Abstract
Objective:
Cinacalcet has been used in controlling secondary hyperparathyroidism (SHPT) in dialysis patients since 2004, but its full economic evaluation has not been conducted from the US perspective. This study assesses the cost-effectiveness of cinacalcet and low-dose vitamin D for the treatment of SHPT in dialysis patients compared with flexible vitamin D.
Methods:
A lifetime patient-level simulation model was developed using ADVANCE trial data, including biomarker levels: parathyroid hormone, calcium, and phosphorus. The impact of the biomarkers on mortality, cardiovascular events, fractures, and parathyroidectomy were estimated from literature: Block, an observational study; Cunningham, a combined analysis of four randomized trials of cinacalcet; and Danese, a study investigating the effect of duration in recommended targets. Baseline event rates were derived from the large dialysis organizations registries. One-way and probabilistic sensitivity analyses (PSA) were conducted.
Results:
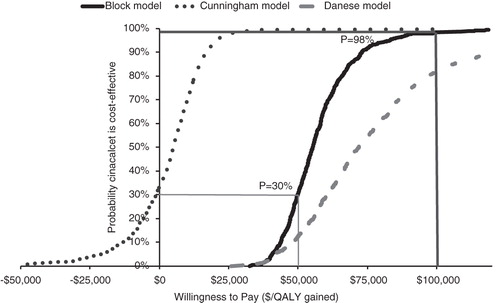
The cost-effectiveness ratio for cinacalcet compared with standard of care (vitamin D and phosphate binders) was $54,560 and $72,456/quality-adjusted-life-year (QALY) gained or an incremental cost of $3155 and $2638 per year alive for the Block and Danese variants, respectively. In the Cunningham variant, cost-effectiveness ratio for cinacalcet was $5064/QALY gained or a cost saving of $1068 per year. The difference in the results of the Cunningham variant vs other variants can be explained by the favorable impact of cinacalcet on outcomes, specifically cardiovascular events observed in the Cunningham study. The PSA showed 98% likelihood for cinacalcet to be cost-effective at $100,000/QALY threshold.
Limitations:
Observational data assessing effects on clinical outcomes, trial restriction to use calcium-containing phosphate binders, no utility data in SHPT dialysis population, and insufficient evidence on long-term impact of cinacalcet and vitamin D on biochemical markers.
Conclusions:
Cinacalcet treatment is cost-effective for treatment of SHPT in the US. Due to cost offsets, cinacalcet can reduce annual costs in some scenarios.
Introduction
Secondary hyperparathyroidism (SHPT) (high levels of parathyroid hormone [PTH] and an imbalance of calcium (Ca) and phosphorous (P) metabolism) is a common complication of end-stage renal disease (ESRD)Citation1,Citation2. Abnormal levels of PTH, Ca, and P in SHPT have been associated with an increased risk of cardiovascular (CV) events, morbidity, mortality, fractures, and parathyroidectomyCitation3–9. In addition, treatment of the complications such as CV disease and fractures carries a significant cost burdenCitation10. A recent analysis (data on file) using data from the published Center for Medical Services’ (CMS) annual expenditures suggests that spending on the management of bone and mineral abnormalities (CV events, fractures, parathyroidectomy and medications) in dialysis patients amounted to almost $1.5 billion, which is 5.3% of the total Medicare spending on dialysis during 2008. In this analysis the full CMS costs of parathyroidectomy and associated medications, and ∼17%Citation5 of the other events costs related to the management of bone and mineral abnormality were attributed to SHPT.
The goal of SHPT treatment has been to control PTH, Ca, and P levels within recommended ranges. Previous recommendation by the National Kidney Foundation Disease Outcomes Quality Initiative (NKF-KDOQI) were 150–300 pg/mL for PTH, 8.4–9.5 mg/dL for corrected serum Ca, 3.5–5.5 mg/dL for serum P and <55 mg2/dlCitation2 for Ca-P product (Ca × P)Citation11. Recently, Kidney Disease: Improving Global Outcomes (KDIGO)Citation12 has recommended somewhat relaxed treatment targets; PTH levels at ∼2–9-times the assay’s upper-normal limit, which translates to 130–600 pg/mL, compared to the KDOQI recommended target of 150–300 pg/mL; lowering P toward the reference range and maintaining Ca within the reference range. Traditional therapies for SHPT have included dietary modification to reduce phosphate intake; use of phosphate binders, calcium supplementation; PTH suppression using Vitamin D or surgical removal of the parathyroid glands (parathyroidectomy)Citation13–16. Vitamin D sterols reduce PTH at the expense of Ca and P, while calcium-containing phosphate binders and calcium supplement may cause hypercalcaemia. Due to the complex metabolic relationship between the various markers, achieving multiple guideline targets simultaneously becomes difficult with traditional therapiesCitation17–19. Recent studies have found that only 7% of patients met all four KDOQI targets (PTH, Ca, P, and Ca × P) simultaneouslyCitation17–21. Achievement of all KDOQI targets has been associated with improved survival after 2 years of dialysis therapyCitation22.
