Abstract
Objective:
To compare the cost effectiveness of prolonged release oxycodone/naloxone (OXN) tablets (Targinact) and prolonged release oxycodone (OXY) tablets (OxyContin) in patients with moderate-to-severe non-malignant pain and opioid-induced constipation (OIC) from the perspective of the UK healthcare system.
Methods:
A cohort model used data from a phase III randomised, controlled trial (RCT). It calculated the cost difference between treatments by combining the cost of pain therapy with costs of laxatives and other resources used to manage constipated patients. SF-36 scores were converted into EQ-5D utility values to calculate the quality-adjusted life-year (QALY) gains. Deterministic and probabilistic sensitivity analyses were performed.
Results:
The incremental cost of OXN versus OXY was £159.68 for the average treatment duration of 301 days. OXN gave an incremental QALY gain of 0.0273. The estimated incremental cost-effectiveness ratio (ICER) was £5841.56 per QALY. Sensitivity analyses gave a maximum ICER of £10,347.03. In some scenarios, OXN dominated with a cost saving of up to £4254.70. Probabilistic sensitivity analysis showed that OXN had approximately 96.6% probability of cost effectiveness at the £20,000 threshold.
Limitations:
The model was conservative in predicting the probability of constipation beyond the 12-week RCT period. UK cost of constipation data were limited and based on primary care physician opinion.
Conclusions:
In the base case, direct treatment costs were slightly higher for patients treated with OXN than for those treated with OXY. However, patients treated with OXN experienced a quality of life gain, and had an ICER considerably below thresholds commonly applied in the UK. The model was most sensitive to the estimated cost of constipation with a number of realistic scenarios in the sensitivity analysis demonstrating a cost saving with OXN (OXN dominant). OXN is therefore estimated to be a cost-effective option for treating patients with severe non-malignant pain and OIC.
Introduction
Persistent pain is a common and disabling illness; it has an estimated prevalence of 19% across EuropeCitation1 and affects around 5 million people in the UKCitation2. It restricts daily activitiesCitation1, reduces quality of lifeCitation1, and carries a significant economic burden in terms of direct costs (e.g., healthcare services, medications)Citation3,Citation4 and indirect costs (e.g., loss of income, decreased productivity)Citation4–6. Although many clinicians are aware of the direct costs, few take account of the wider cost implications of chronic pain.
Patients with severe pain frequently require strong opioids as part of their pain management programme. However, around 80% of patients treated with opioids will experience at least one adverse eventCitation2. Opioids commonly cause opioid-induced bowel dysfunction (OIBD) by binding to μ opioid receptors throughout the gastrointestinal (GI) tract. This leads to a range of symptoms, including constipation, bloating, abdominal pain, cramping and incomplete faecal evacuation. However, the most common (and most disabling) side-effect of opioid treatment is opioid-induced constipation (OIC)Citation7. Binding of opioid receptors in the GI tract reduces peristaltic activity and GI secretions, and increases fluid absorption, leading to harder stools passing through a poorly peristaltic gut, and resulting in classical symptoms of constipation.
OIC is generally managed with stool softeners and laxatives. However, although these offer a degree of symptom control, they do not resolve the underlying problem and many patients will not have their constipation resolved with this therapy. Several studies document patients’ inadequate response to laxatives. In one study, approximately 54% of patients treated for OIC did not achieve the desired result even half of the timeCitation8. Some patients might not be able to tolerate the dose of laxative required to control the constipation (laxatives are associated with a number of side-effects, including nausea, bloating and dehydration). OIC can be such a distressing side-effect that some patients try to avoid it by reducing the dose of their opioid medication; they prefer to be in pain rather than experience severe constipationCitation9. Patients with OIC frequently have a reduced quality of life and may use additional healthcare resources, including primary care physician and district nurse visits. Studies carried out in the US and Sweden showed that patients with OIC had significantly higher total health costs than those who did notCitation10–12.
Oxycodone is a strong opioid agonist that is commonly used to treat non-malignant pain in the UK. Prolonged release oxycodone/naloxone (OXN) tablets (Targinact*) are a fixed-dose, prolonged release formulation of oxycodone and the opioid antagonist naloxone. Naloxone counteracts OIC by blocking the action of oxycodone at opioid receptors in the gut. It does this without compromising the analgesic effect of oxycodone as when taken orally, naloxone is largely metabolised by the liver and does not reach the central nervous system in clinically significant amounts. Clinical studies have confirmed that treatment with OXN provides effective pain control while reducing OICCitation13–16.
