Abstract
Background and objectives:
Secondary hyperparathyroidism (SHPT) is a frequent complication of CKD with incidence, prevalence, and costs increasing worldwide. The objective of this analysis was to estimate therapy cost of SHPT in a sub-population of the FARO study.
Materials and Methods:
In the FARO study, an observational survey aimed to evaluate patterns of treatment in patients with SHPT who had undergone hemodialysis, pharmacological treatments and biochemical parameters evolution data were collected in four surveys. Patients maintaining the same treatment in all sessions were grouped by type of treatment and evaluated for costs from the Italian National Health Service perspective.
Results:
Four cohorts were identified: patients treated with oral (PO) calcitriol (n = 182), intravenous (IV) calcitriol (n = 34), IV paricalcitol (n = 62), and IV paricalcitol + cinacalcet therapy (n = 20); the cinacalcet monotherapy group was not analysed due to low number of patients (n = 9). Parathyroid hormone (PTH) level at baseline and effectiveness of treatments in suppressing PTH level were assessed to test comparability among cohorts: calcitriol PO patients were significantly less severe than others (PTH level at baseline lower than 300 pg/ml; p < 0.0001); calcitriol IV patients did not reach significant reduction in PTH level. Paricalcitol and paricalcitol + cinacalcet treatment groups results were comparable, while only the IV paricalcitol cohort’s PTH level, weekly dosage, and cost decreased significantly from the first to the fourth survey (p = 0.020, p = 0.012, and p = 0.0124, respectively). Total costs per week of treatment (including calcium-based phosphate binder and sevelamer) were significantly lower in the paricalcitol vs paricalcitol + cinacalcet cohort (p < 0.001). Major limitations of this study are related to the survey design: not controlled and lack of comparability between cohorts; however, reflective of true practice patterns.
Conclusions:
The IV Paricalcitol cohort had significantly lower treatment costs compared with patients treated with paricalcitol + calcimemtics (p < 0.001), without a significant difference in terms of baseline severity and PTH control.
Introduction
The incidence, prevalence, and costs related to the treatment of chronic kidney disease (CKD) and particularly to End-Stage Renal Disease (ESRD) are increasing worldwideCitation1. In the US, total Medicare spending in 2006 was nearly $355 billion, while ESRD costs accounted for 6.4% of the entire Medicare budgetCitation2.
In Italy, the prevalence of patients on dialysis in 2006 was above 700 per million population (PMP), while the incidence was ∼150/PMPCitation3, with an expenditure that was estimated to account for 2% of total healthcare expenditureCitation4.
Secondary hyperparathyroidism (SHPT) is one of the most frequent complications of CKD and is mainly characterized by alterations in levels of parathyroid hormone (PTH), calcium, phosphorous, and vitamin DCitation5. High levels of PTH have been shown to be associated with an increased risk of hospitalization and death from cardiovascular diseaseCitation6–10.
The FARO study was a prospective, multi-center, observational study conducted in 28 different dialysis centers across ItalyCitation11. Centers were selected on the basis of geographical distribution and included a relatively high proportion of patients on hemodialysis (HD). Each center collected data on SHPT management (bone and mineral parameters and consumption of medical resources) in HD patients; all HD patients were enrolled.
This analysis aimed to evaluate pharmaceutical cost of treatment and the evolution of bone and mineral parameters in patients who maintained the same SHPT treatment and participated in all surveys.
Materials and methods
A sub-population of patients from the FARO study was selected in order to estimate resource consumption, costs, and clinical consequences related to specific SHPT treatment; the sub-populations were selected on the basis of the consistency of SHTP treatment throughout the study.
The FARO survey was a prospective, multi-center Italian study that was performed from April 2006 to October 2007, involving 28 dialysis centers. In each center, data derived from the monitoring of bone and mineral parameters (PTH and serum levels of calcium and phosphorus) and therapies to control SHPT and other complications (i.e., anemia) were collected from HD patients over four periods of cross-sectional evolution of 1 week every 6 months (baseline, months 6, 12, and 18).
A total of 2637 patients were observed in at least one survey. All patients signed a written informed consent form following the Legislative Decree 196/2003 (patients data used in an anonymous way) and the FARO project is approved by Ethical Committees in each of the 28 participant centers. Since this was an observational study, the choice of treatment was the decision of the physician.