Cinacalcet, a calcimimetic, makes it easier to achieve consistent control of multiple metabolic disordered parameters by acting directly on the calcium-sensing receptors of the parathyroid gland, which is the core of SHPT pathophysiologyCitation23–31. Several interventionalCitation32–39 and observational studiesCitation17,Citation31,Citation40–42 have demonstrated improved control of biomarkers with cinacalcet. Cinacalcet also enables substantially more patients to achieve all four key KDOQI goalsCitation13. Long-term treatment with cinacalcet (range: 1–3.5 years) maintains reductions in PTH, Ca, P, and Ca × P, with no evidence of decreasing effectiveness over timeCitation38,Citation43,Citation44. Several phase IV studies have demonstrated cinacalcet efficacy on biochemical markersCitation23–26 and volume coronary artery calcification (CAC) scores, which are a surrogate marker of cardiovascular diseaseCitation25,Citation26. Lastly, a retrospective analysis of combined phase 3 and phase 2 studies showed an effect on mortality, cardiovascular events, fractures, and parathyroidectomiesCitation39. The impact of cinacalcet on mortality and morbidity are investigated in the on-going Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events [EVOLVE] studyCitation45.
Despite the demonstrated benefit on biomarkers, cinacalcet has been used primarily in more severe cases of SHPT, possibly because treatment with cinacalcet is costly and the lack of data on mortality. Coverage decisions regarding cinacalcet in most countries are based on the clinical effectiveness as well as economic considerations. Three cinacalcet cost-effectiveness models have been published; ACHIEVE46-based model concerning cost (US$) per unit improvement in KDOQI targets; PenTAGCitation47,Citation48, from the UK National Health Service (NHS) perspective; and OPTIMACitation49, from an Italian healthcare perspective, both relating impact of biomarkers on outcomes.
In the US, health economic evaluations are becoming increasingly important. The new prospective payment of dialysis by the CMS (the ‘bundle’) went into effect in January 2011 and included the payment for treatments received in a month. Current treatments included in the bundle are IV drugs given during treatment for ESRD and oral versions of activated vitamin D. Other oral drugs that are currently fee for service, including phosphate binders and cinacalcet, are considered for the inclusion in the bundle over time. The objective of the current study was to assess the cost-effectiveness of cinacalcet as an addition to low-dose vitamin D and phosphate binders for the treatment of SHPT in dialysis patients compared with standard of care (flexible vitamin D and binders) from a US healthcare system using the ADVANCE clinical trial dataCitation25,Citation26. We chose the ADVANCE clinical trial since it is the only US-based trial which evaluates the impact of cinacalcet on biomarkers over a longer time horizon (1 year). It should be noted that the primary outcome of the ADVANCE clinical trial, CAC score, did not reach statistical significance, although some related outcomes (e.g., aortic valve calcification) did.
Methods
A patient-level simulation model was developed from a US healthcare system perspective using Microsoft Office Excel 2007 that estimates the long-term health effects and costs associated with treatment of SHPT in dialysis patients. Each simulated patient was defined with unique characteristics: age; and serum PTH, Ca, and P. The simulation spanning over each patient’s entire lifetime compared the standard of care (flexible vitamin D and phosphate binders) to cinacalcet and low dose vitamin D and phosphate binders in 1-month cycles. Costs and health outcomes (life years [LYs], quality adjusted life years [QALYs]) were discounted to present values at 3% per year.
The model closely reproduced the relevant observed data of individuals who participated in the ADVANCE trial. The ADVANCE trial measured both coronary calcification and biochemical markers. The current model focuses on the biochemical markers end-point from the ADVANCE trial, including serum levels of PTH, Ca, and P as they changed over time, which can be related to cardiovascular and other events, including total mortality. Coronary calcification was not considered in this model as the literature linking CAC to CV disease in SHPT patients is limited. Furthermore, the intermediate outcome (CAC score) can only be related to CV disease, and not to other health outcomes or costs.
After the end of the trial, each life history was projected by expected probabilities of events, similar to a population-level Markov model. Projections of effects were based on relationships of PTH, Ca, and P with mortality, CV events, fractures, and parathyroidectomy, as reported from three different published data sources:
Block model: Laboratory values (PTH, Ca, and P) are related to key outcomes (mortality, CV events, fractures, and parathyroidectomy) based on Block et al.Citation5, a large observational study.
Danese model: Similar to the Block model, except that it relates the number of quarters in a year when PTH, Ca, and P are within the KDOQI target instead of serum lab levels at baseline to mortality; based on Danese et al.Citation22. Outcomes other than mortality are as in the Block model.
Cunningham model: Hazard ratios (HR) for the key outcomes are based on the analysis of four randomized trials of cinacalcet, as reported in Cunningham et al.Citation39. This study represents the only available randomized trial-based evidence of the impact of cinacalcet on clinical end-points to date.
During the first 12 months of the model, representing the duration of the trial,
Reported mortality, individual patient-level PTH, Ca, and P levels and treatment doses were modeled as observed in the ADVANCE trial.
Individual patients’ monthly PTH, Ca, and P lab values were modelled.
Missing observations were filled in by carrying forward the last observation.
Individual patients’ average monthly treatment doses for cinacalcet, vitamin D, and phosphate binders were incorporated, rather than the average doses over the entire trial period.
After the first 12 months of the model,
PTH, Ca, and P levels remained stable (last measurement in ADVANCE) while receiving cinacalcet and deteriorated in the vitamin D arm and in patients who stopped cinacalcet treatment. This agrees with results from cinacalcet RCTsCitation38,Citation45.
Patients who stopped cinacalcet by the end of the trial period remained off treatment for all of the remaining duration of the model. A total of 78 out of 180 patients stopped cinacalcet by the end of the trial.