The objective of this cost-utility study was to evaluate the cost effectiveness of OXN versus prolonged release oxycodone (OXY) tablets (OxyContin†) in patients with moderate-to-severe non-malignant pain experiencing OIC. The study was carried out from the perspective of the UK National Health Service (NHS).
Patients and methods
The model used data from a phase III, randomised, controlled, double-blind, parallel-group study published in 2008 by Simpson et al.Citation14. The study compared OXN with OXY in adults who required continuous opioid therapy for moderate to severe non-malignant pain, and had OIC. After a 7–28-day run-in phase (during which their pre-study opioid was converted to OXY; the protocol required their pain to be controlled at a dose of 50 mg/day or less), 322 patients were randomised to treatment with OXN (n = 162) or OXY (n = 160) and entered a 12-week double-blind phase. The mean dose of oxycodone across both treatment groups was approximately 33 mg/day. Patients were told to take oral bisacodyl as a rescue laxative according to the protocol. The primary objective was to assess whether OXN gave improvements in constipation compared with OXY alone after 4 weeks’ treatment. Constipation was assessed using the Bowel Function Index (BFI). Patients rated the following on a scale of 0–100, where a lower score indicates better bowel function: ease of defecation, feeling of incomplete bowel evacuation and personal judgement of constipation. Their BFI score was defined as the mean of these three scoresCitation17. Normal bowel function is defined as a score of ≤28.8; this was determined in a study that reported that 95% of non-constipated patients had a BFI score ≤28.8Citation18. Patients’ average pain over the last 24 hours was assessed using a 0–10 numerical rating scale (where a lower score indicates less pain). The study showed that although analgesic efficacy was comparable between OXN and OXY, patients in the OXN group had statistically and clinically significant improvements in OIC. After 4 weeks of treatment, mean BFI scores were 34.9 (SD 25.80) in the OXN group and 51.6 (SD 26.78) in the OXY group (p < 0.0001). Mean pain intensity scores were similar between treatment groups and remained stable throughout the study (ranging from 3.3 to 3.5 in the OXN group and from 3.3 to 3.7 in the OXY group). Forty-nine patients (31%) in the OXN group used rescue laxatives in the first 4 weeks, compared with 87 patients (55%) in the OXY group (p < 0.0001). The number of laxative tablets required was significantly lower in the OXN group than in the OXY group (p < 0.0001).
Model structure and overview
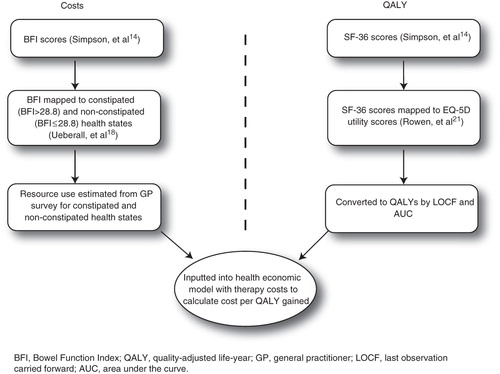
A cohort cost-utility model was developed in Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA) with constipated and non-constipated health states. The model was replicated in SAS v9.2 (SAS Institute, Cary, NC, USA) to audit the calculations and results. The model calculated the incremental cost-effectiveness ratio (ICER) defined as Δcost/Δeffectiveness, where effectiveness was defined in terms of quality-adjusted life-years (QALY) gainedCitation19. The health states were estimated using the BFI data. Utility values were calculated by mapping SF-36 data from the study by Simpson et al.Citation14 to EQ-5D scores. Pain was not included as a health state as based on the study data, it was assumed to be equal between treatments. The model included laxative use based on the use of rescue laxative in the study by Simpson et al.Citation14.
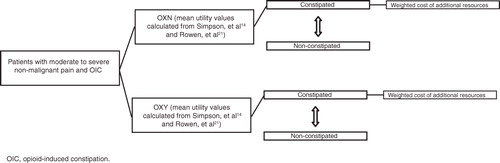
shows the structure of the model. Most patients started in a constipated health state, however over time patient movement occurred between the constipated and non-constipated health states, with the constipated health state incurring an additional cost. The model had weekly time intervals. The duration for base case analysis was 301 days, according to the average duration of treatment with OXY tablets in patients with non-cancer diagnosesCitation20. Cost and effects were not discounted owing to the time horizon being less than 1 year.
