Abstract
Objective:
The 21-gene assay (Oncotype DX Breast Cancer Test (Genomic Health Inc., Redwood City, CA)) is a well validated test that predicts the likelihood of adjuvant chemotherapy benefit and the 10-year risk of distant recurrence in patients with ER+, HER2− early-stage breast cancer. The aim of this analysis was to evaluate the cost-effectiveness of using the assay to inform adjuvant chemotherapy decisions in Germany.
Methods:
A Markov model was developed to make long-term projections of distant recurrence, survival, quality-adjusted life expectancy, and direct costs for patients with ER+, HER2−, node-negative, or up to 3 node-positive early-stage breast cancer. Scenarios using conventional diagnostic procedures or the 21-gene assay to inform treatment recommendations for adjuvant chemotherapy were modeled based on a prospective, multi-center trial in 366 patients. Transition probabilities and risk adjustment were based on published landmark trials. Costs (2011 Euros (€)) were estimated from a sick fund perspective based on resource use in patients receiving chemotherapy. Future costs and clinical benefits were discounted at 3% annually.
Results:
The 21-gene assay was projected to increase mean life expectancy by 0.06 years and quality-adjusted life expectancy by 0.06 quality-adjusted life years (QALYs) compared with current clinical practice over a 30-year time horizon. Clinical benefits were driven by optimized allocation of adjuvant chemotherapy. Costs from a healthcare payer perspective were lower with the 21-gene assay by ∼€561 vs standard of care. Probabilistic sensitivity analysis indicated that there was an 87% probability that the 21-gene assay would be dominant (cost and life saving) to standard of care.
Limitations:
Country-specific data on the risk of distant recurrence and quality-of-life were not available.
Conclusions:
Guiding decision-making on adjuvant chemotherapy using the 21-gene assay was projected to improve survival, quality-adjusted life expectancy, and be cost saving vs the current standard of care women with ER+, HER2− early-stage breast cancer.
Introduction
Breast cancer is the most frequently occurring cancer in women in Germany, with an estimated incidence of 57,000 cases per year, which corresponds to 28% of all registered female cancers in GermanyCitation1. Although the incidence and mortality rates have declined in recent years, ∼18,000 women die each year from breast cancerCitation1. Considerable regional variation exists in Germany in terms of the incidence of breast cancer, with the highest incidence reported in Northern Germany and the lowest in Eastern GermanyCitation1. Variation in mortality rates show similar trends, ranging from 20 per 100,000 in Thuringia to 30 per 100,000 in SaarlandCitation1. Analysis of temporal trends in mortality has shown that, overall, breast cancer mortality has declined over the period 1996–2005 and 5-year survival rates are now in the region of 81%Citation1.
The economic burden of breast cancer is considerable. In 2007, 7.2% of total healthcare expenditure in Germany was spent on breast cancerCitation2. However, there is a relative paucity of data detailing costs at an individual patient level. It is generally well accepted that adjuvant chemotherapy is a key driver of costs, as it is associated with multiple costs from the healthcare payer perspective, in terms of the cost of chemotherapy agents (drug acquisition costs) and administration, as well as the monitoring and management of adverse events. Moreover, adjuvant chemotherapy is associated with both short-term and long-term adverse events, absenteeism/lost workplace productivity, and treatment negatively impacts quality-of-lifeCitation3–5. Consequently, a large proportion of the economic burden associated with breast cancer is thought to be due to the inappropriate prescribing of adjuvant chemotherapy, as well as management of associated adverse events. A very modest proportion of patients (4% in the B20 study) with early-stage ER-positive and HER-2 negative invasive breast cancer derive benefit from adjuvant chemotherapyCitation6–8. However, for over 50% of early-stage ER+, HER2− breast cancer patients chemotherapy is currently recommended in GermanyCitation9,Citation10.
