Abstract
Objective:
This study used simulation to compare the effectiveness of rosuvastatin 20 mg vs atorvastatin 40 mg, and rosuvastatin 40 mg vs atorvastatin 80 mg in preventing MACE in a range of patient populations with varying baseline cardiovascular risk.
Research design and methods:
The Archimedes Model was used to simulate head-to-head clinical trials in nine patient populations: Framingham Risk Score (FRS) ≥ 5%, 5–10%, 10–20%, > 20%, EURO-SCORE ≥ 5% and >10%, diagnosed diabetes, secondary prevention (history of myocardial infarction or stroke, CVD), and acute coronary syndrome (ACS). Simulated patients, aged 45–70 at trial start, were based on the NHANES 1999–2006. Treatments were modeled using results from the STELLAR, JUPITER, CARDS, ASCOT-LLA, and TNT trials. Treatment models were confirmed using trial validations.
Results:
Comparing rosuvastatin 20 mg vs atorvastatin 40 mg, the 5-year numbers needed to treat to prevent one MACE event (NNT) were 525, 70, and 55 for the FRS ≥ 5%, CVD, and ACS groups, respectively. Comparing rosuvastatin 40 mg vs atorvastatin 80 mg the corresponding NNT values were 468, 63, and 51. The 20-year relative risks of MACE in the FRS ≥ 5% population were 0.907 (0.901–0.913) for rosuvastatin 20 mg vs atorvastatin 40 mg and 0.892 (0.884–0.901) for rosuvastatin 40 mg vs atorvastatin 80 mg. The relative risks were similar for the remaining populations.
Conclusions:
This study found that rosuvastatin 20 mg and 40 mg lowers the risk of MACE more than atorvastatin 40 mg and atorvastatin 80 mg. While simulation models cannot replace real-world clinical trials, this study bridges gaps in the evidence, and identifies high risk cohorts that would likely see additional benefit from treatment with rosuvastatin rather than atorvastatin.
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality globally, with an estimated 17 million people dying from CVD each yearCitation1. Elevated serum cholesterol is an important modifiable risk factor in the prevention of CVD, and there is a large body of evidence documenting that lowering cholesterol reduces the risk of major cardiovascular eventsCitation2. Statins (HMG CoA reductase inhibitors) have been demonstrated to both lower cholesterol and prevent major cardiovascular eventsCitation3, and a series of landmark trials (CARDSCitation4, TNTCitation5, PROVE IT-TIMI 22Citation6, and JUPITERCitation7) have established atorvastatin and rosuvastatin as the most effective statins currently available. However, in practice the majority of patients treated with statins fail to reach their LDL goals, likely as a result of conservative initial treatment and low rates of titration at follow-upCitation8,Citation9. To optimally treat patients, healthcare providers should understand the relative benefits of the most effective statin therapies available, and how the benefits differ across patient populations with varying cardiovascular (CV) risk. However, to-date no large, randomized, controlled clinical trial has directly compared the effectiveness of atorvastatin and rosuvastatin in preventing major CV events.
The objective of this study was to use the Archimedes Model to compare the effectiveness of rosuvastatin 20 mg vs atorvastatin 40 mg, and rosuvastatin 40 mg vs atorvastatin 80 mg, in preventing long-term cardiovascular events in a broad population representative of patients eligible for statin therapy in the US. This study also examined the benefit of these treatments to a series of patient populations stratified by baseline CV risk, and patients with Acute Coronary Syndrome (ACS).
Methods
The Archimedes model
The Archimedes Model is a trial-validated, clinically detailed simulation model of human physiology, disease progression, and healthcare deliveryCitation10,Citation11. The core of the Model is a set of algebraic and differential equations representing the physiological pathways pertinent to diseases and their complications. The Model currently includes coronary artery disease, diabetes, and its complications, congestive heart failure, stroke, hypertension, colorectal, lung, and breast cancers, and more, in a single integrated model. Use of a single model enables Archimedes to compare a wide range of treatments, guidelines, and performance measures within an integrated system, and to address co-morbidities, syndromes that span multiple organ systems, drugs that have multiple effects, and combinations of treatments. The use of continuous equations preserves the continuous nature of biological variables and the interactions between them, as well as the continuous nature of chronic disease. Diseases and clinical outcomes are defined in terms of the underlying variables, enabling diseases to occur and progress in the same continuous fashion as in reality, without the need for artificial ‘states’ or annual time-steps. Interventions, both to prevent diseases and to manage them when they occur, are modeled at the level of the underlying biology.
