Abstract
Objective:
Denosumab has been approved in the US for skeletal-related event (SRE) prevention in bone-metastatic prostate cancer on the basis of a phase III clinical trial in which denosumab reduced SREs relative to zoledronic acid. Overall survival, disease progression, and serious adverse events did not differ significantly between groups. This analysis assessed the cost-effectiveness of denosumab vs zoledronic acid in bone-metastatic prostate cancer from a US payer perspective.
Methods:
A literature-based Markov model, wherein inputs were selected to reproduce clinical trial outcomes, was developed to estimate the survival, quality-adjusted life-years (QALYs), number and costs of SREs, and drug and administration costs for patients receiving denosumab or zoledronic acid over 27 months. QALYs were estimated by assigning health-state utilities. SRE-related costs and utilities were literature-based. Outcomes were discounted 3% per annum, and model robustness was tested via scenario, univariate, and probabilistic sensitivity analyses.
Results:
Denosumab resulted in fewer estimated SREs (−0.241; 1.036 vs 1.277), more QALYs (0.0074; 0.9306 vs 0.9232), and lower SRE-related costs (−$2340; $8824 vs $11,164), but higher drug-related costs ($10,181; $23,144 vs $12,963) and total costs ($7841; $31,968 vs $24,127) vs zoledronic acid. The base case estimated cost per QALY-gained was $1,058,741.
Conclusion:
This analysis was limited by the restricted availability of clinical data and the need to use projection methods beyond the trial time frame. However, a wide range of scenarios predicted denosumab to have an incremental cost/QALY gained above what may be considered acceptable value for money in the US. This raises important questions regarding the pharmacoeconomic value of denosumab in bone-metastatic prostate cancer.
Introduction
Approximately 70% of advanced prostate cancer patients will develop bone metastasesCitation1. Subsequent skeletal-related events (SREs; e.g., radiotherapy or surgery to bone, pathological fracture, and spinal cord compression) are associated with increased treatment costsCitation2, decreased survivalCitation3, and impaired quality-of-life (QoL)Citation4.
On the basis of a phase III clinical trial wherein prostate cancer patients with bone metastases were randomized to receive monthly denosumab or zoledronic acid until first on-study SRE or deathCitation5, denosumab was approved by the US Food and Drug Administration for SRE prevention in patients with bone-metastatic prostate cancer; an indication for which zoledronic acid had been the only approved therapy. In the trial, monthly denosumab (120 mg subcutaneously) demonstrated a primary end-point of statistical non-inferiority to monthly zoledronic acid (4 mg intravenously) for time to first SRE (HR = 0.82, 95% CI = 0.71–0.95; p = 0.0002; p = 0.008 for secondary end-point, superiority). Overall survival, disease progression, and adverse event (AE) rates were similar between treatment arms, with the exception of hypocalcemia (denosumab 121 [13%] vs zoledronic acid 55 [6%]; p < 0.0001)Citation5. Denosumab also offers the convenience of subcutaneous injection and no requirement for routine renal monitoring (except in renally compromised patients).
In the US, the monthly wholesale acquisition cost of denosumab is $1650Citation6 compared to $886Citation7 for zoledronic acid. Thus, despite its obvious clinical benefit, concerns have been expressed regarding denosumab’s cost given its clinical benefitsCitation8–10. The present analysis was therefore conducted to estimate the cost-effectiveness of denosumab compared with zoledronic acid in metastatic prostate cancer patients from a US payer perspective.
Methods
A Markov decision model (), representing monthly progression through eight mutually exclusive health states, was developed to estimate the SRE incidence, survival, quality-adjusted life-years (QALYs), SRE-related costs, and drug-related costs for metastatic prostate cancer patients receiving monthly denosumab 120 mg or zoledronic acid 4 mg for up to 27 months (the maximum published duration of patient follow-up data available at the time of this analysis). In a scenario analysis, the duration of follow-up was extended to 60 months using projection methods.
Figure 1. Model structure. Patients occupied one health state at a time and transited monthly according to transition probabilities. ‘No SRE’ makes no assumption whether a patient has had any SRE before model initiation.