For all patients who did not stop treatment by the end of the trial and for all treatments (cinacalcet, vitamin D, and phosphate binders), dose in the post-trial phase was the average of their last four doses during the trial.
Figure 1. Model structure. SHPT: secondary hyperparathyroidism; CV: cardiovascular; PTH: parathyroid hormone; ![]()
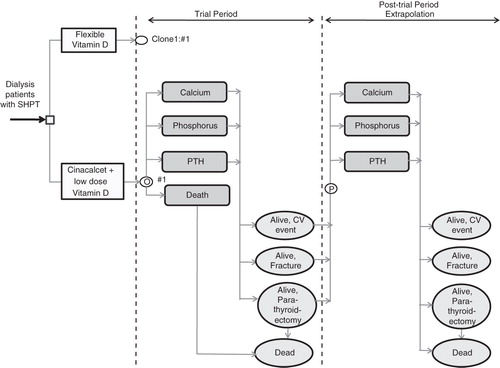
The above assumptions regarding model structure were tested in the sensitivity analysis termed ‘structural’ sensitivity analysis. Some of the additional model assumptions that were not part of sensitivity analysis were:
Mortality was modeled a function of age, and last measured PTH, Ca, and P levels in the Block model and a function of quarters in target in the Danese model.
Costs of dialysis was not included in the base case, as in the PenTAG baseline model. Including costs of dialysis increases the incremental cost-effectiveness ratio (ICER) by ∼$55,000/QALY gained (cost of dialysis/year).
Data sources
Efficacy: Mortality and morbidity data
Patient-level PTH, Ca, and P values and cinacalcet, vitamin D, and phosphate binder doses were taken from the ADVANCE trial dataCitation25,Citation26. The mortality during the ‘post-trial phase’ of the model for each patient was calculated as a function of age and PTH, Ca, and P levels at that time. The impacts of PTH, Ca, and P levels on outcomes (mortality, cardiovascular events, fractures, and parathyroidectomy) were obtained from literatureCitation5,Citation22,Citation50 and modeled as HRs.
The study used for the Block model was chosen based on population size and because it adjusted for the three lab values simultaneously. The study reported the correlations between baseline PTH, Ca, and P, and the risk of death during a 12–18-month follow-up in 40,538 dialysis patientsCitation5. Mortality in the Danese model was based on a study of the same population, but used quarters in target instead of lab values at baseline as explanatory variablesCitation22. We derived the HR estimates of CV events and fracture based on the lab values from the Block et al.Citation5 study. The model also included the effect of prior CV events which have been shown to increase the risk of subsequent CV hospitalizations in dialysis patients, as reported by Trespalacios et al.Citation51,Citation52 (RR = 2.24, 95% CI = 1.78–2.78). Since cinacalcet may reduce parathyroidectomy rates which have an impact on mortality, we included this outcome in our model. Incidence of parathyroidectomy and its correlation with baseline Ca, P, and PTH levels was analyzed in a cohort of 10,588 Medicare patients by Slinin et al.Citation50 Impact of parathyroidectomy on subsequent mortality is also incorporated in the model. Kestenbaum et al.Citation53 reported a short-term increase in 30-day mortality post-parathyroidectomy (RR = 2.72) ().
Table 1. Model inputs: Event rates.
Baseline rates of events (mortality, CV event) were primarily derived from the large dialysis organizations (LDO) registries (event rates derived from subjects with elevated levels of PTH, Ca, and P). Rates of fracture and parathyroidectomy were derived from Gastanaga et al.Citation54 and Li et al.Citation55, respectively (). We calibrated the model by adjusting the baseline rates in the vitamin D arm so that the resulting overall monthly rates of the model extrapolation after the trial are representing rates from these data sourcesCitation50,Citation56. The vitamin D arm was used to represent the monthly rates from literature under the assumption that the patients in those studies were receiving vitamin D. Since the cinacalcet arm uses the same calibrated baseline rates, it was calibrated indirectly.
Costs
Costs were evaluated from the US public healthcare system (Medicare) perspective. Only direct costs were included in the model: acquisition costs for cinacalcet and standard treatment (vitamin D sterols and phosphate binders), management of CV events, fractures, and parathyroidectomy procedures. The dosages considered in the model were derived from actual patterns recorded in the ADVANCE studyCitation25,Citation26. Unit costs for vitamin D were obtained from the CMSCitation57 (average sales price [ASP] + 6%), phosphate binders and cinacalcet from the Red BookCitation58 (average wholesale price [AWP] − 15%). The costs for management of CV events and fractures were calculated based on current Diagnosis Related Group (DRG) tariffs in the US, as reported by Doan et al.Citation59 The authors used the 2001 United States Renal Data System (USRDS) Medicare data to quantify direct medical costs of acute episodic events (acute myocardial infarction, stroke, fractures) and chronic conditions (arrhythmia, peripheral vascular disease, heart valve disease, congestive heart failure, coronary heart disease, and non-acute stroke). The individual costs of a hip, pelvic, or vertebral fracture were derived from Medicare claims dataCitation59. Unit costs for parathyroidectomy were based on estimates from Duh et al.Citation60 and the Healthcare Cost and Utilization Project (HCUPnet)Citation61. All costs were inflated to 2009 US$ using the medical component of the US Consumer Price IndexCitation62 ().
Table 2. Model inputs: Costs and utility.
