Abstract
Objective:
To compare pharmacotherapy adherence, persistence, and healthcare utilization/costs among US patients with chronic hepatitis B (CHB) initiated on an oral antiviral monotherapy recommended as first-line treatment by current national (US) guidelines vs an oral antiviral not recommended as first-line monotherapy.
Research design and methods:
In this retrospective cohort study, patients aged 18–64 with medical claims for CHB who initiated an oral antiviral monotherapy for CHB between 07/01/05 and 01/31/10 were identified from a large US commercial health insurance claims database. Patients were continuously enrolled for a 6-month baseline period and ≥ 90 days follow-up. They were assigned to ‘currently recommended first-line therapy’ (RT: entecavir or tenofovir) or ‘not currently recommended first-line therapy’ (NRT: lamivudine, telbivudine, or adefovir) cohorts.
Main outcome measures:
Multivariate analyses were conducted to compare treatment adherence, persistence, healthcare utilization, and costs for RT vs NRT cohorts.
Results:
Baseline characteristics were similar between RT (n = 825) and NRT (n = 916) cohorts. In multivariate analyses, RT patients were twice as likely as NRT patients to be adherent (OR = 2.09; p < 0.01) and persistent (mean: RT = 361 days, NRT = 298 days; p < 0.01) and half as likely to have an inpatient stay (OR = 0.527; p < 0.01). Between the two oral antivirals recommended as first-line treatment, even though pharmacy cost was higher for entecavir, mean total healthcare costs for entecavir and tenofovir were similar ($1214 and $1332 per patient per month, respectively). Similar results were also observed with regard to adherence, persistence, and healthcare use for entecavir and tenofovir.
Conclusions:
A limitation associated with analysis of administrative claims data is that coding errors can be mitigated but are typically not fully eradicated by careful study design. Nevertheless, the current findings clearly indicate the benefits of initiating CHB treatment with an oral antiviral monotherapy recommended as first-line treatment by current guidelines.
Introduction
Roughly 400 million persons worldwide (∼ 5% of the global population) are chronically infected with the hepatitis B virus (HBV)Citation1–3, a life-threatening infection that can lead to long-term sequelae such as cirrhosis, liver decompensation, and hepatocellular carcinomaCitation4. An estimated 15–25% of patients with chronic hepatitis B (CHB) die each year due to complications of HBV-related chronic liver diseaseCitation5,Citation6. In the US, ∼ 2 million people are living with CHBCitation7. Of the 200,000 people who contract hepatitis B each year in the US, roughly 5–10% go on to develop a chronic form of the diseaseCitation8.
Five oral antiviral medications (nucleoside analogues) have been approved by the US Food and Drug Administration (FDA) for the management of CHB: lamivudine (100 mg/day for 48–52 weeks), adefovir (10 mg/day for 48 weeks), entecavir (0.5 mg/day for 48 weeks), telbivudine (600 mg/day for 52 weeks), and tenofovir (300 mg/day for 48 weeks)Citation9,Citation10. Antiviral medications are typically administered until specific end-points, such as HBsAg seroconversion and HBV DNA suppression, are achievedCitation11,Citation12. Long-term therapy with antiviral medication is usually required to prevent or minimize adverse outcomes associated with HBV, including progression of the disease to cirrhosis, end-stage liver disease, or hepatocellular carcinomaCitation11.
Current treatment guidelines issued by the American Association for the Study of Liver Diseases (AASLD) in December 2009 recommend that monotherapy with either entecavir or tenofovir should be administered for first-line oral antiviral treatment of CHBCitation10. All other FDA-approved oral antiviral medications are not guideline-recommended for first-line treatmentCitation10. Entecavir and tenofovir have been found to have the greatest antiviral potency and are associated with the lowest rates of resistance compared with the other approved CHB medicationsCitation9–15.