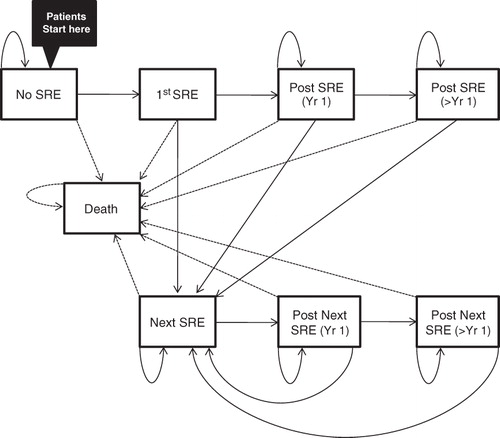
Disease progression and adverse events were not considered, as they did not differ significantly between treatments (with the exception of bypocalcemia)Citation5. It was assumed that including hypocalcemia would have biased the analysis against denosumab because the trial was not powered to detect significant differences for specific adverse events between treatments. Mortality was not assumed to differ between treatments as there was no significant difference reported in the clinical trialCitation5.
Clinical inputs and transition probabilities
Health state transition probabilities were selected to replicate SRE incidence (first and subsequent) and overall survival shown in the Kaplan-Meier curves of the clinical trial (Clinical Trial Number: NCT00321620)Citation5, which displayed data for 27 months (). These curves were approximated using Weibull survival models which allowed for the calculation of absolute SRE/Death risks at any given time point for either treatment armCitation11. This absolute risk, or hazard (r), at any given month was calculated as follows:
where λ and α represent the shape and scale of the curve, respectively. To obtain the transition probability, P, from the hazard for each model month, the following transformation was usedCitation12:
Table 1. Model inputs for this cost-effectiveness analysis.
SRE inputs
Quality-of-life
SREs were expected to negatively impact QoL proportionally to their severity. The calculation of QALYs, a measure of the quality-adjusted duration of life, is the standard approach to quantifying QoL in cost-effectiveness analysisCitation13. QALYs were calculated by multiplying the QoL score (i.e., utility; ranging from 0 = death to 1 = best possible health) assigned to a given health state, by the duration of time spent in that state. Because patients in this analysis had bone-metastatic prostate cancer, their pre-SRE baseline utility was assigned 0.70, equal to the baseline utility value elicited in a previous metastatic prostate cancer patient sampleCitation14.
The decreases in utility for each SRE type (), which vary in severity and level of impairment, were obtained from the same study by Weinfurt et al.Citation14. In this study, a change in utility score (measured in patients who experienced an SRE) reflected the difference between predicted (in absence of SRE) and actual scores reported after the SRE. The changes in utility were −0.07 for radiation to the bone (n = 121, 95% CI: −0.13, −0.02), −0.13 for pathologic fractures (n = 76, 95% CI: −0.20, −0.07), and −0.02 for a combination of events including surgery to the bone, spinal cord compression, and change in antineoplastic therapy to treat bone pain (n = 42, 95% CI: −0.17, +0.13). Since it is likely that spinal cord compression and surgery to the bone would be associated with worse decrease in utility than fractures or radiation to the bone, it was assumed that the disutility for both surgery to the bone and spinal cord compression would be −0.17 (the lowest end of the confidence interval reported by Weinfurt et al.). The use of SRE-related utilities reported by Weinfurt et al. is consistent with a previous cost-effectiveness analysis comparing denosumab vs zoledronic acid in bone-metastatic prostate cancer conducted by an independent assessment group associated with the National Institute of Health and Clinical Excellence (NICE) in the United KingdomCitation15. SRE-related disutility was permanently maintained following an SRE. This assumption was varied in scenario analysis.
Distribution of SREs
The denosumab package insertCitation16 and trial results reported by Fizazi et al.Citation5 list the rates and distribution of first SRE types observed in the trial and the cumulative number of SREs. Neither provided information regarding the type of subsequent SREs. Thus, it was assumed that the distribution of subsequent SRE types was identical to the distribution of first SREs ().