Utility weights
Utility is used to calculate QALY. Since health utilities from SHPT patients are not specifically reported in literature, base utilities from dialysis patients were utilized and additional assumptions made. The utility value used was 0.66 for patients receiving hemodialysis, as reported by deWit et al.Citation63 When utilities associated with events were not available for dialysis patients, we used estimates for these events in the non-dialysis population and adjusted it by the baseline utility for dialysis (). Taylor et al.Citation64 and Brazier et al.Citation65 were used as sources for CV and fracture-related disutility. Reduced QoL associated with uncontrolled PTH levels was derived using the same method as in a recent published economic model for cinacalcet, as described in the UK National Institute for Health and Clinical Excellence (NICE) health technology assessment (HTA) reportCitation47,Citation48 (i.e., multiplying the overall utility at the end of each cycle by −15% for patients with PTH levels above 800 pg/mL). The application of PTH level-specific utility values was intended to reflect bone pain, a common symptom associated with hyperparathyroidism (). Parathyroidectomy was assumed not to influence QoL, because of the short impact of the actual surgery; however, a disutility related to PTH > 800 was assumed in the model.
Analysis
Cost-effectiveness analyses were conducted and are presented as ICER including cost per LY gained or cost per QALY gained (calculated as (Costcinacalcet – CostVitamin D)/(Effect cinacalcet – EffectVitamin D)).
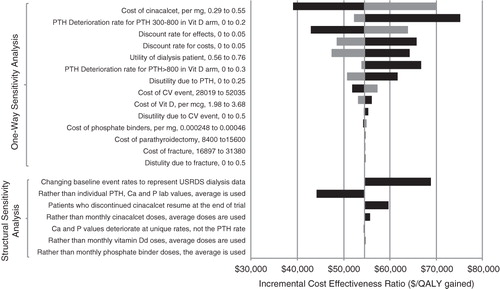
Extensive one-way sensitivity analyses were undertaken to explore which of the input parameters, when varied independently of the other model inputs, have the greatest impact on the incremental cost-effectiveness of cinacalcet. Utilities were varied by ±0.1, and costs were varied by 20% on either side of the value utilized in the model unless otherwise stated. Discounting rate for both costs and effects varied between 0–5%. Structural sensitivity analyses were conducted where model assumptions were varied. For example, we changed the assumption that the patients who stopped cinacalcet during the trial don’t re-start it after the end of the trial. Results are presented as the tornado diagram (). The baseline event rates were also varied as part of the structural sensitivity analysis. Event rates for mortality, CV event, and fracture were varied to represent the US dialysis population by age and sex as reported in the national registry of USRDS dataCitation56, and parathyroidectomy rates as reported in Slinin et al.Citation50
Figure 2. Tornado diagram (one-way and structural sensitivity analysis). CV: cardiovascular; PTH: parathyroid hormone; Ca: calcium; P: phosphorous; QALY: quality adjusted life year; USRDS: United States Renal Data System; Note: The base case is based on the Block variant. For one-way sensitivity analysis, the black (dark) bar indicates that the impact on ICER when model parameter is changed to a lower value and a grey (light) bar indicates impact on ICER when model parameter is changed to a higher value compared to the base case.
The probabilistic sensitivity analysis of the base case (LDO variant) considered uncertainty of the trial results by bootstrapping the set of trial participants as well as uncertainties of the model parameters used to project health effects after the trial. Point estimates were used as median for log-normal uncertainty distributions and upper limits of one-way ranges as 97.5% points. Uncertainties for HRs for mortality and parathyroidectomy were included. Model results were analyzed at net benefit ICER thresholds of $50 k/QALY and $100 k/QALY and presented as a cost-effectiveness acceptability curve (CEAC).
Results
Base case (using LDO data)
The Block model showed that, compared with standard vitamin D treatment, cinacalcet incurred on average additional lifetime costs of $31,708 per person while increasing life expectancy by 0.80 life years and 0.58 QALYs. The resulting ICER was $39,593 per life year gained and $54,560 per QALY gained. A dialysis provider may be more interested in the cost per year alive, which increased by $3155 with cinacalcet compared to vitamin D alone. In the Cunningham model, the ICER was $5064 per QALY gained and the cost per year alive decreased by $1068 with cinacalcet compared to vitamin D alone (). The Danese model showed an ICER of $72,456 per QALY gained and an increase of $2638 in cost per patient year alive.
Table 3. Baseline model results (using various definitions of effectiveness).
Sensitivity analysis (using the Block model)
One-way sensitivity analysis
The biggest impact on ICER was due to the cost of cinacalcet, discount rate for effects, and utility values associated with dialysis. When the cost of cinacalcet was reduced by 30%, the ICER was reduced to $39,064 per QALY gained, whereas increasing the cost by 30% increased the ICER to $70,056 per QALY gained for cinacalcet vs low dose vitamin D treatment. When no PTH deterioration was allowed in the vitamin D arm, the ICER increased to $75,172/QALY gained. When effects were not discounted (0% discount rate), the ICER decreased to $42,923/QALY gained, whereas increasing the discount rate for effects to 5% increased the ICER to $63,837/QALY gained. When costs were not discounted (0% discount rate), the ICER increased to $65,770/QALY gained, whereas increasing the discount rate for costs to 5% decreased the ICER to $48,452/QALY gained. Utility of being on dialysis was varied by ±0.1. Increasing the utility associated with dialysis to 0.76 provided a favorable result for cinacalcet ($47,381/QALY gained). Decreasing the utility of dialysis increased the ICER to $64,303/QALY gained ().
