Abstract
Objective:
Incidence of breast cancer with brain metastases (BCBM) is increasing, especially among patients over-expressing HER2. Epidemiology on this sub-type of cancer is scarce, since cancer registries carry no information on the HER2 status. A retrospective database analysis was conducted to estimate the burden of BCBM, especially among HER2-positive patients in a secondary objective.
Methods:
Patients with a new diagnosis of BCBM carried out between January and December 2008 were identified from the national hospital database using the International Disease Classification. Patients receiving a targeted anti-HER2 therapy were identified from the national pharmacy database. Hospital and pharmacy claims were linked to estimate the burden of HER2-positive patients. Data on hospitalizations were extracted to describe treatment patterns and healthcare costs during a 1-year follow-up. Predictors of treatment cost were analyzed through multi-linear regression analysis.
Results:
Two thousand and ninety-nine BCBM patients were identified (mean age (SD) = 57.8 (13.6)), of whom 12.2% received a targeted anti-HER2 therapy; 79% of patients had brain metastases associated with extracranial metastases, and the attrition rate reached 82%. Patients received mostly palliative care (47.4%), general medical care (40.6%), and chemotherapy (35.0%). The total annual hospital cost of treatment was 8,426,392€, representing a mean cost of 22,591€ (±14,726) per patient, mainly influenced by extracranial metastases, surgical acts, and HER2-overexpression (p < 0.0001).
Conclusions:
The database linkage of hospital and pharmacy claims is a relevant approach to identify sub-type of cancer. Chemotherapy was widely used as a systemic treatment for breast cancer rather than for local treatment of brain metastases whose morbi-mortality remains high. The variability of treatment costs suggests clinical heterogeneity and, thus, extensive individualization of protocols.
Introduction
Breast cancer (BC) is the second primary tumor responsible for the development of brain metastases (BM)Citation1,Citation2. The incidence of BM is increasing due to advances in diagnosis and improvement of treatments which change the natural history of the disease. BM are frequent, occurring in 10–16% of metastatic breast cancer (MBC)Citation3, especially for patients who over-express the Human Epidermal growth factor Receptor 2 (HER2+). The HER2-oncoprotein was identified as a predictive factor of BMCitation4, but data on this sub-population are scarce, as French cancer registries carry no information on the HER2 status. Ten-to-30% of BC patients over-express the HER2-oncoproteinCitation5,Citation6, but the efficacy of therapies targeting HER2 may have changed the clinical course of this sub-set of patients. Because of their intracranial localization, BM result in important functional disorders such as headaches, altered cognitive or motor functions, psychological disorders, and seizuresCitation7. Therapeutic management can be ablative, such as surgery or stereotactic radio-surgery, depending mainly on the number of intracranial metastases. For multiple BM, whole-brain radiotherapy is considered the treatment of referenceCitation3,Citation8. Chemotherapy is generally used in addition to these reference treatments as a systemic treatment, and is a source of important healthcare costs which have not been extensively evaluated. Indeed, more than 70% of patients with HER2-positive breast cancer with brain metastases (BCBM) are also affected by other extracranial metastasesCitation9,Citation10. Major side-effects such as hair loss or transient worsening of neurological symptoms are associated with classical treatments for BM that may have a major impact on patients’ quality-of-life.
The medical need for effective treatments of BM is still unmet and contributes to the high morbidity and poor prognosis associated with this diseaseCitation3,Citation8. Chemotherapy is not considered as an effective standard treatment for BM because most chemotherapeutic agents do not cross the blood–brain barrier to deliver active substance at the tumor site. Nevertheless, the role of chemotherapy is increasingly debated and studiedCitation7,Citation11–15. Recent data from clinical trials suggest that new oral drugs with a low molecular weight are able to cross the blood–brain barrier and may be effective for treating BMCitation16–19. However, the sub-population likely to benefit from these new treatments remains poorly defined. Epidemiological as well as economic data on BCBM are scarce and fragmentary. Further data are needed to improve understanding of the size and the needs of this population. With the emergence of new oral drugs, it is necessary to estimate the healthcare costs associated with classical treatments and to understand its predictors, especially in an economic environment increasingly constrained.