For the cost consequences evaluation, the inclusion criteria were: patients with data collection in all four surveys and receiving only one SHPT treatment regimen in all four surveys (except for the group receiving the association IV paricalcitol and cinacalcet).
Selected patients were grouped by continuous treatment cohorts. Patients who did not have data collected in all four surveys were excluded from the cost analysis.
Bone and mineral parameters considered for the clinical effectiveness evaluation included serum PTH, calcium, and phosphorus. Target levels for serum PTH, calcium, and phosphorus were in accordance with the National Kidney Foundation in the Kidney Disease Outcomes Quality Initiative (K/DOQI) international guidelines (revised in 2003) and with the contemporary national guidelinesCitation12.
For the cost consequence analysis, weekly cost of pharmacological treatment per group was estimated, based on an Italian National Health Service (INHS) perspective; i.e., for hospital drugs, the maximum approved selling prices to INHS were considered (reference year 2007) while, for retail drugs, the approved reimbursement price was considered; all prices are pre-transient compulsory reductions and include negotiated discounts published on the Official Italian Bulletin. Unitary costs for drugs indicated for SHPT treatment are reported in . No discount rate was applied considering that the cost estimated refers to a 1-year period.
Table 1. Reimbursed cost for SHPT drugs.
Comparisons between treatment groups was assessed by measuring baseline disease severity (i.e., baseline PTH levels) and effectiveness of treatment was expressed in terms of PTH decrease (difference from the first to the fourth survey).
Sensitivity analysis
Sensitivity analysis was performed in order to assess the robustness of the results.
Effectiveness in reducing PTH levels, average dosage of the administered drugs in the analyzed sub-populations and related costs were compared with those of the FARO study total population (all patients who received a specific treatment at a specific survey and not necessarily remaining on the same treatment for the entire observational period) to assess if patient selection had an influence on the results.
To estimate potential changes in treatment patterns due to the introduction of new therapies (IV paricalcitol and cinacalcet were launched slightly before the beginning of the FARO study), data from cohorts of patients comparable for severity at baseline and for effectiveness (level of iPTH suppression) were compared with data from patients who started a specific therapy at the second or third survey, and remained on the same treatment for the remaining period.
Finally, since it is recognized that different treatments may influence the concomitant use of other drug types, the use of phosphate binders (from the economic point of view the most relevant was Sevelamer) were also assessed in terms of dosage, percentage of treated patients, and costs.
Results
Five cohorts of patients were identified: PO calcitriol (n = 182), IV calcitriol (n = 34), IV paricalcitol (n = 62), cinacalcet (n = 9), and IV paricalcitol + cinacalcet (n = 20).
Baseline demographic characteristics for the different treatment groups are reported in .
Table 2. Demographic characteristics.
Overall, the majority of patients were male (aged 58.38–66.92 years). The most frequent co-morbidities included cardiovascular disease (frequency 77.8–95%) and hypertension (60–67.7%). Over 92% of patients received dialysis treatment three times per week. Patients receiving only cinacalcet were not included in the analysis due to the limited number of patients (n = 9).
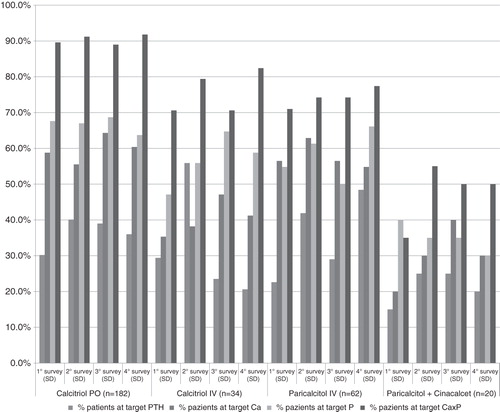
Bone and mineral parameters (PTH, calcium, and phosphorus serum levels) at each survey are reported in .
Table 3. Biochemical parameters.
Average dosages and weekly costs of drugs administered for SHPT treatment are reported in .
PO calcitriol cohort: a significant decrease in the PTH level was observed from the first to the fourth observation (p = 0.039), while the percentage of patients at the target PTH level did not increase significantly from the first to the fourth survey (30.2% and 36.3%, respectively). In this cohort, no significant differences in terms of dosages and weekly costs were observed from the first to the fourth survey.