The following equations were used in the model:
Total cost of drug (laxative and pain treatment)
Cj = total cost of drug in treatment group over the treatment period (j = 1 refers to OXN; j = 2 refers to OXY)
K = the expected duration of each treatment, which is estimated at 43 weeks, hence K = 43
Dj is the average weekly cost of pain therapy in the jth treatment group. Therefore, Dj = cost per mg × average (mean) dose (mg) per day × 7 (days)
Lj is the average weekly cost of laxative use in the jth treatment group. Therefore, Lj = ([mean tablets per 28 days]/4) × Cost per 5 mg tablet
Hence,
Additional healthcare costs
Pij is the proportion of patients with constipation in each treatment group at each week (i)
The total average weekly cost per patient of additional healthcare required is V, where V = (average cost/patient per course of therapy/301 days) × 7 days
Therefore, using a half-cycle correction, the additional healthcare costs (Zj*) is given by:
The incremental cost is therefore:
Utilities
If the utilities for each treatment group at each week are denoted Uij, then defined as Uj*, the total QALY gain across all 43 weeks for a given treatment group, using a half cycle correction is:
The ICER is therefore given by [3] divided by [4]:
Model inputs
shows the major model inputs. Incremental quality-adjusted life-years (QALYs) and costs were calculated using the area under the curve (AUC) method with last observation carried forward (LOCF).
Cost inputs
Unit costs were based on the latest available data at Q1 2011Citation22–24. The pack costs for OXN were £17.56 (5 mg), £35.11 (10 mg), £70.22 (20 mg) and £140.44 (40 mg)Citation22. The pack costs for OXY were £12.46 (5 mg), £24.91 (10 mg), £49.82 (20 mg) and £99.66 (40 mg)Citation22. The model used sales data for OXN and OXY to calculate a weighting that was then used to estimate cost per mg (due to slight variations in the cost per mg of both treatments at different dose strengths, it was necessary to estimate the proportion of patients receiving each of the tablet strengths in the UK setting). The weightings applied were 24% (5 mg), 30% (10 mg), 29% (20 mg) and 18% (40 mg). The cost calculations included 5–40 mg oxycodone in each group. The weighted cost per week of OXY was £12.71; the weighted cost per week of OXN was £17.48.
Laxative use was based on patient data from Simpson et al.Citation14. As data were only available for both treatment groups for the first 4 weeks of treatment, the model assumed that laxative use would remain stable in both treatment groups for the rest of the study period (301 days). shows costs of treatment with OXN and OXY, and laxative costs.
Table 1. Costs of pain and laxative therapy.
Alongside direct pain treatment and laxative costs, a constipated patient may incur other healthcare costs, such as physician consultations and home visits, hospital visits and procedures, outpatient appointments, enemas and manual evacuations. Cost weighting took into consideration the percentage of patients requiring the resource and how often the resource was required over the course of opioid therapy (); this information was based on findings from a specially commissioned survey of UK primary care physiciansCitation25. A limitation of this survey was that it was not explicit about the duration of patients’ treatment, therefore the average resource use per week was also not explicit. The average treatment duration applied in the model (301 days) was used to calculate the weekly resource use. This was potentially conservative as most of the survey respondents said they would treat for less than 14 weeks. Using the figure of 301 days resulted in an average weekly cost of additional therapies for a constipated patient of £5.50 (equating to an average monthly cost of £23.83).
Table 2. Additional healthcare costs.
Inputs for health states
The model included the percentage of constipated and non-constipated patients in both treatment groups throughout the 12-week treatment period (). Constipation status was modelled by defining normal bowel function as a BFI score of ≤28.8Citation18.To allow modelling beyond 12 weeks, it was assumed that the BFI values achieved at the end of 12-week treatment period would remain constant for both treatment groups until the end of the model (301 days). This was a conservative assumption given the results of the extension phase of the study showing the continued benefit of OXN over a 12-month period: patients’ mean BFI score decreased from 35.6 (SD 27.74) at 12 weeks to 20.6 (SD 24.01) after an additional 52 weeks of treatment with OXNCitation26. This equates to 27% of those patients originally randomised to OXN being constipated at the end of the 52-week extension phase. Note that a small percentage of patients in the model did not start in the constipated health state as they were originally diagnosed as constipated according to study entry criteria that were based on complete spontaneous bowel movements (CSBMs), not the BFI threshold. However, in most cases, there is a high level of correlation between the BFI and CSBMsCitation17.
Table 3. Percentage of constipated patients over time [Pij].