The Archimedes Model is well suited to simulating clinical trials. First a study cohort of virtual patients meeting the trial inclusion/exclusion criteria is created by generating simulated individuals with individual characteristics observed in real world patients (in the present case, patients observed in the NHANES 1999–2006 surveyCitation12). Using person-specific data from real people, the Archimedes Model creates simulated populations of virtual individuals with distributions and correlations of risk factors, behaviors, medication usage, and medical histories reflective of real populations. Each patient’s virtual life is then simulated for the trial period, and each person has a simulated physiology that can begin to function abnormally (due to the onset of a disease), triggering signs and symptoms (such as angina in the case of coronary heart disease (CHD)). The virtual patients then may seek care in virtual outpatient clinics or virtual emergency rooms. The patients who receive care will receive virtual diagnoses and be treated with virtual treatments.
The accuracy of the Archimedes Model is checked using a variety of techniques, most importantly the simulation of clinical trials, comparing the Model’s results with the results observed in the trials. More than 50 major clinical trials were used to validate the Model, including several landmark statin trials (HPSCitation13, 4SCitation14, IDEALCitation15, CARDSCitation4, TNTCitation5, and others) that are directly relevant to this project. More details on the Model can be found on the Archimedes website (www.archimedesmodel.com), and in prior Archimedes publicationsCitation11,Citation16.
Simulated study populations
The aim of this study was to compare the effectiveness of rosuvastatin to atorvastatin over a range of patient cohorts eligible for statin therapy. In all trials, populations of simulated patients were created based on actual individuals randomly drawn from the NHANES 1999–2006 databaseCitation12. The first set of simulated trials considered a relatively diverse population of statin therapy candidates with age 45–70 and Framingham 10-year CHD risk score (FRS) of 5% or greater. The benefit of treatment was tracked for the full cohort, and for a series of embedded sub-groups within the cohort. Specifically, patient sub-populations were defined as follows: FRS stratifications of 5–10%, 10–20%, >20%; EURO-SCORE stratifications of ≥5% and >10%; patients with diagnosed diabetes; and secondary prevention patients with a prior diagnosis of myocardial infarction (MI) or stroke at baseline.
The second set of simulated trials enrolled an Acute Coronary Syndrome (ACS) population. Simulated patients were eligible for the ACS trial if they were aged 45–70, and hospitalized for acute MI or high-risk unstable angina in the preceding 10 days. Trial exclusion criteria were percutaneous coronary intervention within 6 months, other than for treatment of the index event, or coronary-artery bypass surgery within the previous 2 months, or in response to the index event. The ACS population analyses were performed as separate sets of simulations because ACS is a rare condition with a short duration, and consequently patients with ACS were not sufficiently represented in the general FRS ≥ 5% population.
Simulation design
The simulations were structured as multi-armed clinical trials comparing the effectiveness of rosuvastatin to atorvastatin. Treatment in all trial arms began with a washout period, where all lipid lowering therapies prescribed prior to trial enrollment were discontinued. Following the washout, the simulated patients were run through a series of trial arms in which they were treated with one of the target therapies. All subjects were considered eligible for the trial treatments and began receiving them at trial start. There were arms for rosuvastatin 20 mg, rosuvastatin 40 mg, atorvastatin 40 mg, and atorvastatin 80 mg. The same simulated individuals were run through atorvastatin and rosuvastatin arms to improve comparability and isolate the treatment effects. In all cases, patients took the target therapy at 100% compliance. Blood pressure and glucose were managed according to the JNC-7Citation17 and ADACitation18 guidelines, respectively. Compliance to these guidelines was set to match levels of medication usage observed in NHANES.
Modeling statin treatments
The statin treatment models were based on published trials. A number of studies have found that the reduction in CV risk associated with statins primarily scales with cholesterol reductions across a wide range of patient populations, and we used this finding in our modelingCitation2,Citation3,Citation19. In the Archimedes Model, statins reduce cardiovascular outcomes by affecting biomarker values (primarily the TC/HDL ratio) and also by providing additional disease-specific benefits referred to as ‘pleiotropic’ effects. These pleiotropic effects are calibrated to match real-world trial results and capture additional benefits of treatments beyond those directly resulting from biomarker changes. The creation of each statin model began with the effects on the lipid panel, taken from published trials. Then, pleiotropic effects on CV outcomes were added to the statin models, so that the total effects of the modeled statins on CV outcomes were consistent with the published trial data. Key statin treatment models were confirmed with dependent trial validations. In these dependent validations, a simulated population with baseline characteristics matched to the published trial was drawn from NHANES and run through a simulated trial approximating the real-world study. The simulated reduction in CV outcomes associated with the modeled treatments was compared to the published results to confirm the benefits of the statin models.