The availability of exhaustive national hospital and pharmacy claims data in France provides an opportunity to assess the burden of BCBM more completely and to estimate the sub-population of HER2-positive patients which is specifically treated with anti-HER2 therapy. The first objective of our study was to estimate the burden of BCBM and the second, the burden of HER2-positive patients with BCBM. Data were collected on incidence, current treatment patterns, and hospital healthcare costs related to their management.
Patients and methods
Study design and data source
A retrospective cohort analysis of BC patients within their first year of a BM diagnosis was conducted using French national hospital and pharmacy claims recorded between 2006–2009. The hospital claims database contains medico-administrative information registered in the patient medical records during 1-day hospital stays (i.e., outpatient hospitalizations) and conventional hospital stays (i.e., inpatient hospitalizations). The pharmacy claims database contains all expensive innovative drugs delivered during each hospitalization that are paid to hospitals by the Health Insurance in addition to the per-case payment. Since 2008, hospitalizations can be linked to pharmacy claims, but only for public hospitals and non-profit private hospitals involved in public hospital duties. This last hospital category includes cancer treatment centers. This new linkage system allows analyzing expensive drug exposure during hospital stays for a given patient, thanks to a unique anonymous patient number.
Selection of the study population and time of follow-up
Patients included in the study were selected from the national hospital claims database using the diagnostic codes of the 10th Revision of the International Classification of Diseases (ICD-10)Citation20. Inclusion criteria were cumulative ():
Female with a primary or secondary diagnosis of BC (C50);
Who developed secondary BM (C79.3) between 01/01/2008 and 12/31/2008 (i.e., inclusion period), including BM only (without extracranial metastases) as well as BM associated with extracranial metastases (i.e., bone, lung, lymph node, skin and liver metastases);
Who had no previous BM from 2006–2007 (i.e.; newly diagnosed for BM); and
Who were treated in public hospital.
Figure 1. Algorithm used for the selection of the study population. The shaded boxes indicate the patient groups analyzed.
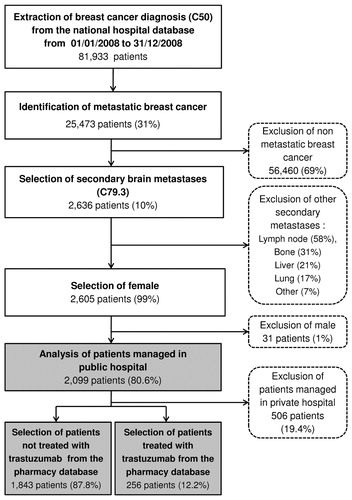
The claims data from 2006–2007 were used to check if patients had a prior hospitalization for BCBM to the inclusion period (01/01/2008 to 12/31/2008). Thus, patients with a hospitalization for BCBM between 01/01/2006 and 12/31/2007 were excluded to fulfill the incidence approach of the analysis. Patients who were included were then followed over a period of 12 months from the date of their inclusion (i.e., date of the diagnosis of BM) until death or last news. For instance, a patient included in July 2008 was followed until July 2009, except if she died before the end of the follow-up period.
Hospitalizations not apparently linked to the BC diagnoses (5.7%) and hospitalizations with no patient identification number (6.5% and 1.8% of hospitalizations, respectively, in 2006 and 2009) were excluded from the database. Patients treated in private hospital were excluded since the information on expensive drugs was not available for these hospitals (i.e., clinics and private centers).
The study cohort was divided into two groups of interest: not trastuzumab treated patients (Group 1) and trastuzumab treated patients (Group 2). The prescription of trastuzumab, a targeted therapy for HER2-positive patients, was used as a surrogate marker for the HER2+ status. The data collected for each patient were anonymous. Since the study was a retrospective analysis of databases, no further specific authorization was required. HEVA (a Contract Research Organization) holds an authorization to extract data from the French hospital and pharmacy claims databases (Authorization CNIL n°1419102).