There is a lack of published research investigating costs and outcomes among CHB patients initiated on a first-line oral antiviral treatment regimen. Therefore, the primary objective of this study was to compare pharmacotherapy adherence and persistence and healthcare utilization and costs among patients with CHB who were initiated on an oral antiviral monotherapy recommended by current guidelines vs CHB patients who were initiated on an oral antiviral monotherapy not recommended by the guidelines. The study’s secondary objective was to compare pharmacotherapy adherence, persistence, healthcare utilization, and healthcare costs between the two AASLD guideline-recommended oral antiviral therapies (entecavir and tenofovir).
Patients and methods
Study design
This was a retrospective cohort study of US patients initiating oral antiviral treatment for CHB between July 1, 2005 and January 31, 2010. Medical and pharmacy claims, enrollment information, and outpatient laboratory data for these patients were obtained from the Life Sciences Research Database, a large, geographically diverse US health insurance claims database affiliated with OptumInsight. At the time of this study, ∼13 million individuals with commercial or Medicare Advantage medical and pharmacy benefit coverage, and 8.6 million with medical coverage only, were enrolled in the plan. Data were determined to be statistically de-identified in accordance with established privacy guidelines under the Health Insurance Portability and Accountability ActCitation16; therefore, separate Institutional Review Board approval was not required.
Study periods and sample selection
Patients were identified during the period July 1, 2005 through January 31, 2010 (identification period). The date of the first observed oral antiviral pharmacy claim was considered the index date. Baseline data were obtained during a baseline period that spanned the 6 months prior to the index date, and outcomes were assessed during a variable follow-up period of at least 90 days following the index date. Patients were followed until the earliest of the following events: the end of the study period (April 30, 2010); disenrollment; or pregnancy.
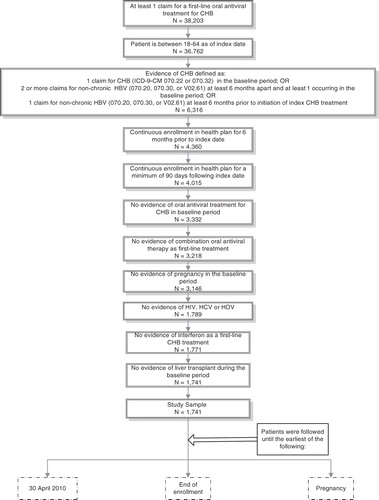
To be eligible for study inclusion, plan members had to be between the ages of 18–64 on the index date and were required to have evidence of CHB as indicated by at least one of the following conditions: (1) at least one claim with a diagnosis code for CHB (ICD-9-CM: 070.22 and 070.32) in the baseline period; (2) two or more claims at least 6 months apart with a diagnosis code for non-chronic hepatitis B (ICD-9-CM: 070.20, 070.30, and V02.61) with at least one of the two services taking place in the baseline period; and (3) one or more diagnosis codes for non-chronic hepatitis B (ICD-9-CM: 070.20, 070.30, and V02.61) at least 6 months prior to the index date. They also had to have at least one pharmacy claim for an oral antiviral agent indicated for treatment of CHB and continuous enrollment with medical and pharmacy benefits during the baseline and follow-up periods. Plan members were also required to have no evidence of an oral treatment for CHB during the baseline period; no evidence of interferon, emtricitabine, or any combination therapy as a first-line CHB treatment; no evidence of pregnancy during the baseline period; no evidence of comorbid human immunodeficiency virus (HIV), hepatitis C virus (HCV), or hepatitis delta virus (based on diagnosis codes or prescriptions for antivirals used to treat HIV or HCV) at any time during the study period; and no evidence of liver transplantation during the baseline period. shows the step-by-step sample selection and attrition process that led to identification of the final study sample.
Cohort assignment
Patients were assigned to one of two cohorts based on whether their first-line oral antiviral monotherapy could be categorized as recommended therapy (RT) (i.e., entecavir or tenofovir) or not recommended therapy (NRT) (i.e., lamivudine, telbivudine, or adefovir) under current AASLD guidelines. For subset analyses, patients in the RT cohort were assigned to the entecavir cohort or the tenofovir cohort, depending on whether they had an index prescription fill for entecavir or tenofovir.