IV calcitriol cohort: patients receiving intravenous administration of calcitriol did not have any significant improvement in PTH level and showed a decrease in the percentage of patients reaching PTH target levels from the first to the fourth survey (from 29.4% to 20.6%). In this cohort, the weekly cost of treatment did not significantly decrease from the first to the fourth survey.
IV Paricalcitol cohort: levels of serum PTH decreased significantly from the first to the fourth survey (p = 0.020) and a significant increase in the percentage of patients reaching target PTH levels from the first to the fourth survey was observed (p = 0.008).
The average dosage and cost of administered drug decreased significantly from the first to the fourth survey (p = 0.012 and p = 0.0124).
Combination-therapy IV paricalcitol + cinacalcet: no statistical changes in the level of PTH and percentage of patients reaching target level of PTH was observed from the first to the fourth survey, possibly due to the low sample size (n = 20).
Table 4. Average dosages and weekly cost of treating SHPT.
In this cohort of patients, dosages of the two compounds have had different trends: the dosage of IV paricalcitol decreased significantly over the observational period (p = 0.04), whereas the dosage of cinacalcet was observed to increase significantly (p = 0.003). The weekly cost of treatment did not show significant differences between the first and the fourth observation.
Comparison among groups
At baseline, patients receiving oral administration of calcitriol had a significantly lower level of PTH compared to the other groups (p < 0.0001), whose average PTH level did not differ from each other (this apparent discrepancy was probably due to the not normal distribution of the values); the percentage of patients with PTH levels within the reference targets was not statistically different among the groups.
At baseline, a higher percentage of patients at target levels of serum calcium levels were observed in the PO calcitriol group vs IV calcitriol group (p = 0.014), PO calcitriol vs association IV paricalcitol + cinacalcet (p = 0.001), and between IV paricalcitol and the IV paricalcitol + cinacalcet combination group (p = 0.005); at the same time, the percentage of patients at serum phosphate target levels were found to be statistically higher in the PO calcitriol group compared to the IV calcitriol group (p = 0.031), without any significant differences among the other groups.
At the fourth survey, the percentage of patients attaining target PTH levels was significantly greater in the IV paricalcitol group than in either the IV calcitriol (p = 0.009) or the IV paricalcitol + cinacalcet groups (p = 0.04).
At the fourth survey, the only significant difference in percentage of patients at target levels for serum calcium were observed between PO calcitriol and IV paricalcitol + cinacalcet (p = 0.015), with a higher percentage in the PO calcitriol cohort; percentage of patient achieving target levels of bone and mineral parameters at each survey per type of treatment are reported in .
Given that PTH is considered the most sensitive parameter of disease severity, PO calcitriol patients at baseline were observed to have significantly lower PTH level vs all other cohorts (p < 0.0001). Considering the change in PTH levels over time as a measure of treatment effectiveness, IV calcitriol-treated patients did not achieve a significant improvement between the first and the fourth survey. The treatment cohorts that were observed to show similar severity at baseline and effectiveness in reducing PTH level were the IV paricalcitol and IV paricalcitol + cinacalcet cohort; the choice among the two treatment options (IV paricalcitol and IV paricalcitol + cinacalcet therapy) appeared to be related to the higher serum calcium and phosphorus levels in the IV paricalcitol + cinacalcet cohort.
Patients treated with IV paricalcitol + cinacalcet did not show a significant improvement in levels of serum PTH from the first to the fourth survey, despite the fact that the absolute values of improvement in PTH level of the IV paricalcitol and IV paricalcitol + cinacalcet cohorts were very similar. This lack of significance may be due to the small number of patients included in the IV paricalcitol + cinacalcet therapy cohort (n = 20), in fact, the percentage of reduction in PTH level from the first to the fourth survey were comparable: 28.4% in the IV paricalcitol cohort and 25.5% in the IV paricalcitol + cinacalcet cohort. Consequently, the IV paricalcitol and IV paricalcitol + cinacalcet cohorts were considered comparable for baseline severity and effectiveness in terms of suppression of serum PTH levels.
Sensitivity analysis
Sensitivity analysis was performed to check the robustness of data.
Dosage information and costs in the identified sub-groups (patients present at all surveys and remained on the same treatment over the course of the study) were compared with data from the total population of the FARO study. The entire cohort of FARO patients were grouped according to type of treatment for SHPT considering that patients received a specific treatment in at least one survey (not necessarily remaining on that same therapy for all surveys). No significant differences in terms of dosage/weekly cost of treatment were observed ().
