness of olanzapine ODT is driven by its lower rates of relapse and its higher proportion of patients who are relapse-free (stable).
Sensitivity analyses results
Three parameters were evaluated in one-way sensitivity analysis: adherence, persistence, and differential rates of relapse between olanzapine ODT and olanzapine SOT (). In real life, patients who are considered poorly adherent can be non-adherent or partially adherent to their medication regimen. It was of interest to assess whether results of the model will change in a meaningful manner if the non-adherent become fully adherent or whether the partially adherent become fully adherent in usual care. The sensitivity analysis varied the proportion of patients who become fully adherent after being previously non-adherent or partially adherent. The analyses on the proportion of fully adherent patients show that olanzapine ODT is cost-effective when this proportion is increased. Additional analysis (data not shown) predicted that olanzapine ODT is cost-effective regardless of the patients’ prior adherence level (partial adherence or non-adherence): olanzapine ODT yielded nearly as many QALYs gained when the proportion of fully adherent patients was 23%, regardless of the category from which patients were taken (partial adherence or non-adherence).
Table 17. One-way sensitivity analysis and QALY ICERs for ODT olanzapine vs olanzapine (standard oral tablet formulation).
The analyses on the absolute rates of annual discontinuation (persistence) show that olanzapine ODT is cost-effective for a 60% annual discontinuation rate as well as a 54% rate of discontinuation and remains cost-effective when the discontinuation is less than the discontinuation rate for olanzapine SOT. The costs in the last two scenarios are a consequence of higher costs due to greater persistence of a slightly more costly therapy. One-way results on the relative risk of relapse showed that, when the relapse rates of olanzapine ODT and olanzapine SOT were the same, olanzapine ODT was more costly, more effective, and cost-effective relative to the $50,000/QALY threshold. Olanzapine ODT afforded greater cost savings as the relative risk of relapse decreased. Furthermore, when medication acquisition costs of olanzapine ODT and oral are equal, there is an ICER of $8071 per QALY, which is half the base case ICER ($19,643). The total healthcare costs are not equal due primarily to the more expensive switch pattern for olanzapine ODT than olanzapine SOT (as the model assumes that switching from any ODT formulation is preferred over another ODT formulation).
The PSA was performed on adherence (proportion of fully adherent patients, ), persistence (annual discontinuation rate, ), and relative risk of relapse () as well. The PSA results are presented as ‘willingness to pay’ curves, which are based upon 1000 simulations of 1000 person cohorts, and they show the proportion of cohorts whose mean cost per QALY was at or below selected threshold levels. Distributions were created for all model parameters except for the aforementioned. Beta distributions were used for probabilities and log-normal distributions for cost parameters. Cost parameters were correlated, as were parameters affecting relapse, adherence, and persistence. The simulation was then executed, changing each of the parameters listed across four values, one of which was the base case.
Figure 6. Proportion of cohorts at or below selected ICER thresholds varying proportion of patients fully adherent. *The increase in full adherence for olanzapine ODT is assumed to come from the partially adherent patients. ICER, incremental cost-effectiveness ratios; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine.
![Figure 6. Proportion of cohorts at or below selected ICER thresholds varying proportion of patients fully adherent. *The increase in full adherence for olanzapine ODT is assumed to come from the partially adherent patients. ICER, incremental cost-effectiveness ratios; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine.](/cms/asset/2d5f08e7-d0bf-41a2-b5d0-0cb8ebee973f/ijme_a_662923_f0006_b.jpg)
Figure 7. Proportion of cohorts at or below selected ICER thresholds varying the absolute difference in annual discontinuation rate. ICER, incremental cost-effectiveness ratios; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine.
![Figure 7. Proportion of cohorts at or below selected ICER thresholds varying the absolute difference in annual discontinuation rate. ICER, incremental cost-effectiveness ratios; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine.](/cms/asset/2973ba23-a497-4878-b79a-f00cfe0717fd/ijme_a_662923_f0007_b.jpg)
Figure 8. Proportion of cohorts at or below selected ICER thresholds varying the relative risk of relapse. Relative risk (RR) is used to calculate ODT OLZ relapse rates relative to OLZ (ODT OLZ = RR * OLZ). ICER, incremental cost-effectiveness ratios; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine.