Variable definitions
Adherence to index therapy was measured using a medication possession ratio (MPR), which represents the proportion of time over the course of the study period that the patient theoretically was in possession of medication. MPR was calculated by dividing the number of days on which medication was available by the number of days between the index date and the end of the observation period.
Persistence with index therapy was measured as the number of days between index therapy initiation and the earliest of: discontinuation (defined as a gap of 30 days in pill supply), switching to another medication, or augmentation to the index therapy.
Healthcare resource utilization was calculated as the number of ambulatory (including office and outpatient) visits, emergency room visits, and inpatient stays during the follow-up period, reported as per-patient-per-month (PPPM) due to variable follow-up periods. Total PPPM healthcare costs were computed as the combined health plan- and patient-paid amounts incurred during the follow-up period. Costs were adjusted to 2010 levels using the annual medical care component of the Consumer Price IndexCitation17 and were categorized as total (medical and pharmacy) costs, medical costs, and pharmacy costs; the medical costs were further categorized as ambulatory costs, emergency costs, inpatient costs, and other costs.
Statistical analysis
Descriptive analysis
Bivariate comparisons were made between the RT and NRT cohorts using chi-squared tests for dichotomous variables, t-tests for continuous variables (except in the case of cost measures), and Wilcoxon rank sum tests for cost variables. ‘n’ values and percentages were examined for dichotomous measures; means and standard deviations were examined for continuous measures.
Multivariate analysis
Multivariate analyses controlling for a total of 21 parameters (including age*, gender, location*, year of index antiviral initiation*, baseline counts of four types of healthcare utilization, baseline Quan-Charlson comorbidity score*, decompensated cirrhosis, and three baseline comorbidities, where * is Categorical: defined with dummy variables) were conducted to assess and compare treatment patterns (adherence and persistence), healthcare utilization, and healthcare costs for the RT vs NRT cohorts and the entecavir vs tenofovir cohorts. Covariates included in the multivariate analyses were selected on the basis of statistical significance observed in the descriptive analyses and on clinical rationale. A p-value of 0.05 was considered statistically significant.
Adherence (MPR ≥ 80% vs < 80%) was modeled using logistic regression. Adjusted results are presented as the odds ratio (OR) of the likelihood of MPR ≥ 80% in the RT cohort compared to the NRT cohort, and in the entecavir cohort compared to the tenofovir cohort. Persistence was modeled using ordinary least squares (OLS) regression with an intercept of 298 days for RT vs NRT and 356 days for entecavir vs tenofovir analysis. Adjusted results are presented as the estimated coefficient for the cohort variable, with NRT and entecavir as the reference groups, respectively. Inpatient admissions were modeled using negative binomial regression. Adjusted results are presented as the incidence rate ratio (IRR) comparing inpatient admissions in the RT vs the NRT cohort and the entecavir vs the tenofovir cohort. Total PPPM healthcare costs were modeled using a generalized linear model with a gamma-distribution and a log link. Adjusted results are presented as a cost ratio comparing costs in the RT cohort compared to the NRT cohort and the entecavir compared to the tenofovir cohort. Adjusted costs were calculated for each cohort.
Results
RT vs NRT cohort
A total of 1741 patients [RT cohort (n = 825), NRT cohort (n = 916)] met all of the eligibility requirements for study inclusion. They had a mean [SD] length of follow-up of 612 [441] days and a median of 490 days. Mean and median follow-up were longer for the NRT cohort than for the RT cohort ().
Table 1. Patient demographic and clinical characteristics at index date, by cohort.
Demographic and clinical characteristics were statistically similar between the two groups at index (). In the overall study population, the most common comorbid conditions at index were cardiovascular disease (present in 27.9% of patients), hypertension (22.3%), metabolic syndrome (13.2%), and diabetes (11.6%). Ninety-one patients (5.2%) had decompensated cirrhosis at index. The mean Quan-Charlson comorbidity score was 1.25 in the RT cohort and 1.22 in the NRT cohort.