Quality of life inputs (utility values)
The model included quality of life data based on patient health survey (SF-36 v2Citation27) responses from patients in the study by Simpson et al.Citation14. General improvements on quality of life were seen for the OXN group. In particular, there were statistically significant improvements in social functioning, vitality and the general health subscale at week 12 (p = 0.012, p = 0.010 and p = 0.039, respectively). As with constipation status, quality of life at 12 weeks was carried forward for the remainder of the model. For the base case, the model applied a mapping method developed by Rowen et al.Citation21 to convert the SF-36 scores to the EQ-5D utility values, which are commonly used in the UK. Subsequently, QALY gains were estimated using the AUC method with adjustment for baseline differences, as described by Manca et al.Citation28, and Willan and BriggsCitation29. Adjusting for baseline differences ensured that any observed differences between treatments (post-baseline) took into account potential differences between treatment groups at baseline. In the sensitivity analysis, QALY gains were also estimated without adjusting for baseline differences. shows the difference in utility scores between OXN and OXY; at week 12, the improvement in quality of life was significantly greater in patients treated with OXN than those treated with OXY (p = 0.0185). The adjusted difference of 0.04 for the OXN group compared with the OXY group is likely to be clinically significant, as a cut-off value of 0.03 is frequently used as the minimum clinically relevant difference for the EQ-5DCitation30–32.
Table 4. Base case EQ-5D utility values.
Deterministic sensitivity analyses
It was important to determine which inputs had the most significant impact on model results and whether particular inputs increased or decreased the ICER. A simple sensitivity analysis involved increasing and decreasing the following key variables by 25% as a change of this magnitude would be sufficient to indicate any trends: incremental QALY gain; total weekly dose of oxycodone; treatment duration, in weeks; cost of additional resources for constipated patients; cost per mg of bisacodyl. Probability of constipation in each treatment group was adjusted by 10% to prevent values exceeding 100%.
It was important to undertake more in-depth analysis on the mapping functions used to convert SF-36 to utility values as a number of mapping functions are available and the choice of function can influence model results. A mapping function by Brazier et al.Citation33 was applied in the sensitivity analysis as it was one of the earliest developed and is still one of the most commonly applied. There have also been several quality of life studies reporting utility values on the impact of constipationCitation34–36. The model compared utility values from the study with published utility values. Applying these values from the literature as a sensitivity analysis was a good way to assess any uncertainty around the quality of life gain reported in the study. The average utility values from the literature were 0.5850 (range 0.31–0.9) for constipation and 0.8200 (range 0.63–1.00) for non-constipationCitation34–36.
Another input that needed to be tested was the cost of treating constipation, especially as the base case was formed on primary care physician opinion. A number of non-UK studies were identified in the literature, most of which involved using extensive databases. Sensitivity analyses were carried out on the cost of constipation using information from studies by Kwong et al.Citation10, Iyer et al.Citation11, and Hjalte et al.Citation12 that included patients with non-malignant pain. In each case, the local currency was converted to GBP and the cost duration converted to weekly costs before being applied to the model. There was no adjustment for inflation as all cost inputs were within a timeframe of one year before the model was built. The analyses included direct costs only; all inputs are shown in .
Table 5. Deterministic sensitivity analysis inputs.
Probabilistic sensitivity analysis
The model conducted probabilistic sensitivity analysis (PSA) on the following major model inputs: utility values; probability of constipation over time; average oxycodone dose; unit cost of resource use in constipated patients; cost of laxatives.
The analysis calculated means and standard errors using data from the study by Simpson et al.Citation14 in the statistical package SAS v9.2. Means and standard errors from the SAS output were manually inputted into the Excel model. Distributions were applied to the variables according to standard methods applied in health economicsCitation19.
The mean weekly dose was considered to be normally distributed because the measures of skewness ranged from 0.08 (little or no skew) to 0.68 (some evidence of positive skew). In addition, the mean and median values were very close to each other suggesting that normality assumptions were not grossly violated. A beta distribution was assumed for EQ-5D utility values, as > 99% of the EQ-5D scores were estimated to be between 0 and 1. Costs were assumed to follow a gamma distribution since costs are positive (>0) and also positively skewed. Gamma distributions are commonly assumed for cost inputs in economic evaluations. Based on a previously described methodCitation19, the model used the following definition of gamma: γ = (1, µ).
A breakdown of the distributions and parameters used for utilities, probabilities of OIC, costs, and average dose of study drug is given in Appendix 1 (Supplementary material).
Results
Base case
shows the base case results. The incremental cost of OXN versus OXY was £159.68 for the average treatment duration of 301 days. OXN gave an incremental QALY gain of 0.0273. The ICER of £5841.56 was well within the £20,000–30,000 thresholdCitation37 often applied in the UK.