Breast cancer is a heterogeneous disease: prognosis, survival, and recurrence rates vary widely and are influenced by a number of factors including disease stage at diagnosis (based on tumor size, lymph node involvement, and distant metastases), presence of particular molecular markers including, in particular, the estrogen and progesterone receptors (ER and PR, respectively) and the human epidermal growth factor receptor (HER2). The ability to predict whether a patient is likely to benefit from chemotherapy would assist physicians in making individualized treatment decisions, thereby improving patient outcomes and minimizing unnecessary exposure to chemotherapy and associated adverse events in patients who are not likely to benefit from treatment. This in turn has implications in terms of reducing the economic and humanistic burden associated with breast cancer. The 21-gene assay (Oncotype DX Breast Cancer Test (Genomic Health Inc., Redwood City, CA)), is a validated diagnostic test that has been shown to successfully predict the likelihood of chemotherapy benefit as well as distant recurrence 10 years after diagnosis in patients with early-stage, node-negative, and node-positive ER+ breast cancer. The assay uses real-time reverse-transcriptase polymerase chain reaction (RT-RCR) to quantitatively examine the expression profile of 21 genes (16 cancer-related genes and five reference genes). The gene expression results are then combined to provide a single recurrence score (RS) between 0–100, which corresponds to a point estimate (with 95% confidence intervals) of the 10-year risk of distant recurrence at an individual patient level. The validity of the 21-gene assay has been demonstrated in a number of clinical studies both for prognosis and prediction of likelihood of chemotherapy benefitCitation6,Citation11–14. A number of studies evaluating the impact of the assay on adjuvant therapy decisions in patients with ER+ early-stage breast cancer have demonstrated that knowledge of RS affects management of patients. Importantly, the studies reveal that every second patient originally recommended adjuvant chemotherapy plus endocrine treatment is recommended endocrine treatment alone after knowledge of the RSCitation15–18. In common with other settings, the use of the 21-gene assay in Germany would be expected to decrease overall chemotherapy usage, which would in turn translate into substantial savings in terms of the overall chemotherapy budgetCitation19,Citation20. The aim of the present study was, therefore, to evaluate the cost-effectiveness of the 21-gene assay in adjuvant chemotherapy decision-making in women with early-stage, ER+, HER2− breast cancer in the German setting.
Methods
Model overview
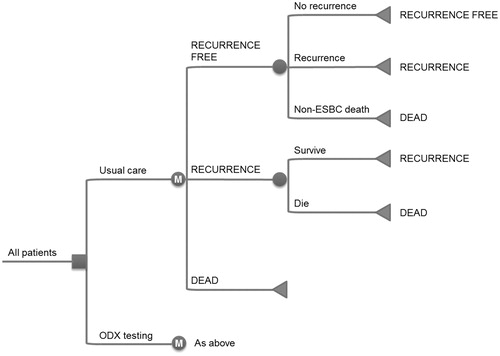
To evaluate the cost-effectiveness of the 21-gene assay in Germany, country-specific data were used to adapt an existing Markov model programmed in Microsoft Excel (Redmond, WA), which was recently developed to support a submission to the National Institute of Health and Clinical Excellence (NICE) in the UK and was developed based on the original analysis by Hornberger et al.Citation21,Citation22. The model made projections of life expectancy, quality-adjusted life expectancy, and direct costs (with the inclusion of indirect costs also possible), based on recurrence rates for low, intermediate, and high-risk patients as well as country-specific mortality data. A Markov model structure was chosen based on suitability for modeling recursive disease processes (e.g. annual risk of recurrence and mortality). Patients in the model are either assigned adjuvant therapy based on the conventional approach in Germany (standard of care) or based on the 21-gene assay RS. There are three states in the model: recurrence-free (in which all patients start the simulation), recurrence (following a distant recurrence event), and dead (following a mortality event) (). In each 1-year cycle of the simulation, patients are exposed to the risk of competing mortality and recurrence. Patients who have a mortality event transition to the dead state (absorbing state). Patients who experience a distant recurrence event transition to the recurrence state, where they are exposed to the risk of breast cancer mortality in each subsequent year of the simulation. Transition probabilities between states are based on published clinical data and are outlined in the Clinical input data section below.