![Figure 8. Proportion of cohorts at or below selected ICER thresholds varying the relative risk of relapse. Relative risk (RR) is used to calculate ODT OLZ relapse rates relative to OLZ (ODT OLZ = RR * OLZ). ICER, incremental cost-effectiveness ratios; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine.](/cms/asset/661f108f-58ff-41bb-a645-4148e108ef1c/ijme_a_662923_f0008_b.jpg)
illustrate the results of each of these simulation groups. When sampling model parameters from distributions, adherence had a large impact on the range of results (). The black line in indicates that when the difference in initial adherence between olanzapine ODT and olanzapine SOT is 30% the proportion of cohorts below any threshold is relatively constant (58–65%). In contrast, the remaining three series in indicate that the proportion of cohorts below a selected ICER threshold increase as the value of the threshold increases.
demonstrates that persistence (annual discontinuation rate) increases the proportion of cost-effective cohorts by ∼10% for each 6% absolute change in annual persistence rate. However, all cohorts for all persistence values were generally cost-effective if the willingness to pay to gain one QALY was $25,000.
Perhaps the greatest range of values can be gained by manipulating the relative risk of relapse. In , the data represented by the black line are based upon equal rates of relapse between olanzapine ODT and olanzapine SOT, where 100% of the simulations were cost-effective at the $40,000 level. However, a relative risk of 0.85 (ODT vs SOT) moves the results significantly, where 100% of the cohorts simulated are cost-effective at the $5000 level. Finally, all olanzapine ODT cohorts were cost-effective if the relative risk of relapse for olanzapine ODT was 75% of the risk of relapse for olanzapine SOT (risk reduction = 25%). A ‘willingness to pay’ curve represents the probability that an intervention is cost-effective as a third-party payer changes the CE threshold at which they accept that a treatment is cost-effective. For example, in the ‘willingness to pay’ curve depicted in , the dependent axis represents the probability that OLZ ODT is cost-effective when compared to OLZ SOT. The black line in shows that if a third-party payer accepts that $50,000/QALY gained is a suitable CE threshold then all of the cases simulated on OLZ ODT are predicted to be cost-effective compared to OLZ SOT. However, if a third-party payer has a lower acceptable threshold of $10,000/QALY gained, then the probability of OLZ ODT being cost-effective is ∼0.20 (20% of cases).
Discussion
This is the first study to compare the cost-effectiveness of an ODT with its respective SOT formulation to help estimate the cost-effectiveness of this innovative drug delivery system in the treatment of schizophrenia. This study compared the cost-effectiveness of olanzapine ODT and olanzapine SOT in the usual treatment of schizophrenia patients from a US healthcare perspective, and further compared olanzapine ODT with two other atypical antipsychotics available in both ODT and SOT formulations—risperidone and aripiprazole. By expanding and updating our previously published micro-simulation economic decision-making modelCitation16, which compared the cost-effectiveness of olanzapine SOT with other atypical antipsychotics in SOT formulations in the treatment of schizophrenia in the US, this study projected that antipsychotics in ODT formulations are more cost-effective than their respective SOT formulations. This micro-simulation model further projects that olanzapine ODT is cost-effective compared with risperidone and aripiprazole in both SOT and ODT formulations.
Base case results of this study, reinforced by results of multiple one-way and PSA (e.g., PSA results based on 1000 simulations of 1000 person cohorts), show that utilization of olanzapine ODT for the treatment of schizophrenia was slightly more costly (by $275 per patient per year), but more effective in terms of a lower relapse and hospitalization rate and better disease-specific QALYs than olanzapine SOT therapy, translating to an ICER of $19,643. Olanzapine ODT was also projected to be more cost-effective compared with risperidone SOT using its generic cost (ICER: $31,966) and less costly and more effective—thus considered a dominant choice—compared with risperidone ODT and aripiprazole in ODT and SOT formulations.
In developing this micro-simulation model, we tried to accurately simulate the dynamic treatment of schizophrenia in usual care settings in the US, where patients may start then switch, continue, or discontinue their medication for a number of reasons, including lack of medication efficacy or treatment-emergent adverse events. Taking into account the heterogeneity of schizophrenia (e.g., differences in patient health states profiles, variations in their adherence levels) and the complexity of its treatment in clinical practice, this micro-simulation model used scientifically sound published data to populate clinical and economic parameters, such as event probabilities, types of resource used and their direct costs, and minimize the need to rely on expert opinion.