Table 6. Base case results.
Deterministic sensitivity analyses
shows the results of the deterministic sensitivity analyses. Simple percentage change adjustments applied to all of the key variables resulted in the ICERs remaining under £8000 in all scenarios. The model was not particularly sensitive to changes in the probability of constipation status as utility values were calculated according to treatment arm, rather than health state, in the base case analysis. When more extensive sensitivity analysis was undertaken on the mapping functions and utility values, the ICER remained under £11,000. Each of the sensitivity analyses on the cost of constipation gave an ICER where OXN was dominant, meaning a total cost saving to the UK is possible if the cost of treating OIC is sufficiently high.
Table 7. Results of sensitivity analyses.
Probabilistic sensitivity analysis
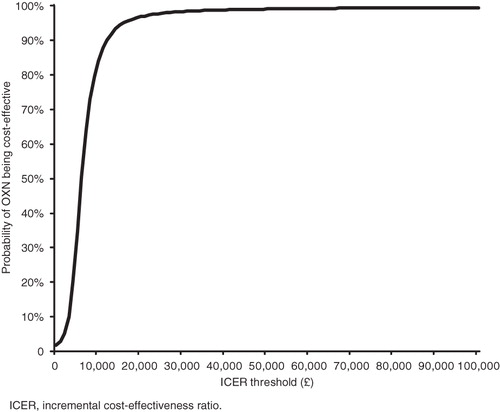
Most of the 10,000 Monte Carlo simulations of the PSA gave an increase in both costs and QALYs. To determine whether the additional benefits are worth the additional cost, the analysis compared the ICERs with the accepted thresholds for cost effectiveness. shows a cost-effectiveness acceptability curve, based on the Monte Carlo simulations and the thresholds usually applied in the UK. The curve shows that the estimated probability of OXN being cost effective is 96.6% at the £20,000 threshold.
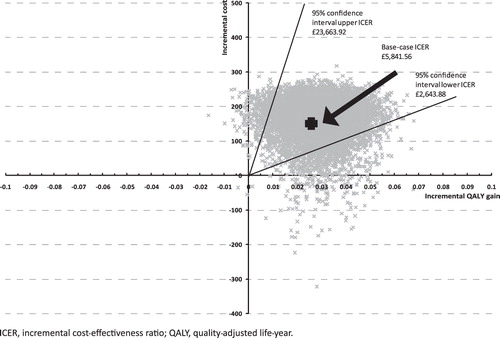
shows the cost-effectiveness plane. The confidence intervals (CIs) were based on calculating the percentiles of the expected net value. The upper 95% CI of £23,633.92 is still within the £20,000–30,000 thresholdCitation37 often applied in the UK.
Discussion
This cost-utility model demonstrates that treatment with OXN generates an ICER well below the commonly applied thresholds in the UK. The ICER is similar to that generated in a previous cost-utility model of OXN compared with OXYCitation38. However, the improved methodology of the current model makes the results more robust. For example, the current model applied the SF-36 quality of life results from the study by Simpson et al.Citation14 rather than using quality of life inputs from other published sources. In addition, the health state of constipation was more accurately defined: rather than using laxative intake to determine whether patients had constipation, the current model applied BFI values to determine the constipation health state (constipated vs. non-constipated). The BFI is a fully validated scoring system and extensive research was undertaken to determine the cut-off value of >28.8 that indicates constipationCitation17,Citation18.
The incremental gain in QALYs, based on the SF-36 results mapped to EQ-5D, implies that patients treated with OXN in real-world clinical practice will experience a quality of life gain. There are two large non-interventional studies in the real-world treatment setting that support improvements in quality of life for patients with chronic pain receiving OXNCitation39,Citation40. Schutter et al. reported that 2023 patients had a 43% improvement in their mean overall quality of life scores (measured using the Brief Pain Inventory social interference from pain domains) after 4 weeks’ treatment with OXNCitation39. Rychlik et al. prospectively followed 757 patients with chronic back pain for 6 monthsCitation40. They were divided into two cohorts: one received OXN and the other received other strong (World Health Organization step III) opioids. At 6 months, direct treatment costs were lower in the OXN cohort and there was a QALY gain compared with the cohort taking other strong opioids. This resulted in OXN dominating the other strong opioids.