In descriptive analyses, compared to the NRT cohort, patients in the RT cohort had a higher proportion of patients with MPR ≥ 80% (54% vs 36%; p ≤ 0.001), and a higher mean MPR (0.65 vs 0.52; p < 0.001; ). Mean length of medication persistence was similar for the two cohorts (282 days and 292 days for the RT and NRT cohorts, respectively).
Table 2. Unadjusted follow-up pharmacotherapy adherence and persistence and healthcare utilization.
Unadjusted counts of inpatient admissions, emergency room visits, and ambulatory visits were similar for the two groups (). Both the RT and NRT cohorts had greater use of ambulatory services than inpatient or emergency room services (mean monthly count of ambulatory visits: RT = 1.031; NRT = 1.088; p = 0.310).
As shown in , compared to the NRT cohort, patients in the RT cohort had similar unadjusted mean PPPM total costs ($1164 vs $1279; p = 0.102). The RT cohort had significantly higher unadjusted mean PPPM pharmacy costs ($603 vs $540; p < 0.001), and significantly lower unadjusted mean PPPM ambulatory costs ($181 vs $302; p < 0.001). Unadjusted medical and inpatient costs were slightly higher for the NRT cohort than for the RT cohort, but they were statistically similar.
Table 3. Unadjusted follow-up healthcare costs.
After adjusting for important confounders in the multivariate analyses, patients in the RT cohort had twice the likelihood of MPR ≥ 80% compared to the NRT cohort (OR = 2.09; p < 0.001) and medication persistence was 63 days longer for the RT cohort (adjusted mean persistence: RT = 361 days, NRT = 298 days; p = 0.001) (). Patients in the RT cohort also had a significantly lower incidence of follow-up inpatient admissions (IRR = 0.53; p = 0.005). Total PPPM healthcare costs were similar for the two cohorts (mean adjusted costs: RT = $1214, NRT = $1332; p = 0.156).
Table 4. Summary of multivariate models*.
Entecavir vs tenofovir cohort
This study subsample included 825 CHB patients who met all eligibility requirements: 609 patients in the entecavir cohort and 216 in the tenofovir cohort. The median length of follow-up for this subsample was 400 days.
Demographic and clinical characteristics were statistically similar between the two groups (). Mean age and mean Quan-Charlson comorbidity score were slightly higher for the tenofovir cohort than for the entecavir cohort, but differences were not statistically significant.
In descriptive analyses, compared to the tenofovir cohort, patients in the entecavir cohort had a lower proportion of patients with MPR ≥ 80% (51% vs 61%; p = 0.011), a lower mean MPR (0.63 vs 0.71; p = 0.007), and longer medication persistence (mean length, 317 days vs 184 days; p < 0.001) ().
Entecavir and tenofovir cohorts had similar unadjusted monthly counts of inpatient admissions and emergency room and ambulatory visits. Use of ambulatory services was greater than use of inpatient and emergency room services for both groups (mean monthly count of ambulatory visits: entecavir = 1.025; tenofovir = 1.046; p = 0.820).
Compared to the tenofovir cohort, patients in the entecavir cohort had significantly lower unadjusted mean PPPM total costs ($1135 vs $1246; p = 0.007) and significantly higher pharmacy costs ($625 vs $539; p < 0.001) during the follow-up period (). Unadjusted medical costs, inpatient costs, emergency room costs, and ambulatory costs were higher for the tenofovir cohort than for the entecavir cohort, but none of these differences were statistically significant. Inpatient costs comprised the bulk of the medical costs for both cohorts (RT: mean $308; NRT: mean $405; p = 0.524).
After adjusting for important confounders in the multivariate analyses, patients in the entecavir and tenofovir cohorts had similar likelihood of MPR ≥ 80% (OR = 1.17; p = 0.402); similar medication persistence (adjusted mean persistence: entecavir = 356 days, tenofovir = 303 days; p = 0.085); similar incidence of follow-up inpatient admissions (IRR = 1.12; p = 0.790); and similar total PPPM healthcare costs (mean adjusted costs: entecavir = $1151, tenofovir = $1213; p = 0.560) ().