Structural sensitivity analysis
Changing the structural model assumptions showed the model results were robust to changes in model assumptions with minimal difference in the ICERs (range between $44,179–$68,907/QALY gained compared to baseline of $54,560) (). We also found that the structural assumptions around post-trial extrapolation of serum levels and therapy doses had limited effect on the cost-effectiveness ratio.
Varying the baseline event rates of the model changed the results slightly. The USRDS data show higher mortality and CV event rates than LDO. The Block-USRDS model variant showed an ICER of $68,907 per QALY gained, and an increase of $2910 in cost per patient year alive for cinacalcet compared to vitamin D alone. The Cunningham-USRDS model variant showed that cinacalcet dominated vitamin D and the cost per year alive decreased by $5564. The Danese model showed an ICER of $78,251 per QALY gained and an increase of $2217 in cost per patient year alive.
Probabilistic sensitivity analysis
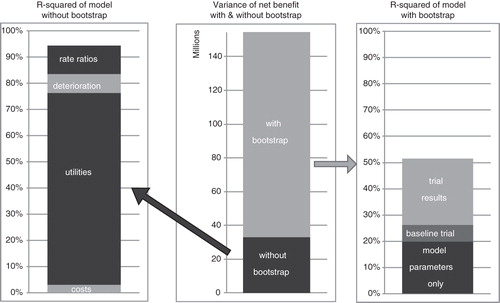
The CEAC () shows a likelihood of 98% for cinacalcet to be cost-effective at a $100,000/QALY willingness to pay threshold. A large part of the uncertainty in estimated net-benefit is due to the limited trial size. The uncertainty caused by the model parameters was limited, of which the largest contributors were (literature-derived) utility of dialysis patients and the rate ratio of impact of calcium levels on mortality ().
Discussion
Several studies have shown that poor control of bone and mineral biomarkers in ESRD patients can adversely affect all-cause mortality and CV eventsCitation3–5,Citation66–72, and that cinacalcet improves biochemical control, although its effects on clinical outcomes have not been tested in a randomized controlled study. A global, double-blind, randomized, placebo-controlled trial (EVOLVECitation45) is currently under way to evaluate the effects of cinacalcet on mortality and CV events in hemodialysis patients with SHPT. A post-hoc analysisCitation39 from four cinacalcet registrational RCTs showed that cinacalcet treatment was associated with a reduced mortality, risk of parathyroidectomy, fracture, and CV event. A recent observational studyCitation73 using DaVita dialysis facilities showed cinacalcet treatment significantly improved all-cause and cardiovascular survival in a large cohort of hemodialysis patients. In the absence of empirical evidence linking cinacalcet to relevant health outcomes, simulation models can estimate the long-term impact of treatments.
Three cinacalcet cost-effectiveness models have been published (ACHIEVECitation46, PenTAGCitation47,Citation48, and OPTIMACitation49). The ACHIEVE model (US payer perspective)Citation46 compared cinacalcet plus low-dose vitamin D vs vitamin D alone (Flex-D), and results were reported as cost (US $) per unit improvement in KDOQI targets. The PenTAGCitation47,Citation48 and OPTIMACitation49 are formed of states representing the main clinical events in the course of SHPT (i.e., CV event, fracture, parathyroidectomy, death). The PenTAG modelCitation47,Citation48 (developed for NICE as part of the HTA process from a NHS perspective) first simulated the effects of cinacalcet on PTH levels (in discrete categories: controlled PTH [<300 pg/mL], uncontrolled PTH [300–800 pg/mL], and very uncontrolled PTH [>800 pg/mL]), and then correlated them with events. The OPTIMA model was from an Italian healthcare perspective, took into account PTH, Ca, and P, rather than PTH alone, and followed the OPTIMA trial. Our model was based on a Phase IV trial for cinacalcet (ADVANCE) and included the impact of PTH, Ca, and P values on outcomes (death, CV events, fractures, and parathyroidectomy) similar to the OPTIMA model. In addition to modeling the impact of patient-level lab values (Block variant), a model variant was considered wherein the impact of time in KDOQI targets on mortality was modeled (Danese variant). Since the Cunningham post-hoc analysis represents the only available trial-based evidence of the impact of cinacalcet on clinical end-points, we also applied a model variant based on the HRs reported by Cunningham et al.Citation39
Results for the models were robust (range $43,529–$93,386/QALY gained) to variants of key input parameters, and model assumptions demonstrating cinacalcet is a cost-effective treatment for SHPT in the US healthcare setting. The difference in the results of the Cunningham variant vs other variants can be explained by the favorable impact of cinacalcet on outcomes, specifically cardiovascular events demonstrated by the Cunningham et al.Citation39 study, a pooled analysis of safety data from four randomized controlled trials. Results were comparable to previously published cinacalcet cost-effectiveness models. The main difference between the OPTIMACitation49 and the current model is the difference in costs of cinacalcet and event rates and the baseline rates for mortality. Therefore, after using the cost inputs from the OPTIMA modelCitation49 and adjusting the baseline mortality rates, results of the ADVANCE model (€25,796/QALY gained) were similar to those of the OPTIMA model (€31,616/QALY gained)Citation49. When treatment doses from the OPTIMA model were used in addition to the above assumptions, the ICER increased to €37,800/QALY gained. Our model results ($54,560/QALY gained) were more favorable compared to the PenTAG model ($98,238/QALY gained)Citation47,Citation48, which might be because our model considers not just PTH but also the impact of Ca and P on relevant outcomes. In addition, the higher unit costs of events (CV, fracture, and parathyroidectomy) may lead to a bigger difference in costs, leading to more favorable results.