Likewise, no information was reported on the type and severity of pathological fractures, which include vertebral and non-vertebral fractures. A pooled analysis of published bisphosphonate trials in cancer patients reporting the distribution of these fracturesCitation17,Citation18 resulted in an estimate of 34.7% for the proportion of vertebral pathological fractures. The effects of vertebral fractures (via costs and quality-of-life) were varied in scenario analysis.
Treatment persistence
Fizazi et al.Citation5 reported that that the median number of doses administered were 13.0 and 10.5 for the denosumab and zoledronic acid groups, respectively. Because patients received treatment approximately once every month, these data acted as proxy measures of median time on study. It was assumed that discontinuations followed an exponential decay function with the constraint that 50% of patients in the denosumab and zoledronic acid groups remained on treatment at 13.0 and 10.5 months, respectively; an assumption consistent with the recent literatureCitation19. Patients who discontinued were assumed not to be on second line therapy of any kind (and no costing of a second line was included. Given the uncertainty regarding the exact nature of treatment discontinuations, an alternate assumption was used for scenario analysis in which patients continued therapy until death.
Costs
SRE costs () and cost ranges for sensitivity analysis () by Barlev et al.Citation20 reporting on the patterns of healthcare utilization and inpatient and outpatient costs associated with SRE episodes in patients with prostate cancer that has metastasized to bone. Administration and renal monitoring costs were drawn from the Ingenix National Fee AnalyserCitation21.
Table 2. Sensitivity analysis inputs.
Wholesale acquisition costs were used for baseline drug acquisition costs. In sensitivity analysis, average wholesale price (equal to wholesale acquisition cost +20%) was also examined. In scenario analysis, the price of zoledronic acid was assumed to be 50% less than its current price to reflect the availability of a generic version in 2013. All model costs were, when necessary, inflated to 2010 prices using the Bureau of Labor Statistics’ Consumer Price Index (all urban consumers, medical care). Costs and QALYs were discounted at 3% per annum.
Analysis
The primary outcome was the discounted incremental cost per QALY gained (i.e., incremental cost-utility ratio, ICUR). In addition to the base case analysis and the scenario analyses described above, univariate and probabilistic sensitivity analyses (PSA) were conducted to identify variables to which model outcomes were most sensitive. PSA parameters are reported in .
Results
Model parameters were estimated to reproduce outcomes from the clinical trial comparing denosumab vs zoledronic acid in bone-metastatic prostate cancer patientsCitation5. The base case model results () were consistent with these findings: denosumab resulted in a reduced proportion of patients experiencing at least one SRE (−5.85%) and resulted in a lower total number of SREs per patient (−0.241). Because denosumab patients had fewer SREs, they also accrued more modeled QALYs (+0.0074) and incurred fewer modeled SRE-related costs (−$2340). However, these benefits were achieved at the expense of higher drug-related costs (+$10,181). Consequently, the net estimated costs were increased by $7841 in denosumab-treated vs zoledronic acid-treated patients, resulting in an ICUR of $1,058,741/QALY ().
Table 3. Base case model results.
In scenario analysis, denosumab’s ICUR was $1,383,251 and $1,051,499 when average wholesale drug price was used and when patients continued therapy until death, respectively. When the duration of analysis was extended to 60 months, the QALYs gained and SREs avoided were +0.0107 and −0.370, respectively; resulting in an ICUR of $933,424. When the estimated generic price of zoledronic therapy was used (e.g., 50% of the base case cost), the ICUR was $1,780,275 (in the 27 month analysis) and $1,516,411 (in the 60 month analysis). In the base case, SRE-related disutility was assumed to be maintained permanently. When this assumption was changed to a duration of 12 months post-SRE (after which the patient returns to the background utility), denosumab’s ICUR increased to $2,878,162/QALY.
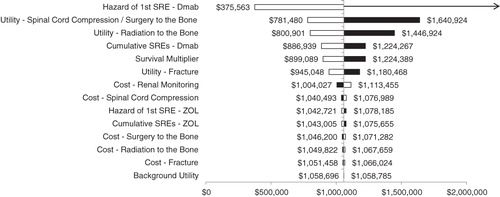
Univariate sensitivity analysis () indicated that results were robust to the hazard of first SRE in the denosumab group, and health state utility values for spinal cord compression/surgery to the bone and bone radiation. With regard to the per-administration prices for drug acquisition, cost neutrality was achieved with a $570/administration reduction in denosumab’s price (i.e., $1650 to $1080) or a $651/administration increase of zoledronic acid’s price (i.e., $886 to $1537).