Discussion
This study is the first to examine the impact on healthcare costs and outcomes associated with adhering to recommended national (US) guidelines for the first-line treatment of CHB. Previously published research has focused on the comparative clinical effectivenessCitation18–23 and/or cost-effectivenessCitation24–28 of oral antiviral agents in the treatment of CHB.
After adjusting for important confounders in multivariate analyses, compared to the NRT cohort, patients in the RT cohort had twice the likelihood of MPR ≥ 80%, medication persistence that was 63 days longer, a significantly lower incidence of follow-up inpatient admissions, and similar total PPPM healthcare costs. We observed no significant differences between the entecavir and tenofovir cohorts with regard to adherence, persistence, healthcare utilization, or total healthcare costs after adjusting for these same confounders. However, in descriptive analyses, entecavir patients were persistent with their medication for 133 days longer than tenofovir patients were. This considerable difference is likely a function of time on the market. Tenofovir was approved for treatment of CHB on August 11, 2008, and entecavir was approved on March 29, 2005. The end date of this study was April 30, 2010. Therefore, the duration of potential follow-up was ∼ 5 years for entecavir patients, whereas the duration of potential follow-up for tenofovir patients was only ∼ 21 months.
The current results showing greater adherence and persistence in the RT cohort are not surprising when one considers the side-effect profile of entecavir and tenofovir compared to other nucleoside analoguesCitation29,Citation30. Adverse effects are a key cause of medication discontinuation or switching, so it is reasonable to speculate that those medications with reportedly lower rates of serious adverse events would be less likely to be discontinued. For example, both Chang et al.Citation29 and Lai et al.Citation30 reported fewer discontinuations due to adverse events for entecavir-treated patients compared to those treated with lamivudine.
In addition, relatively low rates of drug resistance have been reported with the use of guideline-recommended first-line monotherapiesCitation31–34. Lam et al.Citation31 reviewed the efficacy and safety of various antiviral agents and, based on the results of 18 studies, reported that lamivudine was associated with a 39% rate of resistance at 2 years; adefovir, 3%; telbivudine, 10.8–25.1%; entecavir, less than 1%; and tenofovir, 0%. At 3-year follow-up, 60–70% of lamivudine patients were resistant, 20–29% of adefovir patients were resistant, and only 1.2% of entecavir patients had developed resistance. (Three-year follow-up data were not available for telbivudine and tenofovir.)
Since reduced adherence and persistence as well as increased resistance to therapy are associated with poorer clinical outcomes, it is reasonable to speculate that this may have been central to the increased healthcare utilization observed in the NRT cohort. Furthermore, a lower incidence of hepatic flares, cirrhosis, and hepatocellular carcinoma has been reported with the use of entecavir and tenofovir compared with other oral antiviralsCitation30,Citation32. It is possible that the lower incidence of these serious clinical sequelae may have also contributed to the lower likelihood of inpatient visits and the significantly lower (unadjusted) ambulatory and emergency room costs we observed in the RT cohort.
It was recently reported that 85% of worldwide cases of hepatocellular carcinoma are attributable to either HBV or HCV infectionCitation35. This statistic underscores the importance of using antivirals to treat CHB. In a review article, LokCitation36 cited direct evidence from several randomized controlled trials supporting a benefit of antiviral therapy with regard to prevention of hepatocellular carcinoma. He suggested that evidence showing improvements in liver histology with antiviral therapy provide indirect support that antiviral therapy may prevent hepatocellular carcinoma by slowing progression of liver disease and possibly reversing liver damage.
The current study has several important strengths. Administrative data allow for examination of healthcare utilization and expenditure patterns in a real-world setting and offer the advantage of large sample sizes with diverse medical histories. The data sources used for this study are national in scope, contain claims information from some of the largest commercial health plans in the US, and are constructed from a variety of geographic regions and employer groups.
