Abstract
Objective:
This study uses real-world US managed-care claims data to estimate dose escalation rates over the first and second years of therapy among biologic naïve rheumatoid arthritis (RA) patients initiating tumor necrosis factor (TNF) blocker therapy with etanercept, adalimumab, or infliximab.
Methods:
Non-elderly adult (age 18–65 years) RA patients initiating etanercept, adalimumab, or infliximab from July 1, 2005 to April 30, 2009, were identified using the MarketScan Commercial Database. National and regional dose-escalation patterns were evaluated 12 and 24 months after initiation. In the single-instance method, dose escalation was defined as having one average weekly dose 115%, 130%, or 150% greater than the initial average weekly dose. By the two-instances method, dose escalation was defined as having two consecutive claims with an average weekly dose 115% or 130% greater than the initial average weekly dose.
Results:
A total of 2747 patients met the inclusion criteria (mean age 50 years [SD = 10]; 74% female). More patients initiated etanercept (44%) than adalimumab (37%) or infliximab (20%). Using the single-instance method, dose escalation at 12 months ranges were 0.8–1.5% for etanercept, 10.8–12.5% for adalimumab, and 16.4–42.5% for infliximab; ranges at 24 months were 0.8–2.1% for etanercept, 14.3–17.5% for adalimumab, and 26.4–57.6% for infliximab. The two-instances method showed a similar relationship among the treatment cohorts at both 12 and 24 months, with lower dose-escalation rates for etanercept (0.8%, 0.8%) than adalimumab (8.7%, 13.3%) or infliximab (22.9%, 37.6%) at the 130% threshold (p < 0.001). Dose-escalation rates for etanercept, adalimumab, and infliximab were consistent across US geographic regions.
Conclusion:
Patients initiating etanercept had lower rates of dose escalation than patients initiating adalimumab or infliximab in the first and second year following therapy initiation, as well as across US geographic regions. These results may not be generalizable to the entire US RA population.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disorder of unknown etiology that is characterized by pain, inflammation, and progressive joint degenerationCitation1. Biologic therapies that target the proinflammatory cytokine tumor necrosis factor (TNF blockers) have been shown to be effective in the treatment of moderate-to-severe RA.
The three most commonly prescribed TNF blocker therapies are etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira)Citation2–4; golimumab (Simponi)Citation5 and certolizumab pegol (Cimzia)Citation6 are also FDA-approved for the treatment of RA. Etanercept is a recombinant human soluble TNF receptor protein, and adalimumab and infliximab are anti-TNF monoclonal antibodies. Adalimumab and etanercept are administered as a subcutaneous (SC) injectionCitation2,Citation3; infliximab is administered as an intravenous (IV) infusion and is dosed by weightCitation4. For patients with RA, the recommended dose of adalimumab is 40 mg every other week, with the option of increasing the frequency to every weekCitation3, and the recommended dose for etanercept is 50 mg per weekCitation2. Infliximab is administered as an IV infusion of 3 mg/kg at weeks 0, 2, and 6 and then every 8 weeks thereafterCitation4. The RA dose may be adjusted up to 10 mg/kg or the frequency increased to every 4 weeksCitation4.
Neutralizing antibody formation has been observed with both infliximabCitation7,Citation8 and adalimumabCitation7–11, but not with etanerceptCitation12,Citation13. Anti-drug antibodies were reported in 33–43% of RA patients on infliximab therapyCitation7,Citation8 and 12–28% of patients on adalimumabCitation9–11. Presence of anti-infliximab or anti-adalimumab antibodies was associated with a reduced number of clinical respondersCitation8,Citation10 or lower mean responseCitation9 not occurring with etanerceptCitation12,Citation13. Immunogenicity of TNF blockers might play a role in non-response to this treatmentCitation10,Citation14. This diminished treatment response may lead to some of the dose escalation seen with these agents in an attempt to regain or maintain clinical responseCitation8,Citation15,Citation16; however, the literature reports that higher doses seem to elicit effects similar to lower dosesCitation17–20.
Previous studies using US managed-care claims datasets using data from 2002 to 2005 have reported dose-escalation rates of approximately 1–7% for etanercept, 9–21% for adalimumab, and 24–26% for infliximabCitation23–27. Previous studies have shown that patients with dose increases or high starting dose had higher costs compared with patients without high starting dose or dose escalationCitation23,Citation25. In addition to the added costs, increasing the dose of a TNF blocker beyond the minimum recommended label dose increases the risk of side-effectsCitation28–30.