The STELLARCitation20,Citation21 trial was chosen as the primary data source for the biomarker effects because it directly compared the effects of atorvastatin and rosuvastatin. The model of rosuvastatin 20 mg was based on the lipid effects reported from the STELLAR trial and calibrated (via the addition of plieotropic effects) to match the CV outcomes results from the JUPITER trial. Similarly, the model of atorvastatin 80 mg was based on the lipid effects reported from the STELLAR trial and calibrated to match the CV outcome results from the TNT trial. To support the analysis, a model of atorvastatin 10 mg, based on the ASCOT-LLACitation22 and CARDSCitation4 trials, was also used. Regressions were used to estimate the benefit of rosuvastatin 40 mg and atorvastatin 40 mg, because no suitable CV outcomes trials have been published to-date. Again, the effects of rosuvastatin 40 mg and atorvastatin 40 mg on the lipid panel were based on the STELLAR trial. The MI and stroke risk reductions of atorvastatin 40 mg were estimated from interpolations between the atorvastatin 10 and 80 mg models in terms of relative risk (RR) vs TC/HDL change, justified by the fact that the benefits of statins scale primarily with cholesterol reduction across a wide range of patient populationsCitation2,Citation3,Citation19. Similarly, the MI risk reduction of rosuvastatin 40 mg was estimated from a linear regression of MI RR vs TC/HDL change over the series of treatment models: atorvastatin 10 mg, atorvastatin 80 mg, and rosuvastatin 20 mg. The reduction in stroke risk was observed to be incongruent between the rosuvastatin and atorvastatin families, so the stroke benefit of rosuvastatin 40 mg was extrapolated starting from the rosuvastatin 20 mg model, and using the stroke RR vs TC/HDL dose response slope observed for atorvastatin 10 mg and 80 mg.
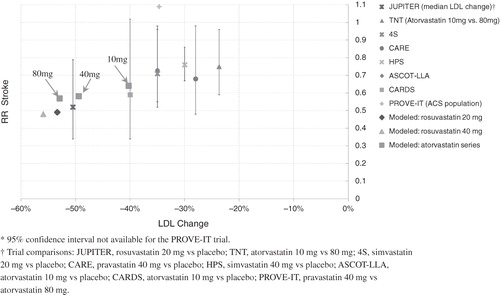
The models of rosuvastatin 20 mg and atorvastatin 40 and 80 mg were confirmed by running a preliminary simulation of the FRS ≥ 5% population and comparing the resulting risk reduction in MI and stroke with the findings of major real-world statin trials. This comparison used the relative risk vs relative change in LDL (as opposed to TC/HDL ratio) because the majority of statin trials have focused on LDL changes and similar comparisons have been previously publishedCitation3,Citation19. The results, shown in and , showed a consistent trend among the major real-world statin trials, and the effects of our models of atorvastatin 10, 40, and 80 mg, as well as rosuvastatin 20 and 40 mg.
Figure 1. A comparison of the MI risk benefit of the statin models with real-world clinical trial results plotted against percent change in LDL. The models of atorvastatin 10 mg, 40 mg, and 80 mg are labeled A 10 mg, A 40 mg, and A 80 mg, respectively.
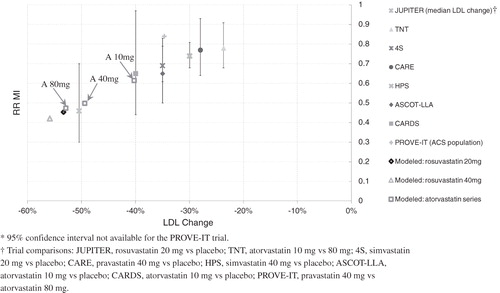
Figure 2. A comparison of the stroke risk benefit of statin models with real-world trial results plotted against percent change in LDL.