As with most health economic analyses, it was necessary to make assumptions that are not directly based on empirical evidence. PTH, Ca, and P levels were assumed to remain stable (last measurement in ADVANCE) while receiving cinacalcet, but were assumed to deteriorate at a steady rate if patients received vitamin D. The model did not assume an increased risk of death after a CV or fracture event. After the end of the trial, mortality was dependent on patient age and projected PTH, Ca, and P levels. History of complications (except immediately after a parathyroidectomy) did not have an impact on mortality. This assumption is likely to have favored vitamin D treatment, as patients in this group had higher PTH, Ca, and P levels and therefore experienced more CV and fracture events. In addition, the risk of a subsequent fracture was not increased in patients who experienced an initial major fracture. Dialysis costs were not included in the base case model as the drug is intended for treatment of SHPT and not the underlying disease which is chronic kidney disease. Clearly, because dialysis is an expensive and hardly cost-effective procedure (Winkelmayer et al.Citation74 reported the ICER of $55,000–$80,000 per LY), extension of life due to cinacalcet will incur addition lifetime costs due to prolongation of dialysis treatment, i.e., survivor effect. However, making a cinacalcet cost-effectiveness conclusion based on a model that includes dialysis costs would likely bias the results against any life extending treatment of dialysis patients. We therefore present the base case results without dialysis costs, and also present additional analysis with dialysis costs included. This approach has been used in the NICE appraisal of cinacalcetCitation47,Citation48 as well as in other analysesCitation49,Citation75,Citation76, and the cost-effectiveness conclusions are made based on the model without dialysis costs. Including costs of dialysis increases the incremental cost-effectiveness ratio (ICER) by ∼$55,000/QALY gained (cost of dialysis/year).
An important limitation of this model is the lack of utility/quality-of-life data for dialysis patients with SHPT with and without other serious morbidity complications such as CV events or fractures. Utility was, therefore, based on a multiplicative assumption of the dialysis utility and event (cardiovascular or fracture) utility from the non-dialysis population. While this was accepted in prior published models, our one-way sensitivity analysis did show a considerable uncertainty around the utility estimates, and we feel that additional utility assessment in the population of interest would be in order, although outside of the scope of the current investigation. Likewise, the 15% utility decrement associated with an uncontrolled SHPT state was adopted from the PenTAG modelCitation15 and represents an expert opinion rather than a measurement. No systematic utility work has been done in SHPT patients to substantiate this estimate, but it is not unreasonable to assume that symptoms related to bone and mineral abnormalities such as bone pain and itching would lead to a reduction in quality-of-life.
It is also important to note that the ADVANCE trial restricted subjects with evidence of baseline CAC to use only calcium-based phosphate binders. This limits generalizability of the study’s results in real world practice where non-calcium containing binders are used, but helped clarify the effect of cinacalcet on vascular calcification by minimizing confounding by co-interventionsCitation26. While we observed broadly consistent results across various model formulations, it is important to recognize that the life time projections in our analyses were based upon a combination of surrogate efficacy measures and epidemiologic studies (Block, Danese), or on a post-hoc analysis of phase 3 trials (Cunningham). In the absence of randomized controlled trials assessing the effects on clinical outcomes, the above approach is the obvious choice. The randomized EVOLVE trial is underway assessing the direct effects of cinacalcet on clinical outcomes, and, when completed, will provide additional data validating modeling results to date.
Conclusion
The cinacalcet ADVANCE economic model was robust to variations in key parameters and structural assumptions used in the cost-effectiveness analysis, demonstrating that cinacalcet treatment could be considered cost-effective for treatment of SHPT in the US healthcare setting. Due to cost offsets in some plausible scenarios, i.e., prevention of events (particularly cardiovascular), cinacalcet can reduce the cost per year alive (as observed in the Cunningham model). Before the availability of results from a long-term clinical trial evaluating the impact of cinacalcet vs vitamin D in patients with SHPT with health impact outcomes, simulation models provide the best method to quantify the cost and health effects associated with these treatments.
Transparency
Declaration of funding
This research was financially supported by Amgen, Inc., Thousand Oaks, CA, and conducted in collaboration with Cerner LifeSciences, Beverly Hills, CA.
Declaration of financial/other relationships
VB is an employee and stockholder of Amgen, Inc. RB and AML are employees of Cerner LifeSciences, Beverly Hills, CA, that provides consulting services to clients, including the pharmaceutical industry.
Acknowledgments
The authors thank Jon Nilsen, PhD (Amgen, Inc) for copy editing and formatting assistance.