Figure 1. Overview of the 21-gene assay cost-effectiveness model structure. ESBC, early-stage breast cancer; ODX, 21-gene assay; M, Markov node. Squares represent decision nodes, circles represent chance nodes (or Markov nodes where designated) and triangles represent transitions to health states. Health states are designated using block capitals.
Clinical input data
To ensure that the modeling analysis was in line with the standard of care in Germany, patients were assumed to receive standard endocrine therapy and chemotherapy regimens in line with data from the decision impact study performed by our groupCitation9. The study was a large, prospective multi-center trial with consecutive enrollment of 379 patients at 15 centers in Germany. A total of 379 patients were recruited with ER+, HER2−, node-negative, and up to 3 node-positive, tumor size ≥1 cm (T1, T2, or T3 tumors with cutaneous infiltration) or <1 cm (if one or more unfavorable histological characteristics present), early-stage breast cancer and no contraindications for chemotherapy. Treatment recommendations before and after knowledge of the RS as well as actual treatment data were recorded. The pre-specified categories for RS were consistent with previous analyses: low (lower than 18), intermediate (18–31), and high (greater than 31). The final analysis set included 366 patients (mean age 56.3 years) as 13 patients were excluded (11 cases where the 21-gene assay could not be performed, one patient withdrew consent, and one patient was HER2+). In total, 244 patients (67%) were node negative and 122 were node positive (33%). The majority of patients had low RS values (54.1%), indicating a low risk of distant recurrence. Approximately 38.0% of patients had an intermediate RS and 7.9% had a high RS. As a result of having the RS, there was a reduction in overall chemotherapy use from 57.1% to 38.3%, with reductions from 50.5% to 13.6% in the low RS group, from 92.6% to 61.2% in the intermediate RS group and an increase from 75.9% to 96.6% in patients with a high RS. Changes in recommended therapy of the overall study population are summarized in .
Table 1. Changes in adjuvant therapy recommendations for breast cancer patients in Germany following 21-gene assay testing.
In each cycle of the model, the risk of recurrence was evaluated for the simulated cohort based on their RS defined category of low, intermediate, or high risk as reported by Paik et al.Citation11 for the NSABP B-20 cohort. The 10-year risk of distant recurrence were 3.2%, 9.1%, and 39.5% in the low, intermediate, and high RS groups. Risk was adjusted based on whether patients were receiving chemotherapy as per the initial recommendations (in the standard of care arm) and based on the RS (in the 21-gene assay arm) based on the same dataset. No risk reduction was assumed in the low RS group and the risk reductions with chemotherapy were 39% and 74% in the intermediate and high RS groups, respectively. For probabilistic sensitivity analysis, recurrence risks and relative risk reductions for chemotherapy were sampled from normal distributions, with variance defined based on the published data. Non-breast cancer death was captured as a competing risk in the model, based on German life tables for females. For patients experiencing distant recurrence, survival was assumed to 3.3 years based on a retrospective analysis of recurrence data from real-life clinical practiceCitation23.
Costs, utilities, and other settings
All costs were expressed in 2011 Euros (€). The costs of adjuvant chemotherapy were derived from a recent study of resource use and costs in 70 early-stage breast cancer patients at seven centers across GermanyCitation24. Resource use was estimated based on a chart review of patients who completed all cycles of adjuvant chemotherapy for the treatment of ER+, HER2−, node-negative, or up to 3 node-positive breast cancer. The analysis was designed to capture the cost associated with chemotherapy drugs and their administration, supportive drugs (to prevent or manage adverse reactions), monitoring of chemotherapy, and management of adverse events. Based on the case report forms completed for each patient, the study assessed patient characteristics, type and costs of chemotherapy regimen, chemotherapy administration costs, follow-up visits, laboratory tests, chemotherapy-specific adverse events, transportation costs, and sick leave (time off work paid for by the sick funds). The analysis showed that 84% of patients were administered one of the four most common chemotherapy regimens: 26% FEC (5-fluorouracil, epirubicin, and cyclophosphamide), 24% FEC + DOC (FEC and docetaxal), 19% TAC (docetaxal, Adriamycin, and cyclophosphamide) and 16% TC (docetaxal and cyclophosphamide). The mean age of the study population was 56 years, 57% were node-negative, and 76% of patients received chemotherapy in the outpatient setting. The analysis reported that the total average costs of chemotherapy were €19,263 from a healthcare payer (sick fund) perspective (). The cost of chemotherapy drugs was the single biggest contributor to the total, accounting for approximately one-third of total direct costs from a sick fund perspective.