Dependent trial validations were also performed to confirm that the rosuvastatin 20 mg model (JUPITER trial), the atorvastatin 10 mg model (CARDS trial), and the atorvastatin 80 mg model (TNT trial) reproduced the benefits observed in the real-world clinical trials. For each trial validation, a virtual population was created that reflected the real-world study population in terms of demographic characteristics and risk factors, and then a simulation was run paralleling the conditions of the trial. In each validation, the hazard ratio reported from the trial was compared to the simulated RR of the specified outcome, as shown in .
Table 1. Results from the trial validations used to confirm the treatment models. The hazard ratio from the trial is compared to the relative risk from the simulation.
Outcomes reported
The primary outcome of this study was the incidence of first major adverse cardiovascular event (MACE), defined as the first occurrence of fatal or non-fatal MI, fatal or non-fatal stroke, or cardiovascular death. The Kaplan-Meier cumulative incidence of MACE for each trial arm was tracked, and the relative risk of MACE was used to compare the relative effectiveness of the treatments. The 95% confidence intervals corresponding to the RR estimates were computed as ±1.96-times the standard error of relative risk (SE(RR)). The SE(RR) was estimated considering the covariance between trial armsCitation23. The Model uses the same individuals in all arms of a given clinical trial, and so there is generally more correlation between events in the arms of an Archimedes simulation than in a real world clinical trial. The number of patients who need to be treated for 5 years to prevent one additional event (number needed to treat; NNT) results were derived with Kaplan-Meier survival estimates using the method of Altman and AndersonCitation24. The 5-year NNT was chosen because it is a time-point commonly reported in real-world clinical trials. In all results reported, the NNT reflects the benefit of treatment with rosuvastatin relative to atorvastatin.
Results
Baseline characteristics
The number of simulated patients in each of the trial populations was determined considering limits in computational resources and estimates of the sample size required to detect the difference in MACE incidence at 5-years with a power of 0.9. The FRS ≥ 5% population consisted of 55,000 simulated individuals. The characteristics for the full population, and the embedded sub-populations defined in terms of baseline disease history and risk score stratifications, are provided in . The baseline characteristics of the ACS population, comprising 16,492 simulated individuals, are also reported in .
Table 2. Baseline characteristics of the FRS ≥ 5% population, embedded sub-populations defined by baseline disease history and risk score stratifications, and ACS population. Values are means (standard deviation) unless indicated as percentages. For the ACS population, disease history does not include the qualifying index event.
Effects of treatments on lipids
Rosuvastatin and atorvastatin both produced substantial improvements in the mean lipid levels of the FRS ≥ 5% and ACS populations. In the FRS ≥ 5% population at 1-year, the reduction in TC/HDL ratio was 43% for rosuvastatin 20 mg, 45% for rosuvastatin 40 mg, 38% for atorvastatin 40 mg, and 41% for atorvastatin 80 mg, as shown in . In the FRS ≥ 5% population at 1-year, the reduction in LDL was 53% for rosuvastatin 20 mg, 56% for rosuvastatin 40 mg, 49% for atorvastatin 40 mg, and 53% for atorvastatin 80 mg. The efficacy trends were similar across the embedded sub-groups of the FRS ≥ 5% population, and in the ACS population. As expected, the mean lipid effects were consistent with the STELLAR trial, which was the primary data source for the modeled lipid effects, as shown in .
Table 3. Percentage reduction in mean TC/HDL ratio and LDL for the full FRS ≥ 5% population at year 1, based on the simulation compared to the STELLAR trial. Larger values indicate greater efficacy.
End points
At 5 years, the Kaplan-Meier cumulative incidence of MACE for the full FRS ≥ 5% population was 3.26% and 3.63% for the rosuvastatin 20 mg and atorvastatin 40 mg trial arms, respectively. The absolute difference in incidence increased with time, as shown in . At 20 years the cumulative incidence of MACE was 17.22% and 18.97% in the rosuvastatin 20 mg and atorvastatin 40 mg arms, respectively.
Figure 3. The Kaplan-Meier cumulative incidence of MACE in the FRS ≥ 5% population. The solid and dashed lines correspond to the rosuvastatin 20 mg and 40 mg arms, respectively. The open squares and solid circles correspond to the atorvastatin 40 mg and 80 arms, respectively. MACE was defined as the first occurrence of MI, stroke, or cardiovascular death.