Figure 2. Univariate sensitivity analysis results. The pivot point in the tornado diagram represents the base case cost per QALY gained with denosumab ($1,058,741). The arrow indicates that denosumab was dominated (i.e., more expensive and produced fewer QALYs) in that scenario. Black and white bars indicate results generated with maximum and minimum values in the sensitivity analysis, respectively. Dmab, denosumab; SRE, skeletal-related event; ZOL, zoledronic acid.
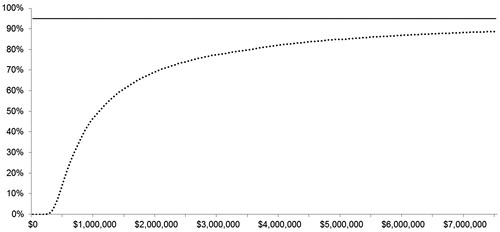
In the PSA, where model parameters were randomly varied 1000-times around pre-specified distributions to generate new model outcomes, the median ICUR was $1,068,037/QALY (95% credible interval: $328,954/QALY-Dominated). None of the PSA simulations had an ICUR less than the $100,000/QALY threshold. That is, this analysis indicates a 0% likelihood that denosumab is cost-effective vs zoledronic acid at that threshold. The cost-effectiveness acceptability curve () indicates that at no point does the probability that denosumab is cost-effective reach 95%, and therefore the upper limit of the 95% up to a threshold of $7.5 million per QALY.
Discussion
This analysis estimated that, relative to zoledronic acid, the use of denosumab for the prevention of SREs in bone-metastatic prostate cancer resulted in more QALYs, fewer SREs and SRE-related costs, and greater overall costs. The resultant base case ICUR was $1,058,741, which exceeds what is traditionally considered good value for money in the US ($50,000–$100,000/QALY). This estimate also exceeds previous ICUR estimates of oncologic supportive care therapies that do not affect survivalCitation22–24, the median ICUR range reported across all non-dominated oncologic therapiesCitation25, and the acceptable threshold for a hypothetical life-extending therapy reported from a survey of oncologists ($300,000/QALY)Citation26. Sensitivity analysis indicated that results were robust to the hazard of first SRE in the denosumab group and the health state utility values associated with spinal cord compression, surgery to the bone, and radiation to the bone.
Cost-effectiveness analyses can be a source of intense debate as they formally assign a value to a therapy. This is particularly true in cases such as the present one, which reports that the cost of a more effective, novel therapy may exceed its benefits. Therefore, great care was taken in the selection of inputs and assumptions for this model so as to provide a defensible estimate of denosumab’s ICUR relative to zoledronic acid. For example, we adopted assumptions that favored denosumab, such as the assumption that all SREs would be sufficiently severe so as to result in substantial additional healthcare costs, even though Hillner et al.Citation27 and other reportsCitation15 have suggested that some SREs may be asymptomatic or have limited or no impact on costs and QALYs. It should also be noted that the protocol of the Fizazi et al.Citation5 clinical trial upon which this analysis is based required that skeletal events be assessed by skeletal surveys every 12 weeks or by radiographic assessments during the course of care. It is possible that this approach resulted in systematic over-identification of asymptomatic or clinically minor events that would not normally be detected and/or treated in routine, non-experimental, clinical practiceCitation15,Citation19,Citation28,Citation29. If true, this would mean that use of the Fizazi et al. data over-estimates the absolute number of clinically symptomatic and/or treatable events prevented by denosumab vs zoledronic acid, thereby over-estimating denosumab’s savings and QALY gains and under-estimating its ICUR.