Cost data and statistical analyses
Costs were estimated from the Health insurance perspective using the national tariffs published in the French Official Journal. Cost analysis included direct medical costs related to treatments occurring in hospital setting (i.e., chemotherapy, radiotherapy, surgery, general medical care, para-clinical care, and palliative care). Costs were calculated over a 1-year period. The cost of treatment per patient (y) included the number of hospitalizations (nh), the per-case payment including physician’s fees (Cs), costs related to additional billing for intensive care or resuscitation (Csup), and costs of expensive drugs (Ced) adjusted for the dose administered (Q):
Descriptive analyses were conducted on the main characteristics of the study population (age, duration of follow-up, metastatic localization, and treatment patterns), and on costs description (distribution). Mean, standard deviation (SD), range, and percentage were calculated. Patients characteristics between group 1 and group 2 were analyzed using ANOVA test for mean comparison and Fisher’s exact test for frequency comparison. Multi-linear regression analyses were conducted on clinical and economic variables to identify the predictors of the mean cost of treatment in each patients group. The following variables were included in the model: mean age, metastatic localization (BM only, BM associated with one extracranial metastasis, BM with multiple extracranial metastases) and treatment pattern (chemotherapy, radiotherapy, surgery, general medical care, para-clinical care, palliative care, expensive innovative drugs, and other treatments). Differences were considered significant at a 5% level. All statistical analyses were performed using the SAS® Software 9.2.
Results
Baseline patients’ characteristics
A total of 2,099 patients with BCBM were included. The median age at diagnosis was 60.0 years (range: 20–98). Groups 1 and 2 accounted for 87.8% and 12.2% of the study population, respectively. Most patients suffered from BM associated with extracranial metastases (79.0%), while 21.0% presented BM only. At the end of the 12-months follow-up period, 84.1% of patients with BCBM had either died or returned home (). The number of patients had decreased from 2,099 (100%), 791 (37.7%), 507 (24.1%) to 371 (17.7%) at 3, 6, 9, and 12 months, respectively.
Table 1. Characteristics of the study population.
Treatment patterns
During the first year following their diagnosis of BM, patients received palliative care (47.4%), general medical care (40.6%), and chemotherapy (35.0%). Radiotherapy and surgery accounted, respectively, for 21.7% and 7.2% of the medical care received (). Local treatment (i.e., surgery and radiotherapy) were mostly used within the first months (). Among the study population, 34.0% of patients received at least one expensive drug therapy. Thirty-seven different expensive innovative drugs were prescribed. Drugs identified in the pharmacy claims database were administered for systemic treatment of BC in 88.0% of the patients. One drug (fotemustin) was approved for the treatment of BM and was prescribed to 0.4% of patients (3/713), and 12.6% of patients had drugs approved for other malignant tumor (i.e., lymphoma, colorectal cancer, …).
Figure 2. Distribution of treatment use during the 1-year follow-up period for the whole population (n = 2,099). This figure presents the distribution of treatment use by 3-month period. For instance, at 3 months of follow-up, 38% of patients had received general medical care, 37% palliative care, 36% chemotherapy and 28% radiotherapy and surgery. The total exceeds 100% since patients may have received one or more types of treatments over the period. Expensive innovative drugs are included in the category chemotherapy.
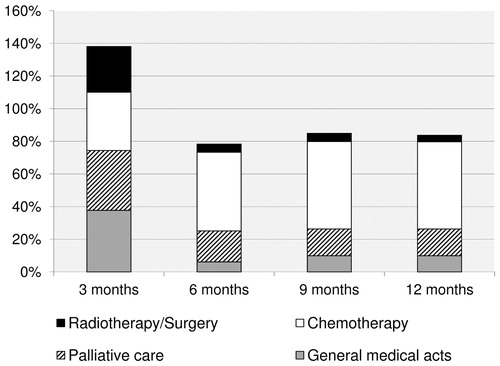
Treatment costs and predictors
The mean annual cost of treatment per patient was 22,591€ (±14,726) and was significantly higher for patients treated with trastuzumab (29,995€ ± 13,484) than for patients not treated with trastuzumab (18,039€ ± 13,587) (p < 0.001) (). The 1-year hospital cost of management of the whole population was 8,426,392€. Expensive drugs accounted for 44.8% of the mean annual cost of treatment per patient. An important variability of costs has been noticed, especially for chemotherapy and palliative care treatments (). Main cost drivers were surgery (p < 0.0001), extracranial metastases associated with BM (p < 0.0001), and the HER2-positive status (p < 0.0001) ().