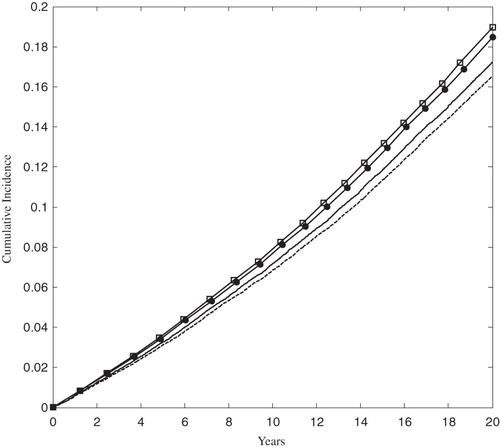
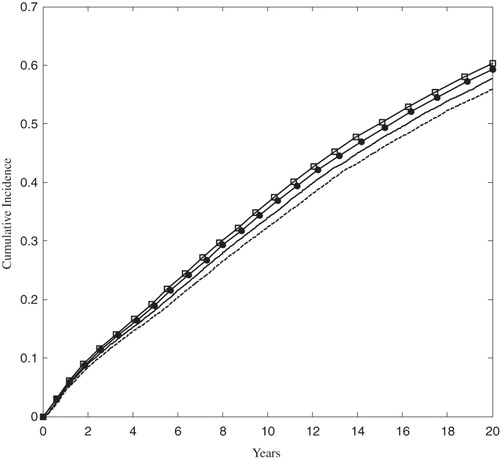
In the rosuvastatin 40 mg and atorvastatin 80 mg arms of the FRS ≥ 5% population, the 5-year cumulative incidence of MACE was 3.12% and 3.50%, respectively. At 20 years the cumulative incidence grew to 16.51% and 18.48% in the rosuvastatin 40 mg and atorvastatin 80 mg arms, respectively. The rate of MACE was sharply higher in the ACS population than in the FRS ≥ 5% population, as shown in , although the relative ranking of treatments remained the same.
Figure 4. The Kaplan-Meier cumulative incidence of MACE in the ACS population. The solid and dashed lines correspond to the rosuvastatin 20 mg and 40 mg arms, respectively. The open squares and solid circles correspond to the atorvastatin 40 mg and 80 arms, respectively. MACE was defined as the first occurrence of MI, stroke, or cardiovascular death.
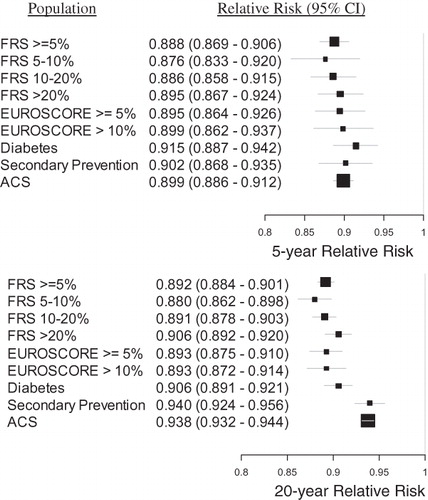
The relative risk of MACE comparing treatment with rosuvastatin 20 mg vs atorvastatin 40 mg for the full FRS ≥ 5% population was 0.897 (0.884–0.911) and 0.907 (0.901–0.913) at 5 and 20 years, respectively. The RR was roughly equivalent across the embedded sub-groups and the ACS population, with a slight decrease in benefit (RR closer to 1) for the higher risk groups, as shown in . The relative risk results were also consistent through the trial duration, with the 5-year results indicating slightly more benefit than the 20-year figures, evident in and . The relative risk of MACE comparing treatment with rosuvastatin 40 mg vs atorvastatin 80 mg for the full FRS ≥ 5% population was 0.888 (0.869–0.906) and 0.892 (0.884–0.901) at 5 and 20 years, respectively.
Figure 5. The relative risk of MACE provided treatment with rosuvastatin 20 mg vs atorvastatin 40 mg, for each population, at 5 and 20 years. The size of each black square is approximately proportionate to the number of patients who had a MACE event in the sub-group; the horizontal lines indicate 95% confidence intervals. A number less than 1 is favorable to rosuvastatin 20 mg.