Four previous pharmacoeconomic evaluations of denosumab vs zoledronic acid in bone-metastatic prostate cancer have been publishedCitation15,Citation30,Citation31. Ford et al.Citation15 reported two UK government payer-perspective cost-effectiveness analyses: an analysis by Amgen-maker of denosumab-submitted to the UKs NICE (subsequently referred to as Amgen Submission) and an analysis by NICE’s independent assessment group (subsequently referred to as NICE AG). Stopeck et al.Citation30 and Lothgren et al.Citation31 reported cost-effectiveness analyses from US managed care and Dutch government payer perspectives, respectively.
All of these analyses found denosumab to be cost-effective: Stopeck et al.Citation30 ($49,405/QALY); Lothgren et al.Citation31 (€42,933/QALY); Amgen submission and NICE models (range of £35,732–£249,575 without discounted denosumab depending on scenarios assumed, but dominant in all scenarios assuming a discounted cost for denosumabCitation15). In contrast, the present analysis estimated denosumab’s cost per QALY to be >$1,000,000.
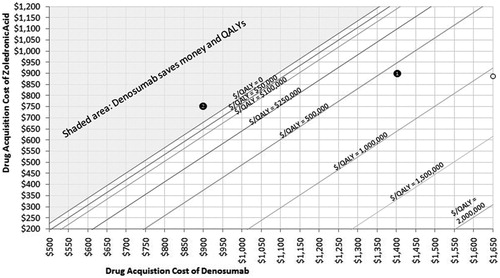
To understand why our cost effectiveness results are radically different from these other analyses, one must consider differences across studies in three main factors that affect the cost effectiveness ratio: total incremental drug cost, total SREs avoided, and the QALYs gained per SRE avoided. compares the five economic analyses (including reported sub-group and scenario analyses) in terms of estimated QALYs gained and SREs avoided with denosumab relative to zoledronic acid. Despite small variations with regard to model structure, background/baseline utility, trial data availability, and handing of adverse events, the results of the present analysis are broadly comparable (and in some cases more favorable towards denosumab) to those of the three European analyses. Thus, the large difference in ICURs may be largely due, in large part, to differences in incremental drug-associated costs. Specifically, in Europe, denosumab’s incremental acquisition cost is substantially lower than in the US. For example, in Lothgren et al.’sCitation31 Dutch analysis the per-administration drug-associated costs for denosumab and zoledronic acid were €486 and €451, respectively (difference = €35). In our US-based analysis, per-administration drug-associated costs are $1682 for denosumab and $1075 for zoledronic acid (difference = $607). In light of this, presents combinations of inputs that result in various ICUR values using the 27-month base case scenario. Using this figure, one can determine the price of denosumab that may be justifiable given a price for zoledronic acid and a given willingness to pay per QALY. For instance (Example 1 on ), in a plan currently paying $900 per infusion for zoledronic acid and $1400 per injection for denosumab, the ICUR for denosumab would be slightly above $500,000. Alternatively, a plan paying only $750 per infusion for zoledronic acid and $900 for denosumab would be saving both money and QALYs and would therefore lie in the shaded area.
Figure 4. Outcomes of cost-effectiveness analyses of denosumab vs zoledronic acid in bone metastatic prostate cancer. The x-axis is organized by Author-Country-Patient Group-Source of SRE Risk-Time Horizon. SRE-experienced and naïve refer to patients who have and have not experienced an SRE at baseline, respectively. In scenarios with sub-group effects, SRE risk/hazard ratios specific to SRE-experienced and naïve groups were applied to those groups in the analysis; whereas otherwise rates were drawn from pooled estimates regardless of whether the analysis referred to a specific patient group.
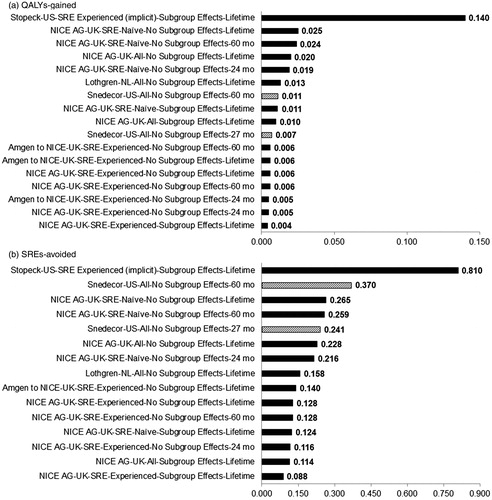
Figure 5. Costs per QALY for combination of denosumab and zoledronic acid acquisition costs. Start with a price for denosumab (on x-axis) and select a cost per QALY level (represented by a line on the graph) gives the associated price of zoledronic acid (on y-axis).