While several studies have described TNF blocker dose escalation among RA patients, there is relatively little information regarding dose escalation after the first year of treatment or within specific regions of the US. As studies have indicated, neutralizing antibodies for adalimumab have been detected up to 3 years following therapy initiation. Accounting for long-term dosing patterns is necessary given the chronic nature of RA.
Methods
Study design
A retrospective, observational study was conducted using US administrative claims data to estimate dose-escalation patterns over the first and second years of therapy among biologic naïve RA patients newly initiating TNF blocker therapy with etanercept, adalimumab, or infliximab.
Data source
The study was conducted using administrative claims from the Thomson Reuters MarketScan Commercial Database. The Commercial Database contains the healthcare experience of privately insured individuals covered under a variety of fee-for-service, fully capitated, and partially capitated health plans. The database is constructed from claims and enrollment data provided by over 130 large employer-sponsored health plans from across the US and includes several million enrollees annually, encompassing employees, their spouses, and dependents. There were nearly 40 million enrollees in the Commercial Database in 2009, an increase from approximately 35 million enrollees in 2008. The Commercial Database is de-identified and fully compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Because this study did not involve the collection, use, or transmittal of individually identifiable data, institutional review board (IRB) review or approval was not required.
Study inclusion criteria
Non-elderly adults with RA, ages 18–65 years, newly initiating TNF blocker therapy with infliximab, etanercept, or adalimumab during the time period from July 1, 2005, through April 30, 2009, were selected for the study. RA patients were identified by the presence of at least one inpatient or two outpatient medical claims containing the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 714.0x. The index date was identified as the date of the first TNF blocker prescription or administration on or after January 1, 2005; the index medication was the TNF blocker prescribed or administered on the index date. Data through April 30, 2010, was used to observe dose-escalation patterns. Patients were required to have 6 months of continuous medical and pharmacy enrollment (i.e., the pre-index period) that was clean of any TNF blocker use, including the index medication.
Patients were required to be continuously enrolled and persistent on their index therapy for 12 months following therapy initiation. Patients were considered to be non-persistent if they switched to a non-index TNF blocker or if they had a gap in their index therapy of greater than 60 days. Dose escalation was evaluated at 24 months following initiation for patients who were continuously enrolled and persistent on index therapy for the entire 2 years. By definition, patients persistent on index therapy for 24 months are a subset of patients who were persistent on their index therapy for 12 months. Patients who were persistent for greater than 12 months but less than 24 months were included only in the 12-month cohort.
Study exclusion criteria
Patients were excluded from the study if they were under 18 or over 65 years of age on the index date. Patients were excluded if they used any of the following medications during the pre- or post-index periods: anakinra (Kineret), rituximab (Rituxan), abatacept (Orencia), tocilizumab (RoActemra, Actemra), certolizumab pegol (Cimzia), or golimumab (Simponi); there was insufficient sample to include these therapies as stand-alone treatment cohorts during the study period. Patients were also excluded if they had the following diagnoses during the pre- or post-index periods: non-Hodgkin’s lymphoma (ICD-9-CM: 200.xx or 202.xx), chronic lymphocytic leukemia (ICD-9-CM: 204.10), psoriasis (ICD-9-CM: 696.1), psoriatic arthritis (ICD-9-CM: 696.0), juvenile idiopathic arthritis (ICD-9-CM: 714.3), Crohn’s disease (ICD-9-CM: 555.x), ulcerative colitis (ICD-9-CM: 556.x), ankylosing spondylitis (ICD-9-CM: 720.0), cancer (ICD-9-CM: 140.xx–209.xx or V10), or HIV (ICD-9-CM: 042). Patients with poor quality claims data (i.e., missing or zero values for quantity or days supply) or outlier doses on any claims (<50% or >200% of labeled dose) were excluded. Lastly, this study was limited to patients receiving greater than or equal to the recommended label dose as their initial dose; patients initiating therapy with suboptimal doses were excluded to ensure that titrating up to the recommended dose was not misclassified as dose escalation.
Outcome variables
Due to the claims database structure, dose was calculated differently for subcutaneously injected and infused products. For etanercept and adalimumab, the initial dose was the dose of the index prescription. The average weekly dose per patient was calculated using the total quantity on the claim divided by the total days’ supply. Since infliximab is infused, it is billed as a medical service and not a pharmacy claim, requiring imputation to determine the dose per claim. The infliximab dose was calculated using the paid amount on the infliximab claim divided by the wholesale acquisition cost (WAC) for that calendar year. Infliximab administrations are scheduled at weeks 0, 2, 6, and then every 8 weeks thereafter; therefore, the initial dose was based on the fourth infusion, which is the first infusion following the loading period.