Figure 3. Box and whisker plot of the distribution of healthcare costs (n = 2,099). The cost shown on the graph is the median cost per treatment category. From top to bottom of the graph: the largest observation (♦), the upper quartile, the median (–), the lower quartile and the smallest observation (▪).
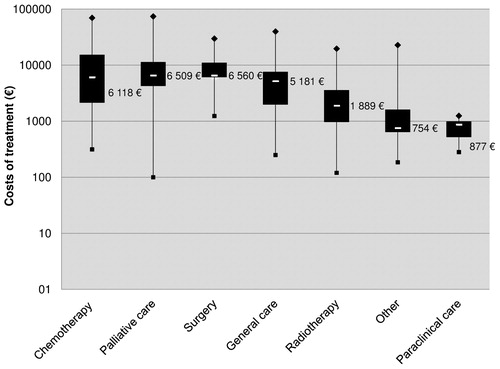
Table 2. Healthcare costs over 1 year of follow-up.
Table 3. Multivariate regression analyses performed on the mean cost of treatment.
Discussion
This study presents data on epidemiology, treatment patterns, and hospital costs of BC over the first year following the diagnosis of BM using the French hospital and pharmacy claims databases from 2006–2009, and provides the first data on the sub-population of HER2-positive patients at a population level.
In our study population, 12.2% of patients were assumed to be HER2+, which is consistent with the available literatureCitation21,Citation22. Nevertheless, we should notice that, despite the implementation of HER2 biological marker diagnosis into routine clinical practice, a slight proportion of patients may have an indeterminate status and, thus, are not treated with targeted therapy (i.e., technical diagnosis failure, changes of serum HER2 status during clinical course). The second factor contributing to under-estimate the HER2+ population may be due to patients who cannot be treated with trastuzumab because of contraindications or severe side-effect (i.e., cardio toxicity) requiring a change of treatment (0.24% according to Horiguchi et al.Citation23). The third reason may be due to patients or clinicians who may refuse the intravenous administration of chemotherapy, preferring the oral route of administration, and which are excluded from hospital careCitation24. So far, the availability of database linkage between hospital and Health Insurance claims, that includes consumptions of non-hospital care, do not allow analyzing the full course of healthcare.
Despite those limitations, epidemiological findings are consistent with data reported by previous studies. The diagnosis of BMs was often associated with extracranial metastases (79.0% of BCBM patients), as reported in a previous retrospective study (74%, n = 207)Citation10. Despite the absence of survival data, results indicate that, after 12 months of follow-up, 84.1% of patients were reported to have died or to have returned home. If we consider the high morbidity on this sub-type of cancer, there is a high probability that the percentage of patients who returned home is marginal compared to the percentage of death. This finding is consistent with a literature review (2004) suggesting a 20% survival rate in BCBMs after 1-year of follow-upCitation3, and with the median survival time reported in the literature (2–16 months)Citation25,Citation26.
Most frequently used chemotherapeutic regimens are consistent with those reported previously reported (i.e., paclitaxel, docetaxel, vinorelbineCitation7). Chemotherapy was offered to 35% of patients in our cohort, while the use of radiotherapy, which is the standard treatment of BM, was only used for 21.7% of patients and appeared lower than the rates having been reported by othersCitation8,Citation25. Indeed, radiotherapy sessions are conducted principally in the private sector (50% in a previous survey and from the Statistique Annuelle des Etablissements databaseCitation27), and therefore are not identifiable through the public hospital claims database.