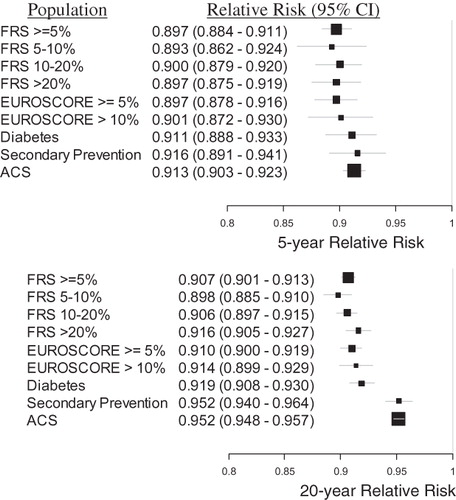
Figure 6. The relative risk of MACE provided treatment with rosuvastatin 40 mg vs atorvastatin 80 mg, for each population, at 5 and 20 years. The size of each black square is approximately proportionate to the number of patients who had a MACE event in the sub-group; the horizontal lines indicate 95% confidence intervals. A number less than 1 is favorable to rosuvastatin 40 mg.
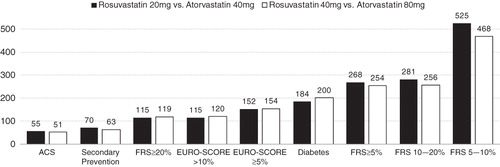
At 5-years, the number needed to treat to prevent one MACE event by treatment with rosuvastatin 20 mg rather than atorvastatin 40 mg was lower among higher risk sub-groups, as shown in . The NNT values indicate the benefit of treatment with rosuvastatin relative to atorvastatin, so smaller positive values indicate greater benefit due to treatment with rosuvastatin. The NNT was lowest in the ACS population, with values of 55 and 51 for treatment with rosuvastatin 20 mg vs atorvastatin 40 mg, and rosuvastatin 40 mg vs atorvastatin 80 mg, respectively.
Sensitivity analysis
It is important to understand the extent to which the findings of this study are sensitive to the assumptions used to model the study treatments. The impact of the study population on the findings can be quantified by examining the sub-group analyses performed in this study. The sub-group analyses explore how the effects of the modeled statins vary across a broad range of patient populations, stratified by baseline CV risk factors. A key assumption in this study was that the effects of statins primarily scale with cholesterol reductions for most patient populations, as found by a number of recent studies. The trend of efficacy vs LDL reduction across our modeled statins, shown in and , is consistent with the results from landmark statin trials. Further, the relative risk associated with treatment with rosuvastatin vs atorvastatin, shown in and , is consistent across the sub-groups examined in this study. Therefore, the variation of the modeled statin effects across a wide range of simulated patient populations stratified by baseline CV risk is consistent with the available real-world evidence.
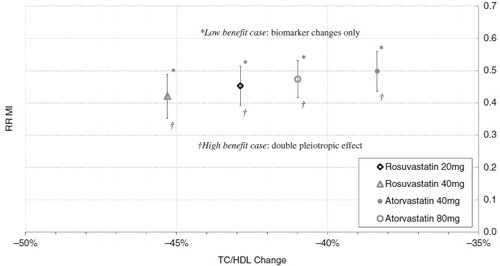
Statin treatments within the Archimedes Model reduce CV outcomes by improving simulated patients’ TC/HDL ratio, and by providing additional pleiotropic benefits calibrated to real-world clinical trial results. Confidence in the benefit provided through biomarker changes that directly enter the Archimedes risk equations (which have been extensively validated) is generally higher than that in benefit provided via pleiotropic effects. This is especially true in the present study since the STELLAR trial reported biomarker changes for each of the statin doses considered. To illustrate the sensitivity of this study’s findings to the assumptions about pleiotropic effects, we explored two additional cases, varying the CHD-specific pleiotropic benefit of each statin while holding the biomarker effects constant:
Low benefit case: the CHD benefit of the treatments due to the biomarker changes only, assuming no pleiotropic effects.
High benefit case: the reduction in CHD events when the ‘pleiotropic’ benefit associated with each treatment is increased by a factor of two.