Table 5. Total population FARO project: average dosages for patients with a determined treatment at least in one survey.
In order to examine potential changes in terms of patterns of treatment (dosage) for patients administered IV paricalcitol + cinacalcet, data were compared with data from patients beginning the treatment with IV paricalcitol + cinacalcet at the second or third survey and remained with the same treatment for the remaining surveys. No differences that may affect cost comparison were reported between the study population and patients who began treatment at the second and third survey ().
Table 6. Association group: average dosages in selected patients compared with incident patients at second and third survey.
Since the majority of patients were treated with other therapies, we wanted to estimate the possible impact of these treatments on pharmaceutical costs. In order to test this, we evaluated the concomitant use of the most frequently used calcium phosphate binders: sevelamer hydrochloride and calcium-based phosphate binders. For these treatments the following items were estimated: percentage of patients treated, dosages, and related impact on costs. The findings from this analysis are reported in . The total cost of treatment (treatment of SHPT, use of sevelamer hydrochloride, and calcium based phosphate binders) remained substantially stable from the first to the fourth survey in the IV paricalcitol + cinacalcet and IV calcitriol cohorts, increased by ∼10% in the PO calcitriol cohort and decreased by ∼11% in the IV paricalcitol cohort (differences not statistically significant in all groups).
Table 7. Cost analysis (for calcium based P binders and Sevelamer) in patients treated with Paricalcitol alone or in combination with Cinacalcet.
Discussion
The main aim of this study was to evaluate resource consumption and costs associated with different SHPT treatments.
The most important finding of this study is that different baseline severity in terms of PTH level was associated with different treatments for SHPT, and higher costs with increasing severity.
PO calcitriol, while a low cost option, was only effective in suppressing PTH level in less severe patients. However, IV paricalcitol, the moderate cost option, and IV paricalcitol + cinacalcet, the high cost option, were effective in the more severe patients. IV Calcitriol was a low cost option, with no evidence of efficacy in suppressing PTH level during the observational period in the analyzed patients.
PO calcitriol patients remained controlled throughout the study period and had a significantly lower level of PTH at baseline compared to other treatment cohorts (p < 0.0001). In contrast, patients treated with IV calcitriol did not achieve a significant improvement, in terms of serum PTH levels reduction. Moreover, the percentage of patients achieving target levels for PTH in this group decreased from the first to the fourth survey. It could be argued that the treatment with IV calcitriol was not followed by a significant PTH control, possibly due to the small sample size and inherent variability within this group of patients.
In terms of SHPT treatment costs, only the IV paricalcitol cohort showed a significant reduction in average dosages of drug and treatment costs over time (p = 0.012 and p = 0.0124, respectively); PO calcitriol, IV calcitriol, and IV paricalcitol + cinacalcet cohort costs did not decrease significantly from the first to the fourth survey. Dose reduction in the IV paricalcitol cohort is consistent with previous publicationsCitation13.
The weekly cost of the IV paricalcitol cohort was at least 50% less than the cost for the IV paricalcitol + cinacalcet therapy cohort for all surveys examined. When sevelamer hydrochloride and calcium-based phosphate binders costs are added, the weekly cost of the IV paricalcitol cohort remained significantly lower than the IV paricalcitol + cinacalcet therapy cohort costs (p < 0.001), without any significant difference in severity at baseline and effectiveness in PTH suppression over the observational period.
Sensitivity analysis comparing data of the selected cohorts with the total population of FARO study, including patients beginning treatment at the second and third survey, confirmed both clinical and economic trends. Even though patients were receiving other treatments that could potentially impact the total cost (calcium-based phosphate binders and sevelamer hydrochloride), the IV paricalcitol cohort had a lower weekly cost than the IV paricalcitol + cinacalcet therapy cohort.
Only patients in the IV paricalcitol cohort had a significant increase in the percentage of patients achieving target levels for PTH between the first and the fourth survey (p = 0.008). However, the percentage of patients reaching target levels for calcium and phosphorus did not attain statistical significance in any cohort. Furthermore, trends towards the improvement of the percentage of patients achieving target levels of serum calcium in the IV paricalcitol + cinacalcet therapy cohort and achieving target levels of phosphorus in IV calcitriol and IV paricalcitol groups were observed, although these data did not reach statistical significance.