The difference of mean costs between the two groups may be due to the use of trastuzumab, which accounted for 57.2% of the overall cost of expensive drugs after a 12-month follow-up. On the whole, the mean cost of treatment reported in our study is difficult to compare with the literature, since studies analyze different costs components and different types of advanced BC, not restricted to BCBMCitation28–31. Drug tariffs vary also from one country to another, depending on the healthcare system, and may contribute to variability in the costs reported. Nonetheless, our results are consistent with those reported by Pelletier et al.Citation32. From a US claims data analysis, they reported a mean annual cost of 23,738$ per patient for hospitalizations within an incident cohort of BCBM. Finally, we found marked variability in hospital costs between and within treatment categories, especially for chemotherapy and palliative care. Clinical heterogeneity between patients and the widespread use of individualized therapeutic protocols for metastatic stage may contribute to the inherent variability in cost analysis.
Nevertheless, this study shares the typical limitations of claims database analysis. First, the number of BCBM cases may be under-estimated due to misclassification or incomplete reporting of BM diagnoses related to BC. Nonetheless, missing data decreased over the period 2006–2009, suggesting an improvement in the quality of hospital data collection. Our economic evaluation was limited to hospital costs, since information on non-hospital costs was not available (e.g., home nursing, oral drugs). These costs are assumed to be important, due to the increasing proportion of patients with cancer who are cared at homeCitation33. Finally, the estimation of expensive drug costs was conducted using national tariffs, which do not necessarily exactly reflect the actual costs of drugs. The latter may vary from hospital-to-hospital, depending on agreements reached between hospital pharmacies and wholesalers. No data on the mismatch between actual costs and published tariffs is available. Direct non-medical costs (i.e., medical transportation, lost wages) would also be important to estimate in future analyses from a societal perspective. BCBM have an impact on patient’s quality-of-life and costs supported by the patient’s family could be high, especially for the end-of-life care.
Despite those limitations, the use of a national database is a relevant approach at several levels. The claims database ensures representativeness of the analyzed population since data are collected from all hospitals, in contrast to the prospective studies performed in selected centers, not necessarily representative of national treatment norms. Secondly, specific patient groups often excluded from clinical trials could be analyzed in such database (i.e., elderly patients or patients with comorbidities). Thirdly, hospital and pharmacy claims studies are used by the health authorities for tracking reimbursement objectives. As a result, data on resource utilization are entered systematically into databases, allowing collection of good quality data and minimizing the risk of under-reporting of costs due to mis-coding. It should also be noted that, in France, regulatory measures ensure, in principle, equal access to innovative drugs for all patients. Therefore, the estimation of populations treated with these drugs is assumed to be relatively exhaustive and consistent with national healthcare practice.
Conclusion
Several randomized and observational studies have described the burden of BCBM, but, so far, no data were available at a population level. Our findings confirm that hospital and pharmacy claims data could be a relevant alternative to a typical epidemiological database such as cancer registries. In addition, these results highlight the lack of chemotherapeutic alternative to radiotherapy and surgery for the local treatment of BM. Given the high morbi-mortality, there is a critical medical need to develop effective therapies to treat or to prevent the occurrence of BM. In patients whose prognosis is in any case poor, the choice of chemotherapy over supportive and palliative care, or conversely, has cost consequences, for which responsibility should be shared between patients, physicians, and stakeholders.
Transparency
Declaration of funding
Funding for the study was provided by GlaxoSmithKline and had no influence on the study design, execution, and publication of results.
Declaration of financial/other relationships
L.B. has a doctoral fellowship financed in part by GlaxoSmithKline and the Association Nationale pour la Recherche et la Technologie (ANRT). At the time of the study, F.-E.C. was employee at GlaxoSmithKline. F.M. (Stat Process) and A.V. (HEVA) are employees of a Contract Research Organization (CRO). G.V.-T. declares no conflicting interests. I.D.-Z. declares having participated to advisory boards for the pharmaceutical industry, including GlaxoSmithKline.
Acknowledgments
The authors wish to thank Baptiste Jouaneton (HEVA, Lyon, France) for technical help in data collection and Adam Doble (Foxymed, Paris, France) for writing assistance of the manuscript. The results of this study were partly presented at the 13th Annual European Congress of International Society for Pharmacoeconomics and Outcomes Research; November 6–9th 2010, Prague, Czech Republic (PCN59).