Dose escalation was evaluated using two separate methods, both of which are based on the average weekly dose for each claim. Under the first method, the single-instance method, dose escalation was defined as having an average weekly dose on a claim that is greater than 115% of the initial claim’s average weekly dose; the percentage of patients with an average weekly dose greater than 130% or 150% of the initial claim was also reported. Under the second method, the two-instances method, dose escalation was defined as having two consecutive claims where the average weekly dose on each claim is greater than 115% of the initial claim’s average weekly dose; a separate threshold of 130% was also used for the two-instances method.
Using both methods, dose escalation was reported separately by geographic region of the US in the 12 months after therapy initiation; national estimates of dose escalation using both methods were reported in the 12 and 24 months after therapy initiation. Geographic regions included the Northeast, South, North Central (including Midwest) and West.
Descriptive analysis
Descriptive statistics were used to summarize the characteristics of patients in the three TNF blocker cohorts. Chi-squared tests were used to test for differences in nominal/categorical variables; t-tests were used to test for differences in interval/continuous variables. Etanercept was the reference group, with an a priori significance level of 0.05. Analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC, USA)Citation31.
Results
Baseline demographics and clinical characteristics
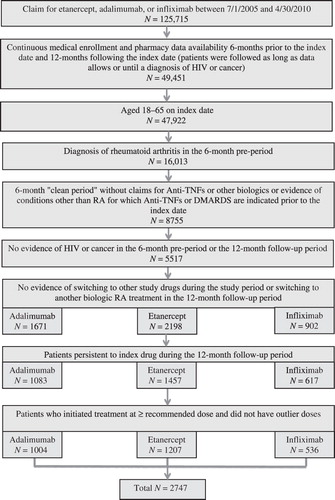
A total of 2747 RA patients newly initiating etanercept, adalimumab, or infliximab between July 1, 2005, and April 30, 2009, met all of the study selection criteria. contains a patient attrition diagram.
Of those meeting the study criteria, 1207 (44%) initiated treatment with etanercept, 1004 (37%) with adalimumab, and 536 (20%) with infliximab. A total of 1125 patients were persistent on their index therapy 24 months following therapy initiation; 447 (42%) of these patients initiated etanercept, 399 (35%) initiated adalimumab, and 250 (22%) initiated infliximab. Patient characteristics were similar across the three treatment groups. Among patients with at least 12 months of follow-up, the mean age was 50 (SD = 10 years), the majority of patients were female (73–75%), and the majority belonged to a non-capitated health plan (79–82%). The highest proportion of patients lived in the South (44.6%), followed by the North Central (28.2%), West (16.5%), and Northeast (10.2%). contains patient demographic and clinical characteristics stratified by index medication, region, and length of index medication persistence (12 or 24 months). Baseline Deyo Charlson Comorbidity Index (DCI) scores were similar across treatments, ranging from 1.07 (SD = 0.29) among adalimumab patients to 1.13 (SD = 0.50) among infliximab patients. Methotrexate (71–75%) and glucocorticoids (57–61%) were the most prevalent concomitant medications used during the pre-index period.
Table 1. Demographic and clinical characteristics of patients persistent with etanercept. Adalimumab, or infliximab far 12 or 24 months.
Initial dose
The average initial weekly dose was 48.7 mg (SD = 2.1 mg) for etanercept, 21.0 mg (SD = 4.8 mg) for adalimumab, and 52.6 mg (SD = 24.3 mg) for infliximab. The majority of patients initiated TNF blocker treatment at the recommended label dose. Nearly all etanercept and adalimumab patients (99% and 93%, respectively) and 72% of infliximab patients initiated therapy within 10% of the recommended label dose; remaining patients initiated therapy with a dose at least 10% higher than the recommended label dose.