However, limitations inherent to the use of administrative claims databases are well recognized and must be considered when interpreting the results of this study. Claims data are collected for the purpose of payment and not research, and are subject to possible coding errors. This limitation is minimized by the study design and inclusion criteria, which require patients to have multiple claims with a diagnosis for CHB and treatment for CHB. In addition, the data used in this study are limited to patients who were privately insured under participating health plans, and therefore the results might not be generalizable to Medicare/Medicaid enrollees or non-insured individuals. In the subanalysis, entecavir patients outnumbered tenofovir patients; further analyses should be conducted using a larger sample of tenofovir patients to enable more proportionate comparisons. And, finally, certain types of patients may have been channeled toward entecavir and others toward tenofovir due to specific characteristics that were not controlled for in the multivariate analyses.
Conclusions
Initiation of an oral antiviral monotherapy recommended as first-line treatment by current AASLD guidelines was associated with better adherence, longer persistence, and lower likelihood of inpatient admission compared with an oral antiviral monotherapy not recommended as first-line treatment by current guidelines. Despite an increase in medication costs resulting from the use of guideline-recommended therapy, there was no significant difference in total healthcare costs between the RT and NRT cohorts after adjusting for key confounders. These results underscore the benefits of initiating CHB treatment with oral antiviral therapy recommended as first-line treatment by current guidelines.
Between the two specific recommended first-line oral antiviral monotherapies, entecavir and tenofovir were comparable as first-line treatment for chronic hepatitis B in terms of persistence, adherence, and healthcare utilization. Even though pharmacy costs were higher for entecavir, the mean total healthcare costs were similar after adjusting for confounders.
Transparency
Declaration of funding
This study was funded by Bristol-Myers Squibb (BMS). OptumInsight (formerly Innovus) was contracted by BMS to conduct this study.
The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Declaration of financial/other relationships
HT, TH, and TJ are employees of BMS. TH, TJ, and HT are BMS stock shareholders. WJ and SH have received lecture honoraria from Gilead Sciences and BMS. EM received lecture honoraria from Genentech, BMS, Gilead, Merck, and Vertex. BP is employed by OptumInsight.
Acknowledgments
The authors thank Victoria Porter, medical writer at OptumInsight, for her assistance with the preparation of this manuscript.
References
- McClune AC, Tong MJ. Chronic hepatitis B and hepatocellular carcinoma. Clin Liver Dis 2010;14:461–76
- Juday T, Tang H, Harris M, et al. Adherence to chronic hepatitis B treatment guideline recommendations for laboratory monitoring of patients who are not receiving antiviral treatment. J Gen Intern Med 2011;26:239–44
- Papatheodoridis GV, Manolakopoulos S, Archimandritis AJ. Current treatment indications and strategies in chronic hepatitis B virus infection. World J Gastroenterol 2008;14:6902–10
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167–85
- Nettleman M. Hepatitis B. emedicine health. http://www.emedicinehealth.com/hepatitis_b/article_em.htm. Accessed December 13, 2011.
- Maddrey WC. Hepatitis B: an important public health issue. J Med Virol 2000;61:362–66
- Lee H, Park W, Yang JH, et al. Management of hepatitis B virus infection. Gastroenterol Nurs 2010;33:120–6
- World Health Organization. Global alert and response: hepatitis B. 2012. http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/index3.html. Accessed May 29, 2012
- Papatheodoridis GV. Treatment of HBeAg-negative chronic hepatitis B patients with nucleos(t)ide analogues. Liver Int 2011;31:748
- Lok ASF, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–2
- Lin CL, Kao JH. Recent advances in the treatment of chronic hepatitis B. Expert Opin Pharmacother 2011;12:2025–40
- Fuentes Olmo J, Uribarrena Amezaga R. [Current treatment of hepatitis B infection: where do the new nucleos(t)ide analogues fit in?]. Gastroentrol Hepatol 2011;34:492–503
- Colonno R, Rose R, Baldick C, et al. Resistance after two years of entecavir treatment in nucleoside-naïve patients is rare. Hepatology 2006;45:1656–65
- Ristig MB, Crippin J, Abert JA, et al. Tenofovir disoproxil fumarate therapy for chronic hepatitis B in human immunodeficiency virus/hepatitis B virus-coinfected individuals for whom interferon-alpha and lamivudine therapy have failed. J Infect Dis 2002;186:1844–7
- Ono SK, Kato N, Shiratori Y, et al. The polymerase L528M mutation cooperates with nucleoside binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest 2001;107:449–55
- Health Insurance Portability and Accountability Act of 1996. Public law 104-191, 104th Congress, U.S. Department of Health and Human Services. 1996. http://www.cms.hhs.gov/HIPAAGenInfo/Downloads/HIPAALaw.pdf. Accessed December 13, 2011
- US Bureau of Labor Statistics. Consumer Price Index. Chained Consumer Price Index for all urban consumers (C-CPI-U) 1999-2008, Medical Care. Washington, DC: US Department of Labor 2011.