Importantly, one of the core assumptions of the model was that better adherence on ODT would lead to more favourable clinical outcomes, including a lower risk of relapse and hospitalization, and thereby alter the cost-effectiveness ratio. The robust link between medication adherence and outcomes has been repeatedly demonstrated in numerous schizophrenia studies, using various methods that range from double-blind randomized trialsCitation26 to prospective observational studiesCitation20,Citation23 and retrospective claims database analysesCitation3,Citation4,Citation31. In addition, the assumption that patients are more adherent on ODT formulations than on SOT formulations of the same antipsychotic medication was based on data from a published 16-week randomized double-blind, double-dummy study that compared olanzapine ODT and olanzapine SOT in the treatment of patients with schizophreniaCitation13. Although that study had a robust experimental design, it is unknown whether its findings will extrapolate to long-term adherence in real life. Further naturalistic observational research will be needed to address this important question. While this model’s core assumption was based on data from a single study, the only randomized study to offer a head-to-head comparison of adherence levels of the two formulations, this study is augmented by other studies in which olanzapine ODT was associated with improved patient attitudes toward medicationCitation11,Citation12 and with improved medication adherence at inpatient and outpatient settingsCitation11–13. These findings are also consistent with a published randomized, open-label, cross-over study that compared patient preference for olanzapine ODT vs olanzapine SOTCitation14, which found the majority of patients (61%) preferred olanzapine ODT, whereas only 27% preferred olanzapine SOT, and 12% expressed no preference. Taken in conjunction with the high probability of cost-effectiveness predicted by the model, the scientific literature shows consistent support for this model’s core assumptions, although additional supportive data will be needed from usual care settings.
To maximize the model’s validity and transparency, we examined the uncertainty in the model and the stability of the results using one-way sensitivity analysis and PSA for the model’s core assumptions on differential adherence, persistence, and rates of relapse, showing the robustness of the base case findings. Although adherence had a relatively large impact on the range of results, the main driver of the model’s findings was relapse requiring inpatient hospitalization, which is the costliest component in the treatment of schizophrenia. However, adherence and relapse are related, as better adherence is linked to a lower risk of psychiatric hospitalization in the treatment of schizophrenia. Generally, prior schizophrenia researchCitation3,Citation4,Citation20,Citation23 has shown that, compared to adherent patients, the non-adherent are about twice as likely to have psychiatric hospitalizations over a 1-year period. Considering that hospitalization (i.e., relapse requiring inpatient hospitalization) was the core driver in this model, it is important to underscore that psychiatric hospitalization rates in this model were based on the National Institute of Mental Health-sponsored CATIE trialCitation26, in which olanzapine-treated patients had the lowest annual rate of hospitalization for exacerbation of schizophrenia. While the CATIE trial showed that atypical antipsychotics significantly differ from each other on effectiveness as well as on safety and tolerability profiles, it is important to note that differential efficacy among atypical antipsychotics has also been shown in a recent meta-analysis. In their comprehensive meta-analysis, Leucht et al.Citation46 included 293 publications of 78 studies, with 13,558 participants manifesting a relatively chronic course of schizophrenia, and found the SOT formulation of olanzapine to be superior to aripiprazole and risperidone (also superior in comparisons with quetiapine and ziprasidone). Their sensitivity analyses showed that results were robust with regard to the effects of pharmaceutical industry sponsorship of some studies, antipsychotic dosages, study quality, and trial duration. Findings of our cost-effectiveness study—as they specifically pertain to olanzapine and aripiprazole in SOT formulations—are consistent with a recent cost-effectiveness study comparing these two antipsychoticsCitation47. That study used patient-level data from a randomized, double-blind study comparing olanzapine and aripiprazole in the treatment of patients with schizophrenia. Olanzapine was found to be a dominant cost-effective choice, because it was associated with greater effectiveness at lower total healthcare costsCitation47.