References
- Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl 1999;73:S14-S19
- Owda A, Elhwairis H, Narra S, et al. Secondary hyperparathyroidism in chronic hemodialysis patients: prevalence and race. Ren Fail 2003;25:595-602
- Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 2005;16:1788-93
- Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001;12:2131-8
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208-18
- Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006;70:1358-66
- Tentori F, Blayney MJ, Albert J. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008;52:519-30
- Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 2011 Jun;26(6):1948-55
- NIH. USRDS 2010 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Disease, 2010
- Doan QV, Gleeson M, Kim J, et al. Economic burden of cardiovascular events and fractures among patients with end-stage renal disease. Curr Med Res Opin 2007;23:1561-9
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Am J Kidney Dis. 2003;42(suppl 3):S1-202
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1-130
- Moe SM, Chertow GM, Coburn JW, et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 2005;67:760-71
- Aparicio M, Combe C, Lafage MH, et al. In advanced renal failure, dietary phosphorus restriction reverses hyperparathyroidism independent of changes in the levels of calcitriol. Nephron 1993;63:122-3
- Andress DL, Norris KC, Coburn JW, et al. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med 1989;321:274-9
- Martin KJ, Gonzalez EA, Gellens M, et al. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 1998;9:1427-32
- Arenas MD, varez-Ude F, Gil MT, et al. Implementation of 'K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease' after the introduction of cinacalcet in a population of patients on chronic haemodialysis. Nephrol Dial Transplant 2007;22:1639-44
- Arenas MD, Alvarez-Ude F, Torregrosa V, et al. Consequences of the implementation of K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease in a population of patients on chronic hemodialysis. J Nephrol 2007;20:453-61
- Arenas MD, Alvarez-Ude F, Gil MT, et al. Application of NKF-K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease: changes of clinical practices and their effects on outcomes and quality standards in three haemodialysis units. Nephrol Dial Transplant 2006;21:1663-8
- Al AZ, Gonzalez EA, Martin KJ, et al. Achieving K/DOQI laboratory target values for bone and mineral metabolism: an uphill battle. Am J Nephrol 2004;24:422-6
- Young EW, Akiba T, Albert JM, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004;44(5 Suppl 2):34-8
- Danese MD, Belozeroff V, Smirnakis K, et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 2008 Sep;3(5):1423-9
- Messa P, Macário F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 2008;3:36-45
- Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008;3:1718-25
- Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011;26:1327-39
- Floege J, Raggi P, Block GA, et al. Study design and subject baseline characteristics in the ADVANCE Study: effects of cinacalcet on vascular calcification in haemodialysis patients. Nephrol Dial Transplant 2010;25:1916-23
- Akizawa T, Koshikawa S. Clinical study of cinacalcet in Japan. Ther Apher Dial 2008;12(1 Suppl):S13-S15
- Akiba T, Akizawa T, Tsukamoto Y, et al. Dose determination of cinacalcet hydrochloride in Japanese hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial 2008;12:117-25
- Meola M, Petrucci I, Barsotti G. Cinacalcet reduced glandular volume in patients with SHPT [Abstract MP409]. Presented at the XLV ERA-EDTA Congress, May 10--13 2008, Stockholm, Sweden
- Os I, Bencova V, Banos A, et al. Achievement of KDOQI targets with cinacalcet (mimpara/sensipar) according to length of time on dialyses [Abstract SP394]. Presented at the XLV ERA-EDTA Congress, May 10--13 2008, Stockholm, Sweden
- Arenas MD, Rebollo P, Alvarez-Ude F, et al. Is cinacalcet a cost-effective treatment in severe secondary hyperparathyroidism in patients on hemodialysis? Nefrologia 2008;28:511-6
- Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol 2005;16:800-7
- Block GA, Martin KJ, de Francisco AL. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. New Engl J Med 2004;350:1516-25
- Lindberg JS, Moe SM, Goodman WG, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium x phosphorus in secondary hyperparathyroidism. Kidney Int 2003;63:248-54
- Quarles LD, Sherrard DJ, Adler S, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol 2003;14:575-83
- Goodman WG, Hladik GA, Turner SA, et al. The Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 2002;13:1017-24
- Lien YHH, Silva AL, Whittman D. Effects of cinacalcet on bone mineral density in patients with secondary hyperparathyroidism. Nephrol Dial Transplant 2005;20:1232-7
- Moe SM, Cunningham J, Bommer J. Long-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCL. Nephrol Dial Transplant 2005;20:2186-93
- Cunningham J, Danese M, Olson K, et al. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 2005;68:1793-800
- Lucchi L, Stipo L, Perrone S, et al. Early initiation of cinacalcet (cn) for the treatment of secondary hyperparathyroidism (shpt) in hemodialysis patients [Abstract MP398]. Presented at the XLV ERA-EDTA Congress, May 10--13 2008, Stockholm, Sweden
- Portoles J, Tato A, Lopez-Sanchez P, et al. Cinacalcet in patients on peritoneal dialysis with moderate to severe hyperparathyroidism resistant to conventional treatment. A one-year, prospective study. Nefrologia 2008;28:419-24
- Meier PD, Hertner H, Jungbluth M, et al. KDOQI target achievement is improved with cinacalcet in clinical practice – the Swiss OPTIMIZE survey. [Abstract P54]. Presented at the 41st Annual Meeting Swiss Society of Nephrology. Kursaal Interlaken December 2--4, 2009