Limitations
In the base case, estimated constipation costs were based on data collected from a survey of UK primary care physiciansCitation25; no other sources of UK costs could be identified. Applying results from other studies that estimate the cost of constipation showed the sensitivity of the model to the cost of treating constipation and the potential for substantial cost savings. The sensitivity analyses included US and Swedish costings, which were significantly higher than the UK costingsCitation10–12. The US costings are based on insurance registry data and the Swedish costings on patient case histories, therefore both may be more accurate. In addition, the UK costings were based on the perceptions of primary care physicians; other groups of healthcare professionals, particularly community-based nurses and secondary care specialists treating constipation, may report different resource use and costs. The survey of primary care physicians did not explicitly define the duration of treatment and subsequent resource use per unit of time. Given that the cost of managing constipation could substantially alter the cost-effectiveness results and the applied UK costings may be an underestimate of the true cost, it is possible that with more accurate UK-derived data, OXN could become dominant (i.e., cost saving to the UK NHS) when compared with OXY. The non-interventional study comparing OXN with other strong opioids suggests that these savings are also likely to be demonstrated in the real-world settingCitation40. The model may have further underestimated the cost effectiveness of OXN by assuming that patients’ BFI and quality of life remains at the level seen at week 12 of the study by Simpson et al.Citation14 for the duration of treatment. However, data collected during an extension phase of the study show that the BFI score continues to improve up to 12 months of treatmentCitation26, again suggesting that OXN may be more cost effective than in the current model. A potential area for future research is to develop parametric survival curves to more accurately estimate the treatment benefits beyond 12 weeks.
One additional limitation of this model is that the health state was based on constipation, the most common side-effect of opioid treatment. However, OXN may counteract other aspects of OIBD (such as abdominal pain, cramping and bloating) that may require additional healthcare resources. It is therefore possible that a model examining all aspects of OIBD, rather than just constipation, may show a greater incremental QALY gain from OXN compared with OXY. Also, the analysis was focused on non-malignant pain, but in broad terms the model and results could potentially be applied to other areas such as malignant pain. Given that OIC can impact on patients’ productivityCitation4–6, an important area of future research is to develop a health economic model from a societal perspective, in which the potential increased productivity associated with OXN treatment is assessed.
Appropriateness of the comparator
For a health economic analysis to be relevant it is imperative that the comparator is representative of standard clinical practice. Identifying the appropriate comparator is always a challenge in the area of treating OIC. This analysis used OXY as a comparator and allowed rescue laxative use in both treatment groups. Changing the comparator to OXY and regular laxatives was discounted for the following reasons. Firstly, there is limited evidence on laxatives for the management of constipationCitation41. Secondly, there is a lack of UK guidelines on the treatment of OIC, particularly in patients with non-malignant pain. Those guidelines that do exist (for palliative careCitation42 and patients with malignant painCitation43) recommend the use of a stimulant and a softener, but acknowledge that this recommendation is based on ‘common sense’ rather than evidence. Thirdly, it seems that these guidelines are seldom followed. Information from the UK-based General Practice Research Database showed that between January 2004 and May 2009, only 3% of patients prescribed an opioid were prescribed concomitant laxativesCitation44. An audit of clinical practice in Fife, Scotland showed that primary care physicians’ prescribing habits did not change following publication of guidance that stated that patients receiving opioids must have access to regular prophylactic laxativesCitation45. In the 6 months before and the 18 months after publication of the guidance, 98% of prescriptions for patients needing opioids were written for the opioid alone (i.e., laxatives were not prescribed)Citation45. Even when a laxative is prescribed, it is highly likely that patients ‘individualise’ their regimen, rather than take them every day. This means that the pattern of laxative use permitted in the study by Simpson et al.Citation14 (largely individualised due to patients’ monitoring of bowel movements, and judgement of constipation and related discomfort, triggering the use of rescue laxative under protocol-specified conditions) may not differ greatly from that observed in clinical practice. In addition, the regular use of a stimulant and softener may result in significant side-effects caused by over-laxation in some patients. This would be of particular concern for elderly patients, in whom dehydration and electrolyte imbalance can add significant morbidityCitation46. All of the above issues with laxative therapy in OIC means there is a need for innovative therapies like OXN that fundamentally reduce the probability of constipation developing in the first place. The laxative regimen used in the study was a stimulant laxative taken as rescue medication as defined by the study protocol, in addition to any existing and stable doses of fibre supplementation or bulking agents. On balance, it therefore appears to be an accurate reflection of laxative use in UK clinical practice. This means that the favourable cost-effectiveness ratio for OXN is based on a relevant comparator.