Our model has, however, a number of limitations. First, lack of published medical literature for some model input parameters (e.g., QALYs by health states and adherence levels) required using expert panel opinions. In addition, lack of head-to-head randomized studies comparing all three studied atypical antipsychotics (i.e., olanzapine, risperidone, and aripiprazole) required making input assumptions that need further study (e.g., that aripiprazole and ziprasidone are similar on clinical and safety features). Second, the model does not include all antipsychotics currently available in the US in ODT formulation, thus excludes clozapine ODT. This exclusion was made a priori, as clozapine is used infrequently in the US and is often reserved for treatment-resistant patients with schizophrenia.
Third, the model used a 1-year time horizon, although schizophrenia is a life-long illness. While this follow-up duration is used in most other schizophrenia cost-effectiveness models, it may not be sufficiently long to observe changes in costs and outcomes over the course of a chronic illness or the potential long-term medical and economic impact of metabolic changes such as weight gain, diabetes, and hyperlipidemia. Moreover, it may also not allow for accurate assessment of specific treatment-emergent adverse events such as tardive dyskinesia which could take longer to develop. However, the use of a 1-year time horizon is often deemed to be sufficient for demonstrating the cost implications of treatment strategies for US payers who typically work with annual budgets and to be also clinically meaningful, as schizophrenia patients tend to frequently change their medication regimensCitation26.
A fourth limitation of the model is its focus on direct cost and exclusion of indirect cost, which can be substantial in the treatment of schizophrenia. However, this study considered the perspective of the healthcare payers in the US, thus excluded indirect, non-medical costs, such as cost of lost productivity or cost of patient involvement with the criminal justice system. Had indirect costs been considered in the model, it was hypothesized that treatment with olanzapine ODT would have resulted in more favourable results, because the indirect costs of relapse (the major cost driver in the model) would have been much greater. Another limitation is that this model used the 2010 prices and changes in the price of antipsychotics have occurred since that time. To address this issue, we re-ran the model using the generic Net Wholesale Price (accessed December 19, 2011) of risperidone ODT, and found that the results (not shown) were essentially unchanged, except that olanzapine ODT no longer dominated (was less costly and more effective) risperidone ODT, but was more cost-effective than risperidone ODT at ICER of $34,062/QALY. This was not unexpected, as this model’s results appear to be driven primarily by the cost of relapse. We also re-run the model using generic NWP (accessed December 19, 2011) of both olanzapine (ODT; SOT) and risperidone (ODT; SOT), as the US patent for branded olanzapine (Zyprexa®) has expired recently in the US (October 23, 2011). Again, results (not shown) remained essentially unchanged; only this time olanzapine ODT was found to dominate—to be less costly and more effective—all of the studied comparators.
Finally, the model did not take into account that some patients may have pre-existing adverse events and medical conditions, including diabetes and hyperlipidemia, which may impact future costs and outcomes. Additional research is needed to help identify which patients with what profiles respond best to which antipsychotic after failure on specific medications for what reasons.
Conclusions
Results from this micro-simulation model, which are evaluated from the perspective of payers in the US healthcare system, suggest that utilization of an antipsychotic in its ODT formulation is more cost-effective than using its SOT formulation in the treatment of schizophrenia. More specifically, olanzapine ODT was found to be more cost-effective than olanzapine SOT and more cost-effective than risperidone and aripiprazole in either ODT or SOT formulations. This model simulates real-world treatment processes and provides projections that should be used only to inform decision-making processes from the US healthcare system perspective. As with any other economic model, current findings will require future revision and validation of baseline assumptions when new and additional relevant scientific data are available.
Transparency
Declaration of funding
This study was funded by Eli Lilly and Company.
Author contributions
HAS initiated the model, helped with model development, interpretation of the results, and preparation of the manuscript. NMF developed the model, conducted the sensitivity analyses, interpreted the results, and helped draft the manuscript. AHL, RRC, and SDC helped interpret the results and assisted with manuscript preparation and revision. RWK and LJS helped develop the model and its sensitivity analyses. All authors read and approved the final manuscript.
Declaration of financial and other relationships
Haya Ascher-Svanum, Anthony Lawson, and Robert Conley are all full-time employees and minor shareholders of Eli Lilly and Company. Nicolas Furiak, Robert Klein, Lee Smolen, and Steven Culler have had consulting agreements with Eli Lilly and Company.
Acknowledgments
The authors thank Angela Lorio, ELS, Senior Medical Editor at i3, an Inventiv Health Company, for her assistance with formatting and editing of the manuscript.