Together, the point estimates and these low and high benefit cases illustrate the sensitivity of the comparative effectiveness conclusions to the modeling assumptions used, as shown in . In the low benefit case, rosuvastatin 20 mg reduces MI more than atorvastatin 40 mg. The STELLAR trial found that rosuvastatin 20 mg provided a significantly greater reduction in TC/HDL than atorvastatin 40 mg (p-value < 0.002Citation21), and this larger TC/HDL reduction translates directly to a greater reduction in MI risk as predicted by the Archimedes Model. Similarly, rosuvastatin 40 mg reduces MI more than atorvastatin 80 mg when only biomarker changes are considered. The comparative effectiveness conclusions are also unchanged in the high benefit case, suggesting that the comparisons are reasonably insensitive to the assumptions made about pleiotropic effects. Although not derived explicitly, the general level of sensitivity demonstrated for MI should be applicable to the remaining health outcomes considered in this study.
Discussion
This study compared the effectiveness of some of the most potent statin doses currently available, across a range of populations with varying CV risk. Despite the widespread availability of these treatments, a large body of clinical evidence demonstrating the safety and efficacy of statins, and national guidelines recommending statinsCitation25, many patients remain under-treated and fail to reach their lipid goalsCitation8,Citation9. Modifying treatment patterns to increase the proportion of patients who meet their LDL goals would likely yield substantial improvements in cardiovascular health. Recognizing both the pattern of under-treatment and the safety of statins across approved doses, the strategy of initiating patients on higher starting doses (chosen according to individual patient lipid levels, goal, and CV risk profile) appears a promising alternative to the more widely practiced ‘start-low-and-titrate’ approachCitation26.
This simulated study found that rosuvastatin (20 and 40 mg) provided a greater benefit than atorvastatin (40 and 80 mg) in all comparisons and populations considered. The relative benefit differences between rosuvastatin and atorvastatin varied modestly across all populations considered. For example, the 5-year relative risk of MACE given treatment with rosuvastatin 20 mg vs atorvastatin 40 mg varied only from 0.893 (0.862–0.924) for the FRS 5–10% population (which had the lowest rates of MACE) to 0.916 (0.903–0.923) in the Secondary Prevention population (which had one of the highest rates of MACE).
The cardiovascular benefit of rosuvastatin relative to atorvastatin was also quantified in absolute terms. For the lower risk populations the absolute difference in the benefit between rosuvastatin and atorvastatin was modest, as evidenced by NNT values in the FRS ≥ 5% population of 525 and 468 comparing rosuvastatin 20 mg to atorvastatin 40 mg, and rosuvastatin 40 mg to atorvastatin 80 mg, respectively. However, the higher risk groups (such as ACS, Secondary Prevention, FRS ≥ 20%, and EURO-SCORE > 10%) saw a substantially greater absolute benefit from treatment with rosuvastatin, most likely because of their higher MACE rates. The 5-year NNT comparing rosuvastatin 20 mg to atorvastatin 40 mg was 55 and 70 for the ACS and secondary prevention populations, respectively. Further, the 5-year NNT comparing rosuvastatin 40 mg to atorvastatin 80 mg was 51 and 63 for the ACS and Secondary Prevention groups, respectively. It should be noted that these NNT figures quantify the difference between two highly effective treatments, rather than the more common case of a treatment compared with placebo. Also, these NNT values correspond to the case of 100% compliance to both atorvastatin and rosuvastatin, and, under the more realistic scenario of imperfect compliance, the NNT values would be higher.
Healthcare providers seek to maximize patient health by efficiently allocating finite healthcare resources. The number needed to treat is a metric of absolute benefit that can help healthcare providers identify treatment groups that should receive the most aggressive available treatments. This study found that in low-to-moderate risk patient groups, the additional benefit of rosuvastatin relative to atorvastatin is likely not large enough—in absolute terms—to justify additional resources. However, high risk patient groups, like ACS and secondary prevention, did see substantial additional benefit, which suggests that these patient groups should be considered for treatment with rosuvastatin.