Conclusions
This cost-utility analysis examined patients with non-malignant pain suffering from OIC and was based on common UK clinical practice. The base case analysis showed slightly higher direct costs for OXN but this was accompanied by a quality of life gain, meaning that OXN demonstrates cost effectiveness at the commonly applied threshold. There is good evidence for a sufficient quality of life gain to offset the cost of OXN, with all sensitivity analyses resulting in ICERs below the commonly applied threshold. However, there is still a lack of clarity about the incremental cost of OXN given the uncertainty around the costs of treating OIC. A number of plausible scenarios explored within the sensitivity analysis showed that OXN was cost-saving (dominant). Overall, assuming a threshold of £20–30,000, OXN is estimated to be a cost-effective option for managing OIC in patients with severe non-malignant pain.
Transparency
Declaration of funding
This cost-utility study was sponsored by Napp Pharmaceuticals Limited.
Declaration of financial/other relationships
At the time of the study, W.D. was an employee of Napp Pharmaceuticals Limited; he is currently an employee of Mundipharma International Limited. R.U. is an employee of Mundipharma Research GmbH & Co. KG. I.K. is an employee of Mundipharma Research Limited. A.T. is an employee of Napp Pharmaceuticals Limited. Napp Pharmaceuticals Limited, Mundipharma Research GmbH & Co. KG, Mundipharma Research Limited and Mundipharma International Limited are independent associated companies. G.B. is employed by the University of East Anglia (UEA). UEA Consulting Limited received funding from Napp Pharmaceuticals Limited in order to critically appraise the model outlined in this paper.
Acknowledgements
Itrat Iqbal (Napp Pharmaceuticals Limited) provided support with the economic analyses. Dr Joanna Todd and Iain Leslie (Napp Pharmaceuticals Limited) provided medical writing services.
Notes
*[Targinact, Napp Pharmaceuticals, Cambridge, UK]
†[OxyContin, Purdue Pharma LP, Stamford, CT, USA]
*Exchange rates taken from http://www.xe.com. $1 US = £0.615574023 (2nd March 2011). €1 = £0.887653 (10th June 2011)
References
- Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333
- British Pain Society. Opioids for persistent pain: good practice. The British Pain Society, 2010;1-36
- Belsey J. Primary care workload in the management of chronic pain. A retrospective cohort study using a GP database to identify resource implications for UK primary care. J Med Econ 2002;5:39-50
- Wenig CM, Schmidt CO, Kohlmann T, et al. Costs of back pain in Germany. Eur J Pain 2009;13:280-6
- O'Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009;27:95-112
- Gupta A, Mehdi A, Duwell M, et al. Evidence-based review of the pharmacoeconomics related to the management of chronic nonmalignant pain. J Pain Palliat Care Pharmacother 2010;24:152-6
- Kurz A, Sessler DI. Opioid-induced bowel dysfunction. Pathophysiology and potential new therapies. Drugs 2003;63:649-71
- Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 2001;182:S11-18
- Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 2007;61:1181-7
- Kwong W, Diels J, Kavanagh S. Costs of gastrointestinal events after outpatient opioid treatment for non-cancer pain. Pain Management 2010;44:630-40
- Iyer S, Davis KL, Candrilli S. Opioid use and patterns and health care resource utilization in patients prescribed opioid therapy with and without constipation. Manag Care 2010;19:44-51
- Hjalte F, Berggren A-C, Bergendahl H, et al. The direct and indirect costs of opioid-induced constipation. J Pain Symptom Manage 2010;40:696-703
- Meissner W, Leyendecker P, Mueller-Lissner S, et al. A randomised controlled trial with prolonged-release oral oxycodone and naloxone to prevent and reverse opioid-induced constipation. Eur J Pain 2009;13:56-64
- Simpson K, Leyendecker P, Hopp M, et al. Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Curr Med Res Opin 2008;24:3503-12
- Vondrackova D, Leyendecker P, Meissner W, et al. Analgesic efficacy and safety of oxycodone in combination with naloxone as prolonged release tablets in patients with moderate to severe chronic pain. J Pain 2008;9:1144-54
- Löwenstein O, Leyendecker P, Hopp M, et al. Combined prolonged release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: a randomised controlled trial. Expert Opin Pharmacother 2009;10:531-43
- Rentz AM, Yu R, Müller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ 2009;12:371-83