Limitations of this study are related to the structure of the FARO study: not controlled and lack of comparability between cohorts, however reflective of true practice patterns. To address the issue of continuity, cohorts were limited to those patients who remained on the same therapy, and sensitivity analyses were performed. Moreover, a priori, we hypothesized that patients who received the same treatment at all surveys would remain on the same treatment for the entire study period. Considering the modality of data collection (one survey every 6 months collecting clinical/consumption data regarding the current week), data was not available on resource consumption for the period between two different surveys. Finally, data of bone and mineral parameters, average dosage, and pharmaceutical costs were examined for patients that remained on the same treatment at all surveys. While the selection of homogeneous treatment cohorts resulted in a reduction in sample size, it was necessary to conduct the economic evaluation for the different treatment cohorts.
Conclusions
The present cost consequences analysis performed on a sub-population of the FARO survey was conducted to evaluate the bone and mineral consequences and costs of pharmacological treatment of patients affected by SHPT in dialysis. The results of this analysis showed that different treatments were associated with different cost levels, and were likely to be used in patients with different severity disease levels; particularly, the most severe group is associated with a more aggressive and costly treatment regimen. Finally, considering that IV paricalcitol and IV paricalcitol + cinacalcet cohorts showed similar effectiveness in suppressing PTH level without significant differences in baseline severity, IV paricalcitol treatment was associated with lower costs.
Transparency
Declaration of funding
An unrestricted educational grant for this study was provided by Abbott S.r.l, Italy.
Declaration of financial/other relationships
AMC is the Head Medical Affairs at Abbott Italy. ULP is Medical Director at Abbott Italy. DC is Health Economics & Regional Development Manager at Abbott Italy. SM is on the FARO Steering Committee for Abbott Laboratories, and has received lecture fees from Shire and Amgen. MC is on the FARO Steering Committee for Abbott Laboratories, and has received lecture honoraria from Abbott, Shire, Amgen. DB is on the FARO Steering Committee for Abbott Laboratories, and serves as a Consultant for Abbott; he has also received lecture honoraria from GlaxoSmithKline, Amgen, and Shire. GC is on the FARO Steering Committee for Abbott Laboratories, and has received honoraria for lectures from Abbott, Janssen Cilag, and Amgen. PM is on the FARO Steering Committee for Abbott Laboratories, and has received lecture fees from Amgen. DPR has received consultancy fees from Abbot Laboratories. MDL and MM declare no conflict of interest.
CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
References
- United States Renal Data Systems, the concise 2008 annual data report. USRDS 2008 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. http://www.usrds.org/2008/usrds_booklet_08.pdf. Accessed 08 May 2012
- United States Renal Data Systems. USRDS 2008 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. http://www.usrds.org/2008/pdf/V2_11_2008.pdf. Accessed 08 May 2012
- Società Italiana di Nefrologia. Registro Italiano di Dialisi e Trapianto. http://www.sin-ridt.org/sin-ridt.org.htm. Accessed 07 May 2012
- Rombol`a G. Dialysis for everybody? At any cost? J Nephrol 2002;15(6 Suppl):S33-S42
- Slatopolsky E, Delmez JA. Pathogenesis of secondary hyperparathyroidism. Am J Kidney Dis 1994;23:229-36
- Marco MP, Craver L, Betriu A, et al. Higher impact of mineral metabolism on cardiovascular mortality in a European hemodialysis population. Kidney Int Suppl 2003;63:S111-4
- Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, Q6 and cardiovascular disease in hemodialysis patients: the USRDS Waves 1, 3, and 4 Study. J Am Soc Nephrol 2005;16:1788-93
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208-18
- Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2005;67:1179-87
- Block GA, Port FK. Re-evaluation of risks associated with hyperphsphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Disease 2000;35:1226-37
- Mazzaferro S, Brancaccio D, Messa P, et al. Management of secondary hyperparathyroidism in Italy: results of the Italian FARO survey. J Nephrol. 2011 Mar-Apr;24(2):225-35
- Mazzaferro S, Cozzolino M, Marangella M, et al. Calcimimetics, phosphate binders, vitamin D and its analogues for treating secondary hyperparathyroidism in chronic kidney disease: guideline from the Italian Society of Nephrology. G Ital Nefrol 2007 (24 Suppl)37:107-24. Italian. PubMed PMID: 17347960
- Llach F, Yudd M. Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis 2001;38(5 Suppl):S45-50