Dose escalation
contains the dose-escalation results for both the single- and two-instances methods. Under the single-instance method, patients in the adalimumab (12.5%) and infliximab (42.5%) cohorts were both more likely than etanercept (1.5%) patients to have an average weekly dose that was greater than 115% of the initial dose (p < 0.001 for both comparisons). Under the single-instance method, patients in the adalimumab (12.0%) and infliximab (30.2%) cohorts were both more likely than etanercept (1.2%) patients to have an average weekly dose that was greater than 130% of the initial dose (p < 0.001 for both comparisons); 0.8% of etanercept patients, 10.8% of adalimumab patients, and 16.4% of infliximab patients had dose escalation in the 12 months following therapy initiation using the 150% threshold (p < 0.001 for adalimumab versus etanercept and infliximab versus etanercept, separately). Using the two-instances method, 0.8% of etanercept patients had dose escalation using the 130% threshold in the 12 months following therapy initiation, compared with 8.7% of adalimumab patients and 22.9% of infliximab patients (p < 0.001 for both comparisons). For patients persistent on their index therapy for 24 months, 1.5% of etanercept patients had dose escalation using the 130% threshold for the single-instance method, compared with 16.5% of adalimumab patients and 41.2% of infliximab patients (p < 0.001 for both comparisons); 0.8% of etanercept patients had dose escalation in the 24 months following therapy initiation at the 150% threshold compared with 14.3% of adalimumab patients and 26.4% of infliximab patients (p < 0.001 for both comparisons). Using the two-instances method, 1.0% of etanercept patients had dose escalation in the 24 months following therapy initiation at the 130% threshold, compared with 13.8% of adalimumab patients and 51.2% of infliximab patients (p < 0.001 for both comparisons).
Table 2. Dose escalation in the first 24 months of etanercept, adalimumab, or infliximab use.
Regional analysis of dose escalation
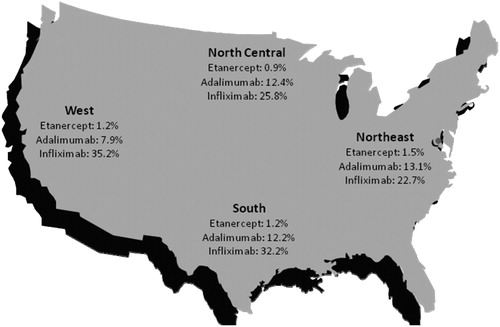
Rates of dose escalation by geographic region were similar to the national rates. Using the single-instance method and 150% threshold, dose-escalation rates for etanercept ranged from 2.3% in the West to 3.9% in the Northeast in the 12 months following therapy initiation. Using the same criteria, dose-escalation rates ranged from 8.5% in the West to 13.1% in the Northeast for adalimumab and from 26.5% in the Northeast to 39.5% in the West for infliximab. depicts regional dose-escalation rates.
Discussion
This study is a comprehensive assessment of dose escalation among patients initiating TNF blockers, providing recent estimates of dose escalation among biologic naïve RA patients newly initiating TNF blocker treatment with employer-paid commercial healthcare insurance in the 12 and 24 months following treatment initiation. This study used two methods and multiple thresholds for defining dose escalation. Using the most stringent definition (two instances, 130% threshold) and least stringent definition (single instance, 115% threshold), dose-escalation rates ranges were 0.8–1.5% for etanercept, 8.7–12.5% for adalimumab, and 22.9–42.5% for infliximab in the year following therapy initiation. This is similar to a previous study evaluating dose escalation among TNF blocker naïve RA patientsCitation24, which reported that dose escalation was lowest among patients treated with etanercept and highest among those treated with infliximab. Harrison and colleagues used commercial healthcare claims data from January 2004 to December 2005 to evaluate dose escalation among biologically naïve RA patients initiating TNF blocker therapy and reported that 2% of etanercept patients had a dose increase during the 12-month observation period compared with 14% of adalimumab patients and 60% of infliximab patientsCitation24. Harrison defined dose escalation as having a subsequent dose that is 10% greater than the index dose, which may explain why Harrison’s reported rates are slightly higher than this current analysis. Other published studies have shown higher dose-escalation rates in the 12 months following therapy initiation. Huang and colleagues used multiple methods for evaluating dose escalation and reported dose-escalation rates in the ranges 5.5–10.3% for etanercept patients and 14.8–33.6% for adalimumab patients using the same, albeit older, managed-care data source as this current analysisCitation26. Using a large national managed-care database from April 2007 through March 2009, Khanna and colleagues found that 3% of etanercept patients increased their weekly dose from 50 mg to 100 mg, 13% of adalimumab patients increased their biweekly dose from 40 to 80 mg, and 39% of infliximab patients either added another vial or reduced the time between infusion administrations to less than 6 weeksCitation32. Some of the variation in the dose-escalation estimates in previous research may be due to the method of calculating dose escalation, differences in the patient population, or differences in trends over time. However, despite these differences in treatment period and operational definitions of dose escalation, there is a consistent trend of higher rates of dose escalation among adalimumab and infliximab patients compared with etanercept patients.