Nowhere is the importance of a lower incremental drug cost more apparent than in Amgen’s own analysis submitted to NICE. Before discounting denosumab’s cost with a confidential patient access scheme required by NICE for reimbursement approval, denosumab’s ICUR for bone-metastatic prostate cancer patients was £157,276Citation1Citation5. With the discounted price, zoledronic acid was dominated; i.e., denosumab cost less and saved more QALYs. In fact NICE AGCitation15 specifically noted that even a slight price reduction for zoledronic acid would render a discounted denosumab cost-ineffectiveCitation15, highlighting the importance of denosumab’s price relative to zoledronic acid in this setting.
However, differences in incremental drug acquisition cost alone cannot explain the difference in results between this analysis and the other US-based analysis conducted by Stopeck et al.Citation30 given that denosumab was associated with an incremental per-administration drug-associated cost of $607 in both analyses. To some degree, the difference may be driven by Stopeck et al.’s application of utilities to modes of administration, with denosumab’s administration being associated with a smaller utility loss than zoledronic acid’s. The present analysis did not incorporate these values because it is unclear how such gains in utilities can be derived with a reasonable degree of certainty and validity. (Of note, such effects were not collected directly in the clinical trial upon which the present analysis was based, and Stopeck et al. do not provide sufficient details of their estimation methods in the analysis). The difference in cost effectiveness between the this analysis and that by Stopeck et al. appears to be driven largely by differences in estimated SREs avoided and QALYs gained. Stopeck et al. reported 5–35-times more QALYs gained and 2–9-times more SREs avoided than the other analyses (). These large differences appear to stem from Stopeck et al.’s adjustment of the SRE rates in their analysis to reflect a US ‘real-world’, managed-care perspective as opposed to a within trial analysis. Specifically, zoledronic acid SRE rates were derived by comparing published rates from an insurance claims database analysis by Hatoum et al.Citation32 (among metastatic breast, prostate, and lung cancer patients) to SRE rates reported in the denosumab clinical trials. On the basis of this calculation, the authors report “an adjustment factor of 2.01 was derived and was used to adjust the trial-based SRE rates for zoledronic acid-treated patients in the model. The SRE rates for denosumab-treated patients were then calculated by applying the treatment effects from the phase III clinical trials. The adjusted annual SRE rates in clinical practice for denosumab and zoledronic acid were 1.500 and 1.903, respectively”.
The higher SRE-rate assumption made by Stopeck et al.Citation30 is not problematic in and of itself, as it is arguably possible that patients in the US routine clinical setting who initiate SRE-limiting therapy do so after experiencing an SRE. However, while Stopeck et al. upwardly adjusted the SRE rate to implicitly reflect a SRE-experienced cohort, they appear not to have downwardly adjusted survival and utility assumptions to correspond to this patient populationCitation15. Instead survival and baseline utility assumptions from the phase III clinical trial (where only 24% of patients were SRE-experienced) were applied. Therefore, the net effect of Stopeck et al.’s selective adjustment is the creation of a ‘hybrid’ population which simultaneously had high SRE rates reflecting a 75% SRE-experienced cohort as in Hatoum et al.Citation32 coupled with survival rates and baseline/background utilities reflecting a 24% SRE-experienced cohort as in Fizazi et al.Citation5. This is problematic because the occurrence of an SRE is associated with lower survival and quality-of-lifeCitation15.
Consequently, the modeled population in Stopeck et al.Citation30 experienced an artificially high number of SREs over a longer survival time than would otherwise be expected for an SRE-experienced population, leading to higher estimated SRE-related utility loss and costs due to excess SREs. Because Stopeck et al. applied the treatment effects from Fizazi et al.Citation5 to the population at artifically high risk for SREs, the magnitude of SRE burden avoided by denosumab was proportionally over-estimated.