References
- Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv 1998;49:196-201
- Ascher-Svanum H, Zhu B, Faries D, et al. A comparison of olanzapine and risperidone on the risk of psychiatric hospitalization in the naturalistic treatment of patients with schizophrenia. Ann Gen Hosp Psychiatry 2004;3:11
- Law MR, Soumerai SB, Ross-Degnan D, et al. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry 2008;69:47-53
- Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Medical Care 2002;40:630-9
- Marcus SC, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr Bull 2008;34:173-80
- Chue P, Jones B, Taylor CC, et al. Dissolution profile, tolerability, and acceptability of the orally disintegrating olanzapine tablet in patients with schizophrenia. Can J Psychiatry 2002;47:771-4
- Keith S. Advances in psychotropic formulations. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:996-1008
- Wertheimer AI, Santella TM, Finestone AJ, et al. Drug delivery systems improve pharmaceutical profile and facilitate medication adherence. Adv Ther 2005;22:559-77
- Bogner RH, Wilkosz MF. Fast-dissolving tablets: new dosage convenience for patients. US Pharmacist 2002;27:34-43
- Kuchekar BS, Badhan AC, Mahajan HS. Mouth dissolving tablets: a novel drug delivery system. Pharma Times 2003;35:7-9
- Kinon BJ, Hill AL, Liu H, et al. Olanzapine orally disintegrating tablets in the treatment of acutely ill non-compliant patients with schizophrenia. Int J Neuropsychopharmacol 2003;6:97-102
- Czekalla J, Wagner T, Schact A, et al. Effectiveness and medication acceptance of olanzapine disintegrating tablets compared to standard olanzapine tablets in acutely treated psychiatric patients. Patient Prefer Adherence 2007;1:19-27
- Karagianis J, Grossman L, Lee B, et al. A 16-week, randomized, double-blind, double-dummy trial of sublingual orally disintegrating olanzapine vs standard olanzapine tablets in patients who gained weight during olanzapine. Schizophr Res 2008; 102:Suppl 2. http://www.sciencedirect.com/science/article/pii/S0920996408707175 [Accessed February 2012]
- Ciorabai EM, Oyffe I, Dilbaz N, et al. Patients preference of olanzapine orodipersible tablets compared with olanzapine classic oral tablet in a multinational randomized crossover study. Eur Psychiatry 2008;23(2 Suppl):S150-1
- San L, Casillas M, Ciudad A, et al. Olanzapine orally disintegrating tablet: a review of efficacy and compliance. CNS Neurosci Ther 2008;14:203-14
- Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness model comparing olanzapine and other oral atypical antipsychotics in the treatment of schizophrenia in the United States. Cost Eff Resour Alloc 2009;7:4
- Bettonvil B, Kleijnen JPC. Searching for important factors in simulation models with many factors: sequential bifurcation. Eur J Oper Res 1997;96:180-94
- Zhao Z. A retrospective economic evaluation of olanzapine versus risperidone in the treatment of schizophrenia. Manag Care Interface 2002;15:75-81
- Rascati KL, Johnsrud MT, Crismon ML, et al. Olanzapine versus risperidone in the treatment of schizophrenia: a comparison of costs among Texas Medicaid recipients. Pharmacoeconomics 2003;21:683-97
- Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004;161:692-9
- Gibson PJ, Damler R, Jackson EA, et al. The impact of olanzapine, risperidone, or haloperidol on the cost of schizophrenia care in a medicaid population. Value Health 2004;7:22-35
- Ascher-Svanum H, Zhu B, Faries DE, et al. Adherence and persistence to typical and atypical antipsychotics in the naturalistic treatment of patients with schizophrenia. Patient Prefer Adherence 2008;2:67-77
- Ascher-Svanum H, Zhu B, Faries DE, et al. Medication adherence levels and differential use of mental-health services in the treatment of schizophrenia. BMC Res Notes 2009;2:6
- Karagianis J, Grossman L, Landry J, et al. A randomized controlled trial of the effect of sublingual orally disintegrating olanzapine versus oral olanzapine on body mass index: the PLATYPUS Study. Schizophr Res 2009;113:41-8