Dose escalation is an important outcome due to its relationship with costs and the similar percentage of patients achieving clinical improvements with increased dose. Previous studies have reported a relationship between dose escalation and higher costsCitation23,Citation24, while other studies have shown that treatment groups with higher rates of dose escalation have increased therapy costs, whereas low population rates of dose escalation are associated with stable costsCitation25,33. BlomCitation17 and MootsCitation18 both reported higher dose-escalation rates among adalimumab and infliximab users compared with etanercept users; however, changes in disease activity were similar across treatment groups. Likewise, Schabert and colleagues found similar disability outcomes among US patients in an RA registry treated with etanercept, adalimumab, or infliximab, even though self-reported dose escalations of the index medication were observed more often among infliximab and adalimumab patients than among etanercept patientsCitation21. In a study using rheumatologist chart assessment data, patients on etanercept were less likely to have dose escalation compared with patients on adalimumab or infliximab; however, there were no statistically significant differences in percentage achieving clinical improvement between treatment groups or between dose escalators and non-escalatorsCitation22.
While previous studies have reported dose escalation in the first 12 months following therapy initiation, there is relatively little data regarding dose escalation over longer time periods. Given the chronic nature of TNF blocker use and the fact that secondary resistance to TNF blocking agents can develop, a longer time period is useful for better understanding dose-escalation rates in the real world. This study found that dose escalation with etanercept was relatively stable from the first to second year, increasing by 0.3% (from 1.2 to 1.5%) using the single-instance method and 130% threshold. By comparison, dose escalation increased from 12.0 to 16.5% for adalimumab patients and from 30.2 to 41.2% for infliximab patients using the same measure.
This study also provides regional estimates of dose escalation, which can be used by managed-care organizations to compare their dose-escalation rates to regional and national benchmarks and to inform formulary decisions. Results of the regional analysis were directionally consistent with the national dose-escalation rates. Dose-escalation rates among etanercept patients were low compared with adalimumab and infliximab and remained stable across all four geographic regions. Dose-escalation rates for adalimumab and infliximab showed more regional variation, but were directionally consistent with national dose-escalation rates.
Limitations
This study was subject to several limitations. Newer biologic RA therapies (such as certolizumab and golimumab) were not included in this analysis due to insufficient sample size within the study time period. While requiring patients to be continuously enrolled and persistent on therapy for 12 (or 24) months created a homogenous patient population and allowed for equitable comparisons of dose escalation across treatments, they also limit the generalizability of the study findings. For example, the results of this analysis may not apply to patients with shorter treatment durations or characteristics that may lead to unstable insurance coverage. Similarly, the data source includes only patients with commercial health coverage; therefore, the results may not be generalizable to the entire US RA population. The data source is also limited to information found on administrative healthcare claims; disease-specific clinical information (e.g., disease remission status, disease-related quality of life, etc.) could not be included in this analysis.
Conclusion
RA patients newly initiating etanercept had significantly lower rates of dose escalation compared with patients initiating adalimumab or infliximab (p < 0.001 for all comparisons). This finding was consistent across dose-escalation measures in both the first and second year following therapy initiation, as well as across geographic regions of the US. These findings should be taken into consideration while making formulary decisions regarding TNF blocker therapy, given the lack of evidence of greater effectiveness of dose escalating agents and the fact that increased dose is associated with higher costs and increased risk of adverse eventsCitation23,Citation25,Citation28–30.
Transparency
Declaration of funding
This research was funded by Immunex Corporation, a wholly owned subsidiary of Amgen, Inc., and by Wyeth, which was acquired by Pfizer Inc in October 2009.
Declaration of financial /other relationships
S.G. is an employee of Amgen, Inc. and has received Amgen stock/stock options; M.B. and K.W. are employees of Thomson Reuters, which received a research contract to conduct this analysis. K.F. received research funds from Amgen, Inc. as a consultant.
Acknowledgments
The peer reviewers on this manuscript have disclosed any relevant financial relationships.