- Ueberall M, Müller-Lissner S, Buschmann-Kramm C, et al. The Bowel Function Index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J Int Med Res 2011;39:41-50
- Briggs A, Claxton K, Sculpher M. Decision Making for Health Economic Evaluation. Oxford: Oxford University Press, 2006
- Napp Pharmaceuticals Limited. Data on file. Average length of treatment on oxycodone (data obtained from CSD)
- Rowen D, Brazier J, Roberts J. Mapping SF-36 onto the EQ-5D index: how reliable is the relationship?. Health Qual Life Outcomes 2009;7:27-38
- British National Formulary 60 (September 2010). London: British Medical Association/Royal Pharmaceutical Society, 2010
- Curtis L. Unit costs of health and social care 2010. Available at: http://www.pssru.ac.uk/pdf/uc/uc2010/uc2010.pdf [last accessed June 2011]
- Department of Health. National Tariff 2010-2011. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/[last accessed June 2011]
- Napp Pharmaceuticals Limited. Data on file. Opioid induced constipation – market research with GPs (n = 100)
- Sandner-Kiesling A, Leyendecker P, Hopp M, et al. Long-term efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of non-cancer chronic pain. Int J Clin Pract 2010;64:763-74
- Ware Jr JE, Kosinski M, Dewey JE. How to score Version 2 of the SF-36® Health Survey. Lincoln, RI: QualityMetric Incorporated, 2000
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14: 487-96
- Willan AR, Briggs AH. Statistical Analysis of Cost Effectiveness Data. Chichester: John Wiley and Sons, 2006
- Gaujoux-Viala C, Rat A-E, Guillemin F, et al. Comparison of the EQ-5D and the SF-6D utility measures in 813 patients with early arthritis: results from the ESPOIR cohort. J Rheumatol 2001;38:8;doi:10.3899/jrheum.101006
- Marra CA, Woolcott JC, Kopec JA, et al. A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med 2005;60:1571-82
- Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care 2005;43:736-49
- Brazier JE, Roberts JR. The estimation of a preference-based index from the SF-12. Med Care 2004;42:851-9
- Penning-van Beest FJA, van den Hank P, Klok RM, et al. Quality of life in relation to constipation among opioid users. J Med Econ 2010;13:129-35
- Guest J, Clegg J, Helter M. Cost-effectiveness of macrogol 4000 compared to lactulose in the treatment of chronic functional constipation in the UK. Curr Med Res Opin 2008;24:1841-52
- Van der Linden MW, van den Haak P, Penning-van Beest FJA, et al. Patient reported quality of life in cancer patients on opioid therapy is influenced by constipation. Value Health 2008;11:A485
- National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London: NICE, 2008. Available at: http://www.nice.org.uk/aboutnice/howwework/devnicetech/guidetothemethodsoftechnologyappraisal.jsp [last accessed January 2012]
- Scottish Medicines Consortium. Advice on oxycodone/naloxone 10 mg/5 mg and 20 mg/10 mg prolonged release tablets (Targinact®). February 2009. Available at: http://www.scottishmedicines.org.uk/files/oxycodonenaloxone_Targinact_.pdf [last accessed January 2012]
- Schutter U, Grunert S, Meyer C, et al. Innovative pain therapy with a fixed combination of prolonged-release oxycodone/naloxone: a large observational study under conditions of daily practice. Curr Med Res Opin 2010;26:1377-87
- Rychlik R, Kiencke P, Kresimon J. [Healthcare research study into quality of life and pharmacoeconomic aspects of patients with chronic back pain being treated with oxycodone/naloxone or other WHO step III opioids. Interim analysis]. Gesundh Ökon Qual Manag 2011;16:10-9. German
- Candy B, Jones L, Drake R, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care (review). The Cochrane Library 2011, Issue 1. Available at: http://www.thecochranelibrary.com [last accessed January 2012]
- NHS Clinical Knowledge Summaries. Palliative care - pain - management. Available at: http://www.cks.nhs.uk/palliative_cancer_care_constipation [last accessed January 2012]
- Scottish Intercollegiate Guidelines Network. Control of pain in adults with cancer. November 2008. Available at http://www.sign.ac.uk/pdf/SIGN106.pdf [last accessed January 2012]
- Napp Pharmaceuticals Limited. Data on file. Prescribing of laxatives with opioids – GPRD study
- Lanza P, Carey M. The impact of opioid and laxative prescribing habits on constipation in the primary care setting before and after the introduction of SIGN 44: Control of pain in patients with cancer. Primary Health Care Research and Development 2006;7:3-9
- Weinberg AD, Minaker KL, AMA Council on Scientific Affairs. Dehydration. evaluation and management in older adults. JAMA 1995;274:1552-6