- Ahn J, McCombs JS, Jung C, et al. Classifying patients by antipsychotic adherence patterns using latent class analysis: characteristics of nonadherent groups in the California Medicaid (Medi-Cal) program. Value Health 2008;11:48-56
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia [Erratum]. N Engl J Med 2010;363:1092-3
- Zimbroff D, Warrington L, Loebel A, et al. Comparison of ziprasidone and aripiprazole in acutely ill patients with schizophrenia or schizoaffective disorder: a randomized, double-blind, 4-week study. Int Clin Psychopharmacol 2007;22:363-70
- Agency for Healthcare Research and Quality (AHRQ). HCUP Nationwide Inpatient Sample (NIS), 2004. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality. Rockville, MD: AHRQ, 2004. http://hcupnet.ahrq.gov/HCUPnet.jsp. [Accessed 9 December 2010]
- Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv 2000;51:216-22
- Tiihonen J, Wahlbeck K, Lonnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ 2006;333:224
- Edwards NC, Locklear JC, Rupnow MF, et al. Cost effectiveness of long-acting risperidone injection versus alternative antipsychotic agents in patients with schizophrenia in the USA. Pharmacoeconomics 2005;23(1 Suppl):75-89
- Siris SG. Suicide and schizophrenia. J Psychopharmacol 2001;15:127-35
- Lambert BL, Cunningham FE, Miller DR, et al. Diabetes risk associated with use of olanzapine, quetiapine, and risperidone in veterans health administration patients with schizophrenia. Am J Epidemiol 2006;164:672-81
- Fleischhacker WW, McQuade RD, Marcus RN, et al. A double-blind, randomized comparative study of aripiprazole and olanzapine in patients with schizophrenia. Biol Psychiatry 2009;65:510-7
- Carlson CD, Cavazzoni PA, Berg PH, et al. An integrated analysis of acute treatment-emergent extrapyramidal syndrome in patients with schizophrenia during olanzapine clinical trials: comparisons with placebo, haloperidol, risperidone, or clozapine. J Clin Psychiatry 2003;64:898-906
- Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr Res 2004;71:155-65
- Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001;158:765-74
- Kern RS, Green MF, Cornblatt BA, et al. The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacology (Berl) 2006;187:312-20
- Analysource Data. AWP/NWP cost of antipsychotics. www.Analysource.com. Accessed December 9, 2010
- Tunis SL, Faries DE, Nyhuis AW, et al. Cost-effectiveness of olanzapine as first-line treatment for schizophrenia: results from a randomized, open-label, 1-year trial. Value Health 2006;9:77-89
- Bureau of Labor Statistics. Consumer Price Index – Medical Services Component. United States Department of Labor, 2010. http://data.bls.gov/cgi-bin/surveymost?cu. Accessed 6 September 2011
- Vera-Llonch M, Delea TE, Richardson E, et al. Outcomes and costs of risperidone versus olanzapine in patients with chronic schizophrenia or schizoaffective disorders: a Markov model. Value Health 2004;7:569-84
- Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-49
- drugstore.com. 2011. http://www.drugstore.com/pharmacy/prices/drugprice.asp?ndc=00006074954&trx=1Z5006; Accessed 7 September 2011
- Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 2009;166:152-63
- Ascher-Svanum H, Stensland MD, Peng X, et al. Cost-effectiveness of olanzapine vs. aripiprazole in the treatment of schizophrenia. Curr Med Res Opin 2011;27:115-22

![Figure 3. Base case clinical outcomes—Inpatient relapses. Mean number of inpatient relapses per patient. ARIP, aripiprazole; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine; RIS, risperidone.](/cms/asset/6204c810-9953-464e-a500-f8c4b81a411d/ijme_a_662923_f0003_b.jpg)
![Figure 4. Base case clinical outcomes—Mean QALYs gained. ARIP, aripiprazole; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine; QALYs, quality-adjusted life years; RIS. risperidone.](/cms/asset/03e09d5f-c397-47db-af70-69d73832956e/ijme_a_662923_f0004_b.jpg)
![Figure 5. Base case economic outcomes. ARIP, aripiprazole; ODT, orally disintegrating tablet [formulation]; OLZ, olanzapine; RIS, risperidone.](/cms/asset/90263d2e-e8b9-467d-a276-baffac3226b5/ijme_a_662923_f0005_b.jpg)