- Shamiliyan TA, Johnson JR, MacDonald R, et al. Systematic review of the literature on comparative effectiveness of antiviral treatments for chronic hepatitis B infection. J Gen Intern Med 2011;26:326–39
- Fung J, Lai CL, Yuen J, et al. Randomized trial of lamivudine versus entecavir in entecavir-treated patients with undetectable hepatitis B virus DNA: outcome at 2 years. Hepatology 2011;53:1148–53
- Lam YF, Yuen MF, Seto WK, et al. Current antiviral therapy of chronic hepatitis B: efficacy and safety. Curr Hepat Rep 2011;10:235–43
- Yoon EL, Yim HJ, Lee YS, et al. Comparison of clevudine and entecavir for treatment-naïve patients with chronic hepatitis B virus infection: two-year follow-up data. J Clin Gastroenterol 2011;45:893–9
- Liaw YF, Raptopoulou-Gigi M, Cheinquer H, et al. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology 2011;54:91–100
- Zhao SS, Tang LH, Dai XH, et al. Comparison of the efficacy of tenofovir and adefovir in the treatment of chronic hepatitis B: a systematic review. Virol J 2011;8:111
- Almeida AM, da Silva AL, Cherchiglia ML, et al. Chronic hepatitis B treatment: the cost-effectiveness of interferon compared to lamivudine. Value Health 2011;14(5 Suppl 1):S24–8
- Wiens A, Venson R, Correr CJ, et al. Cost-effectiveness of telbivudine versus lamivudine for chronic hepatitis B. Braz J Infect Dis 2011;15:225–30
- Spackman DE, Veenstra DL. A cost-effectiveness analysis of currently approved treatments for HBeAg-positive chronic hepatitis B. Pharmacoeconomics 2008;26:937–49
- Arnold E, Yuan Y, Iloeje U, et al. Cost-effectiveness analysis of entecavir versus lamivudine in the first-line treatment of Australian patients with chronic hepatitis B. Appl Health Econ Health Policy 2008;6:231–46
- Chen W, Hou JL. [Pharmacoeconomic evaluation of telbivudine vs. lamivudine in treating the patients with HBeAg-positive and negative chronic hepatitis B.] Zhonghua Gan Zang Bing Za Zhi 2009;17:569–73
- Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001–10
- Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011–20
- Lam YF, Yuen MF, Seto WK, et al. Current antiviral therapy of chronic hepatitis B: efficacy and safety. Curr Hepat Rep 2011;10:235–43
- Papatheodoridis GV. Treatment of HBeAg-negative chronic hepatitis B patients with nucleos(t)ide analogues. Liver Int 2011;31(1 Suppl):95–103
- Zoulim F, Locarnini S. Management of treatment failure in chronic hepatitis B. J Hepatol 2012;56(Suppl):S112–22
- Leung N. Treatment of HbeAg-positive chronic hepatitis B with nucleos(t)ide analogues. Liver Int 2011;31(1 Suppl):85–9
- Hiotis SP, Rahbari NN, Villanueva GA, et al. Hepatitis B vs. hepatitis C infection on viral hepatitis-associated hepatocellular carcinoma. BMC Gastroenterol 2012;12:64
- Lok AS. Does antiviral therapy for hepatitis B and C prevent hepatocellular carcinoma? J Gastroenterol Hepatol 2011;26:221–7
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care 2005;43:1130–9
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40:373–83