- Spasovski GB. Bone health and vascular calcification relationships in chronic kidney disease. Int Urol Nephrol 2007;39:1209-16
- Sterrett JR, Strom J, Stummvoll HK, et al. Cinacalcet HCI (Sensipar/Mimpara) is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol 2007;68:10-17
- Chertow GM, Pupim LB, Block GA, et al. Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE): rationale and design overview. Clin J Am Soc Nephrol 2007;2:898-905
- Shireman TI, Almehmi A, Wetmore JB, et al. Economic analysis of cinacalcet in combination with low-dose vitamin D versus flexible-dose vitamin D in treating secondary hyperparathyroidism in hemodialysis patients. Am J Kidney Dis 2010;56:1108-16
- Garside R, Pitt M, Anderson R, et al. The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation. Health Technol Assess 2007;11(18)
- Garside R, Pitt M, Anderson R, et al. The cost-utility of cinacalcet in addition to standard care compared to standard care alone for secondary hyperparathyroidism in end-stage renal disease: a UK perspective. Nephrol Dial Transplant 2007;22:1428-36
- Eandi M, Pradelli L, Iannazzo S, et al. Economic evaluation of cinacalcet in the treatment of secondary hyperparathyroidism in Italy. Pharmacoeconomics 2010;28):1041-54
- Slinin Y, Foley RN, Collins AJ. Clinical epidemiology of parathyroidectomy in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Hemodial Int 2007;11:62-71
- Trespalacios FC, Taylor AJ, Agodoa LY, et al. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis 2003;41:1267-77
- Trespalacios FC, Taylor AJ, Agodoa LY, et al. Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int 2002;62:1799-805
- Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int 2004;66:2010-6
- Gastanaga V, Zhao S, Stuccio-White N, et al. Incidence rates of hospitalization-associated bone fractures in dialysis patients in the United States Renal Data System. Presented at the 22nd International Conference on Pharmacoepidemiology & Therapeutic Risk Management. August 24--27, 2006. Lisbon, Portugal
- Li S, Chen Y-W, Peng Y, et al. Trends in parathyroidectomy rates in US hemodialysis patients from 1992 to 2007. Am J Kidney Dis. 2011 Apr; 57(4):602-11
- US Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive Kidney Diseases, 2009
- US Department of Health and Human Services. Centers for Medicare and Medicaid Services. Healthcare Common Procedure Coding System. Medicare Part B Drugs Average Price (ASP). 2009. http://www.cms.hhs.gov/McrPartBDrugAvgSalesPrice/ [Last accessed May 20 2010]
- 2009 Drug Topics Red Book. Montvale, NJ: Medical Economics, 2009
- Doan QV, Gleeson M, Kim J, et al. Economic burden of cardiovascular events and fractures among patients with end-stage renal disease. Curr Med Res Opin 2007;23:1561-9
- Duh Q-Y. Current investigation for primary hyperparathyroidism. Hyperparathyroidism 8th Postgraduate Course in Endocrine Surgery, September 22, 2006, Capsis Beach, Crete
- HCUPnet. Healthcare Cost and Utilization Project (HCUP). 1993-2008. Rockville, MD: Agency for Healthcare Research and Quality. http://hcupnet.ahrq.gov/. Accessed May 5, 2010
- US Department of Labor. Bureau of Labor Statistics. http://www.bls.gov/cpi/home.htm#overview. Accessed February 23, 2009
- de Wit GA, Ramsteijn PG, de Charro FT. Economic evaluation of end stage renal disease treatment. Health Policy 1998;44:215-32
- Taylor DC, Pandya A, Thompson D, et al. Cost-effectiveness of intensive atorvastatin therapy in secondary cardiovascular prevention in the United Kingdom, Spain, and Germany, based on the Treating to New Targets study. Eur J Health Econ 2009;10:255-65
- Brazier JE, Green C, Kanis JA. A systematic review of health state utility values for osteoporosis-related conditions. Osteoporos Int 2002 Oct;13:(10):768-76
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998;32(5 Suppl 3):S112-9
- Foley RN, Parfrey PS. Cardiovascular disease and mortality in ESRD. J Nephrol 1998;11:239-45
- Cases A, Vera M, Lopez Gomez JM. [Cardiovascular risk in patients with chronic renal failure. Patients in renal replacement therapy]. Nefrologia 2002;22(1 Suppl):68-74
- Suliman ME, Qureshi AR, Barany P, et al. Hyperhomocysteinemia, nutritional status, and cardiovascular disease in hemodialysis patients. Kidney Int 2000;57:1727-35
- Marco MP, Craver L, Betriu A. Higher impact of mineral metabolism on cardiovascular mortality in a European hemodialysis population. Kidney Int 2003;63:S111-4
- Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2005;67:1179-87
- Spiegel DM, Raggi P, Mehta R, et al. Coronary and aortic calcifications in patients new to dialysis. Hemodial Int 2004;8:265-72
- Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 2010 Sep;78(6):578-89
- Winkelmayer WC, Weinstein MC, Mittleman MA, et al. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making 2002;22:417-30
- Iannazzo S, Pradelli L, Chiroli S. A cost-utility analysis of cinacalcet in secondary hyperparathyroidism (SHPT) in five European countries. Presented at the International Society for Pharmacoeconomics and Outcomes Research 13th Annual European Congress, Toronto, ON, Canada, May 3--7, 2008
- Amgen. Advisory board to explore the relationship between vascular calcification and clinical and health outcomes in dialysis patients. Amgen data on file. 2010
- Stevenson M, Lyold Jones M, De Nigris E, et al. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess 2005 Jun;9(22):1-160
- Kim J, Dylan M, Doan Q, et al. Association of elevated serum parathyroid hormone (PTH) and calcium with hip, vertebral, or pelvic fracture in hemodialysis patients [Abstract S026]. Presented at: ERA-EDTA Congress, 15--18 May 2004, Lisbon, Portugal