References
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72
- Kaal EC, Niel CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol 2005;4:289-98
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol 2004;22:3608-17
- Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 2006;17:935-44
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92
- Drappatz J, Wen PY. Chemotherapy and targeted molecular therapies for brain metastases. Expert Rev Neurother 2006;6:1465-79
- Patchell RA. The management of brain metastases. Cancer Treat Rev 2003;29:533-40
- Ono M, Ando M, Yunokawa M, et al. Brain metastases in patients who receive trastuzumab-containing chemotherapy for HER2-overexpressing metastatic breast cancer. Int J Clin Oncol 2009;14:48-52
- Rades D, Lohynska R, Veninga T, et al. Evaluation of 2 whole-brain radiotherapy schedules and prognostic factors for brain metastases in breast cancer patients. Cancer 2007;110:2587-92
- Fenner MH, Possinger K. Chemotherapy for breast cancer brain metastases. Onkologie 2002;25:474-9
- Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res 2007;13:1675-83
- Peak S, Abrey LE. Chemotherapy and the treatment of brain metastases. Hematol Oncol Clin North Am 2006;20:1287-95
- Tomasello G, Bedard PL, de AE, et al. Brain metastases in HER2-positive breast cancer: the evolving role of lapatinib. Crit Rev Oncol Hematol 2009;75:110-21
- Tosoni A, Franceschi E, Brandes AA. Chemotherapy in breast cancer patients with brain metastases: have new chemotherapic agents changed the clinical outcome? Crit Rev Oncol Hematol 2008;68:212-21
- Addeo R, De RC, Faiola V, et al. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer 2008;113:2524-31
- Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2008;26:1993-9
- Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol 2010;22:625-30
- Sutherland S, Ashley S, Miles D, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases–the UK experience. Br J Cancer 2010;102:995-1002
- World Health Organization. International Classification of Diseases - 10th Revision (ICD-10). WHO, 2007. http://www.who.int/classifications/icd/en/. Accessed February 15, 2010
- Koninki K, Tanner M, Auvinen A, et al. HER-2 positive breast cancer: decreasing proportion but stable incidence in Finnish population from 1982 to 2005. Breast Cancer Res 2009;11:R37
- Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 2007;369:29-36
- Horiguchi H, Yasunaga H, Hashimoto H, et al. Incidence of severe adverse events requiring hospital care after trastuzumab infusion for metastatic breast cancer: a nationwide survey using an administrative claim database. Breast J 2011;17:683-5
- Benjamin L, Cotte FE, Philippe C, et al. Physicians’ preferences for prescribing oral and intravenous anticancer drugs: a discrete choice experiment. Eur J Cancer 2011;October 25 [Epub ahead of print]
- Harputluoglu H, Dizdar O, Aksoy S, et al. Characteristics of breast cancer patients with central nervous system metastases: a single-center experience. J Natl Med Assoc 2008;100:521-6
- Pez E, Gauchez AS, Payan R, et al. Brain metastases exploration in metastatic breast cancer treated with Herceptin®: a place for biological tools? Immuno-Anal Biol Spéc 2007;22:151-5
- Remy V, Mathevet P, Vainchtock A. Vulvar and vaginal cancers and dysplasia in France–an analysis of the hospital medical information system (PMSI) database. Eur J Obstet Gynecol Reprod Biol 2009;147:210-4
- Allen JM. Economic/societal burden of metastatic breast cancer: a US perspective. Am J Manag Care 2010;16:697-704
- Dahlberg L, Lundkvist J, Lindman H. Health care costs for treatment of disseminated breast cancer. Eur J Cancer 2009;45:1987-91
- Galy G, Labidi-Galy SI, Perol D, et al. Chemotherapy for metastatic breast cancer. Comparison of clinical practice and cost of drugs in two cohorts of patients: 1994–1998 and 2003–2006. Breast Cancer Res Treat 2010;128:187-95
- Remak E, Brazil L. Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer 2004;91:77-83
- Pelletier EM, Shim B, Goodman S, et al. Epidemiology and economic burden of brain metastases among patients with primary breast cancer: results from a US claims data analysis. Breast Cancer Res Treat 2008;108:297-305
- Halbert RJ, Zaher C, Wade S, et al. Outpatient cancer drug costs: changes, drivers, and the future. Cancer 2002;94:1142-50