It is unclear why Stopeck et al.Citation30 did not derive SRE rates from Hatoum et al.’sCitation33 2011 analysis in which the authors provided real-world SRE rates specific to bone-metastatic prostate cancer as well as rates specific to SRE-naïve (low-risk) and SRE-experienced (high-risk) patients. In their analysis, Hatoum et al.Citation33 reported SRE rates in SRE-naive and SRE-experienced patients receiving zoledronic acid annualized to be 0.36/year and 1.00/year, respectively. The authors also reported that 243 SRE-naïve and 218 SRE-experienced patients received zoledronic acid. Therefore, the true annualized SRE rate for bone metastatic prostate cancer patients treated with zoledronic acid can roughly be calculated as follows: (243*0.36 + 218*1)/(243 + 218) = 0.66. Based on Stopeck et al.’s 2.01 adjustment, their estimated annualized SRE rate of 1.903 for zoledronic acid-treated patients appears to be ∼3-times higher than should reasonably be expected.
Stopeck et al.’sCitation30 SRE and QALY outcomes are patently discordant with similar analyses of SRE-experienced patients conducted by Amgen and NICE’s independent assessment group, whereas the results of the present analysis are consistent with all but Stopeck et al. (). Thus, taken as a whole, it appears that the US cost-effectiveness ratio reported in Stopeck et al. is too optimistic due to its excessively large estimated number of QALYs gains and SREs avoided relative to all other analyses.
The present study was limited by the restricted availability of clinical data and the need to use projection methods to model beyond the trial time frame. Survival and SRE data were derived from figures of the Fizazi et al.Citation5 trial report, where exact values were unavailable. Although our estimated curves closely approximated the reported Kaplan-Meier curves (data available upon request), the potential for inaccuracy remains. In addition, treatment discontinuation data were not reported in a manner allowing for exact calculation of the proportion of patients still on treatment after a specified amount of time, and therefore the reported median number of doses was a surrogate for the median time on-study. It is also important that the present analysis did not incorporate an effect of treatment administration on utility.
Current published drug prices were used in this analysis, although it is likely the incremental cost of denosumab will soon become much greater, as zoledronic acid is expected to lose patent protection in 2013. An influx of less-expensive, generic forms of zoledronic acid will widen the cost differential between the two drugs, thereby increasing denosumab’s ICUR.
This analysis should not be interpreted as evidence that denosumab is ineffective in the prevention or delay of SREs in patients with bone-metastatic prostate cancer, as clinical trials have shown that denosumab is superior to zoledronic acid across a number of solid tumor typesCitation5,Citation34,Citation35. This analysis sought to determine the value of denosumab vs zoledronic acid from a pharmacoeconomic perspective, and therefore is not intended to determine individual patient treatment decisions, but rather to inform formulary and reimbursement decisions. In that regard, we found that the use of denosumab may disproportionally increase treatment costs without commensurate clinical benefits.
Conclusion
While denosumab may provide benefits relative to zoledronic acid in terms of preventing SREs, increased convenience of administration, and a possibly lower toxicity profile, the present analysis indicates that it results in an ICUR of $1,058,741, which is above what is typically considered good value for medical interventions in the US. This suggests that the choice of denosumab over zoledronic acid for the prevention of SREs in metastatic prostate cancer patients should be carefully considered.
Transparency
Declaration of funding
SJS, JAC, and MFB received a consulting fee from Novartis Pharmaceuticals (maker of zoledronic acid) related to the development of this analysis.
Declaration of financial/other relationships
SK was an employee of and stock owner in Novartis Pharmaceuticals at the time that the analysis was first drafted. SK is now an employee of Celgene Corporation and no longer owns stock in Novartis Pharmaceuticals. The peer reviewers on this manuscript have disclosed that they have no other relevant financial relationships.
Acknowledgements
No additional funding or outside assistance was obtained for this analysis and/or manuscript. An earlier version of this analysis was presented as a poster (#4581) at the 2011 ASCO Annual Meeting; June 3–7, 2011; Chicago, IL.