This study is based on a mathematical model and is subject to all the assumptions used to create it. Simulation modeling will never be a replacement for real-world clinical trials, but it can provide valuable insight in areas where clinical trial evidence is unavailable or where performing a trial would not be feasible. This study used the Archimedes Model to perform head-to-head comparisons of statin therapies for which there are currently no published results from real-world CV outcomes trials. However, the approach is limited by its dependence on available evidence and the inherent uncertainty in that evidence. This analysis used three outcomes trials (CARDS, ASCOT, and TNT) to model atorvastatin, but JUPITER is currently the only suitable CV outcomes trial of rosuvastatin. Consequently, this study has a particularly strong dependence on the JUPITER trial findings. The treatments examined in this study were modeled using results from published trials, yet these results generally had large confidence intervals, as shown in and . For example, the JUPITER trial reported a 95% confidence interval for the MI hazard ratio of 0.3–0.7. These uncertainties in the trial findings propagate through our analysis, and result in some uncertainty in the relationship between the treatments compared here. Further, due to the lack of suitable CV outcomes trials, this study modeled rosuvastatin 40 and atorvastatin 40 by relating biomarker changes and CV benefits associated with treatments already tested in CV outcomes trials (namely, rosuvastatin 20 mg and atorvastatin 10 mg and 80 mg). Thus, this study’s models of rosuvasatin 40 mg and atorvastatin 40 mg include both the uncertainly in published trial results, as well as the uncertainty in the placement of the treatment on a range of benefit defined by the better documented therapies. Recently, SATURN demonstrated a numerically greater reduction in plaque atheroma volume, the primary end-point, in favor of rosuvastatin vs atorvastatin, but did not reach statistical significance (atorvastatin −0.99% [95% CI = −1.19 to −0.63], rosuvastatin −1.22% [95% CI = −1.52 to −0.90], p = 0.17). For a secondary intravascular ultrasound end-point, change from baseline in total atheroma volume, rosuvastatin demonstrated a statistically significant reduction compared with atorvastatin (astorvastatin −4.42 mmCitation3 [95% CI = −5.98 to −3.26], rosuvastatin −6.39 mmCitation3 [95%CI = −7.52 to −5.12], p = 0.01)Citation27. The failure of the SATURN trial to detect a statistically significant difference in the primary end-point between the effects of rosuvastatin 20 mg and atorvastatin 40 mg further emphasizes the current gap in conclusive clinical evidence differentiating the effectiveness of these treatmentsCitation27.
Conclusion
This study used data from published clinical trials and the Archimedes Model to compare the effectiveness of rosuvastatin 20 mg vs atorvastatin 40 mg, and rosuvastatin 40 mg vs atorvastatin 80 mg, across a range of patient populations stratified by baseline CV risk. In all comparisons, rosuvastatin provided a greater benefit than atorvastatin, and the results suggest that high risk patients—such as secondary prevention and ACS populations—should be considered for preferential treatment with rosuvastatin.
Transparency
Declaration of funding
This research was supported by funding from AstraZeneca Pharmaceuticals Ltd.
Declaration of financial/other relationships
CAS, AvH, PA, and BP are employees of Archimedes Inc., who were paid consultants to AstraZeneca in connection with the development of the manuscript. JH and SG are employees of AstraZeneca.
The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Acknowledgment
This study has previously been presented at ISPOR 16th Annual International Meeting, Baltimore, MD, USA, May 21–25, 2011; and ISPOR 14th Annual European Congress, Madrid, Spain, November 5–8, 2011.
References
- World Health Organization. The atlas of heart disease and stroke. 2004. http://www.who.int/cardiovascular_diseases/resources/atlas/en. Accessed January 2012
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227-39
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685-96
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-207
- Willey VJ, Bullano MF, Shoetan NN, et al. Therapy modifications and low-density lipoprotein cholesterol goal attainment rates associated with the initiation of generic simvastatin. Curr Med Res Opin 2010;26:121-8
- Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation 2009;120:28-34
- Eddy DM, Schlessinger L. Validation of the Archimedes diabetes model. Diabetes Care 2003;26:3102-10
- Eddy DM, Schlessinger L. Archimedes: a trial-validated model of diabetes. Diabetes Care 2003;26:3093-101
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data. 1999-2006. http://www.cdc.gov/nchs/nhanes.htm. Accessed January 2012
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005;294:2437-45
- Schlessinger L, Eddy DM. Archimedes: a new model for simulating health care systems–the mathematical formulation. J Biomed Inform 2002;35:37-50
- Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. In: National Institutes of Health N, ed. Bethesda, MD: NIH Publication, US Department of Health and Human Services, 2003
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008;31:173-5
- Robinson JG, Smith B, Maheshwari N, et al. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005;46:1855-62
- Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 2003;92:152-60
- Jones PH, Hunninghake DB, Ferdinand KC, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther 2004;26:1388-99
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149-58
- Casella G, Berger RL. Statistical inference. 2nd ed. Pacific Grove, CA: Thomson Learning, 2002
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492-5
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–97
- Jones PH. Statins: the case for higher, individualized starting doses. Cleve Clin J Med 2005;72:811-6
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078-87