References
- Centers for Disease Control and Prevention. 2010. Available at http://www.cdc.gov/arthritis/basics/rheumatoid.htm
- Enbrel® (etanercept) Prescribing Information, Immunex Corporation, Thousand Oaks, CA, USA
- Humira® (adalimumab) Prescribing Information, Abbott Laboratories, Abbott Park, IL, USA
- Remicade® (infliximab) Prescribing Information, Centocor, Inc., Malvern, PA, USA
- Simponi® (golimumab) Prescribing Information, Centocor, Inc., Malvern, PA, USA
- Cimzia® (certolizumab pegol) Prescribing Information, UCB, Inc., Smyrna, GA, USA
- Pascual-Salcedo D, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology 2011;50:1445-1452
- Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:711-15
- Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis 2007;66:921-6
- Bartelds GM, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long- term follow-up. JAMA 2011;305:1460-8
- van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 2004;63:508-16
- Foerder CA, Rogge MC. Enbrel (etanercept). Dev Biol (Basel) 2002;109:99-102
- Klareskog L, Moreland L, Cohen S, et al. Efficacy and safety of over 8 years of continuous etanercept therapy in patients with rheumatoid arthritis in North America and Europe. Ann Rheum Dis 2006;65(Suppl II):326
- Jamnitski A, Bartelds GM, Nurmohamed MT, et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis 2010;70:284-8
- Haraoui B, Cameron L, Ouetliet M, et al. Anti-infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J Rheumatol 2006;33:31-6
- Finckh A, Dudier J, Wermelinger F, et al. Influence of anti-infliximab antibodies and residual infliximab concentrations on the occurrence of acquired drug resistance to infliximab in rheumatoid arthritis patients. Joint Bone Spine 2010;77:313-18
- Blom M, Kievit W, Kuper HH, et al. Frequency and effectiveness of dose increase of adalimumab, etanercept, and infliximab in daily clinical practice. Arthritis Care Res (Hoboken) 2010;62:1335-41
- Moots RJ, Haraoui B, Matucci-Cerinic M, et al. Differences in biologic dose-escalation, non-biologic and steroid intensification among three anti-TNF agents: evidence from clinical practice. Clin Exp Rheumatol 2011;29:26-34
- Van Vollenhoven RF, Brannemark S, Klareskog L. Infliximab dosage and infusion frequency in clinical practice: experiences in the Stockholm biologics registry. STURE. Scand J Rheumatol 2007;36:418-23
- Pavelka K, Jarosova K, Suchy D, et al. Increasing the infliximab dose in rheumatoid arthritis patients: a randomized, double blind study failed to confirm its efficacy. Ann Rheum Dis 2009;68:1285-9
- Schabert VF, Bruce B, Ferrufino CF, et al. Disability outcomes and dose escalation in rheumatoid arthritis patients treated with tumor necrosis factor blockers: a comparative effectiveness analysis. ISPOR European Congress, Prague, Czech Republic, November 6–9, 2010 (abstract)
- Segal SD, Power DJ, Smith DB, et al. Comparative effectiveness analysis of TNF blockers in rheumatoid arthritis (RA) patients in US community practice. EULAR, London, UK, May 25–28, 2011(abstract)
- Gu NY, Huang XY, Fox KM, et al. Claims data analysis of dosing and cost of TNF antagonists. Am J Pharm Benefits 2010;2:9
- Harrison D, Huang X, Globe D. Dosing patterns and costs of tumor necrosis factor inhibitor use for rheumatoid arthritis. Am J Surg Pathol 2010;67:1281-7
- Bullano MF, McNeeley BJ, Yu YF, et al. Comparison of costs associated with the use of etanercept, infliximab, and adalimumab for the treatment of rheumatoid arthritis. Manag Care Interface 2006;19:47-53
- Huang X, Ning G, Fox K, et al. Comparison of methods for measuring dose escalation of the subcutaneous TNF antagonists for rheumatoid arthritis patients treated in routine clinical practice. Curr Med Res Opin 2010;26:1637-45
- Ollendorf D, Kingman D, Hazard R, et al. Differences in annual medication costs and rates of dosage increase between tumor necrosis factor-antagonist therapies for rheumatoid arthritis in a managed care population. Clin Ther 2009;31:821-35
- Alonso-Ruiz A, Pijoan JI, Ansuategui E, et al. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and meta-analysis of efficacy and safety. BioMed Central Musculoskeletal Disorders 2008;9:52
- Zintzaras E, Dahabreh IJ, Giannouli S, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis: a systematic review and meta-analysis of dosage regimens. Clin Ther 2008;30:1939-55
- Perdriger A. Infliximab in the treatment of rheumatoid arthritis. Biologics 2009;3:183-91
- SAS. Version 9.2. Cary (NC): SAS Institute Inc., 2002-2008
- Khanna D, Cyhaniuk A, Bedenbaugh A. Use of TNF inhibitors in the US: Utilisation patterns and dose escalation from a retrospective US RA population. EULAR, London, UK, May 25–28, 2011 (abstract)