References
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76
- Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm 2010;16:693-702
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 2011;14:177-83
- Bouganim N, Clemons MJ. Bone-targeted agents in the treatment of bone metastases: RANK outsider or new kid on the block? Future Oncol 2011;7:381-3
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-22
- Amgen. FDA Approves Amgen’s XGEVA(TM) (Denosumab) for the prevention of skeletal-related events in patients with bone metastases from solid tumors. Amgen Press Release 2010. URL:http://www.amgen.com/media/media_pr_detail.jsp?releaseID=1498709. Accessed 9 March 2012
- Drug Topics Red Book. Montvale, NJ: Thomson Healthcare; 2010
- Aapro MS. Denosumab for bone metastases from breast cancer: a new therapy option? J Clin Oncol 2011;29(14):e419-20
- Aragon-Ching JB. Unravelling the role of denosumab in prostate cancer. Lancet 2011;377:785-6
- West H. Denosumab for prevention of skeletal-related events in patients with bone metastases from solid tumors: incremental benefit, debatable value. J Clin Oncol 2011;29:1095-8
- Wahed AS, Luong TM, Jeong JH. A new generalization of Weibull distribution with application to a breast cancer data set. Stat Med 2009;28:2077-94
- Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med 1982;73:883-8
- Reed SD, Radeva JI, Glendenning GA, et al. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J Urol 2004;171:1537-42
- Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005;16:579-84
- Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost effectiveness and economic evaluation of denosumab for the treatment of bone metastases from solid tumors. Aberdeen: Aberdeen HTA Group, Institure of Applied Sciences, University of Aberdeen, 2011
- Denosumab [Package Insert]. Thousand Oaks, CA: Amgen Incorporated, 2010
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94:1458-68
- Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol 2003;21:4277-84
- Snedecor SJ, Carter JA, Kaura S, et al. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther 2012;34(6):1334-49
- Barlev A, Chung K, Delea T, et al. Healthcare and utilization costs associated with skeletal related events in prostate cancer patients with bone metastases. J Manag Care Pharm 2010;16(9):693-702
- National Fee Analyzer. Eden Prairie, MN: Ingenix Inc, 2011
- Neighbors DM, Bell TJ, Wilson J, et al. Economic evaluation of the fentanyl transdermal system for the treatment of chronic moderate to severe pain. J Pain Symptom Manage 2001;21:129-43
- Moore S, Tumeh J, Wojtanowski S, et al. Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health 2007;10:23-31
- Klarenbach S, Manns B, Reiman T, et al. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer. Cancer 2010;116:3224-32
- Greenberg D, Earle C, Fang CH, et al. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst 2010;102:82-8
- Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist 2006;11:90-5
- Hillner BE, Weeks JC, Desch CE, et al. Pamidronate in prevention of bone complications in metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 2000;18:72-9
- Sartor O. Denosumab in bone-metastatic prostate cancer: known effects on skeletal-related events but unknown effects on quality of life. Asian J Androl 2011;13:612-3
- Carter JA, Botteman MF. Health-economic review of zoledronic acid for the management of skeletal-related events in bone-metastatic prostate cancer. Expert Rev Pharmacoecon Outcomes Res 2012preprint [Epub ahead of print]
- Stopeck A, Rader M, Henry D, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ 2012;15(4):712-23
- Lothgren M, Bracco A, Lucius B, et al. Cost-Effectiveness of denosumab vs zoledronic acid (ZA) for the prevention of skeletal-related events (SRE) in patients with bone metastases from solid tumors in the Netherlands. International Society for Pharmacoeconomics and Outcomes Research 14th Annual European Congress in Madrid, Spain. 2011
- Hatoum HT, Lin SJ, Smith MR, et al. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer 2008;113:1438-45
- Hatoum HT, Lin SJ, Guo A, et al. Zoledronic acid therapy impacts risk and frequency of skeletal complications and follow-up duration in prostate cancer patients with bone metastasis. Curr Med Res Opin 2011;27:55-62
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132-9
- Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011;29:1125-32