Table 2. Summary of mean costs of adjuvant chemotherapy per patient in breast cancer patients in Germany.
The costs of endocrine therapy were estimated based on the costs of tamoxifen therapy as previously published by Lux et al.Citation25. Although these estimates may under-estimate the true cost of endocrine therapy in Germany (due to use of aromatase inhibitors), it will have no impact on incremental costs (or cost-effectiveness), as these costs would be the same in both of the modeled comparisons (standard of care and standard of care with the 21-gene assay). The costs of distant recurrence were assumed to be €20,955, also based on data published by Lux et al.Citation25. The cost of the 21-gene assay was taken from the published list price (€3180). Costs were inflated to 2011 values as required using the published indices for Germany (Verbraucherpreisindex für Deutschland) available at www.destatis.de.
Quality-of-life utility scores were based on published literature. Patients in the recurrence free state had an annual utility score of 0.78 and in the recurrence state this value was 0.60Citation26,Citation27. For patients in the dead state, the utility score was zero. A disutility of 0.07 was applied to capture the health-related quality-of-life impact of chemotherapy in the first model cycle (for those patients recommended chemotherapy in each arm of the model) based on data published by Peasgood et al.Citation28.
Future costs and clinical benefits were discounted at 3% per annum in line with published guidance for the German settingCitation29. The analysis reported in this paper took the perspective of the healthcare payer (sick funds) and as such only reports direct medical costs. The time horizon for the base case analysis was set to 30 years to capture the benefits of reduced distant recurrence over the long-term.
Sub-group and sensitivity analysis
To investigate the cost-effectiveness of the 21-gene assay in patients with node-positive breast cancer, sub-group analysis was performed in patients with node-negative and node-positive disease. Data from treatment decisions in node-positive and node-negative patients in the German setting were derived from our decision impact study ()Citation9. Rates of disease-free survival specific to node-positive patients were derived from Albain et al.Citation14, from a report in post-menopausal women with ER+, node-positive early-stage breast cancer receiving tamoxifen or chemotherapy followed by endocrine therapy (CAF-T: cyclophosphamide, doxorubicin, and fluorouracil before tamoxifen). An improvement in disease-free survival was observed in the high RS group (p = 0.033), but no significant improvement was observed in the low or intermediate RS groups (p = 0.97 and 0.48, respectively). The relative risk reductions describing the benefit of chemotherapy treatment used as model inputs were, therefore, 0%, 0%, and 41% for low, intermediate, and high RS groups, respectively (i.e. the modeling analysis captured only statistically significant benefits). For the analysis, sub-group specific costs were used for chemotherapy and adverse events based on data collected from node-negative and node-positive patients in the recent Eiermann et al.Citation24 cost of illness study in adjuvant chemotherapy.
Probabilistic sensitivity analysis (PSA) was performed to provide a measure of the likely variance around the reported base case cost-effectiveness outcomes. For the PSA, sampling was performed from distributions around clinical and cost parameters using either normal, beta, or gamma distributions as appropriate (). Where reported, distribution parameters (such as standard error (SE) and minimum and maximum values) were taken from the literature. Where insufficient information was available, it was assumed that the standard error was equal to 10% of the point estimate value and that maximum and minimum values lay 50% above and below the mean value. These assumptions were considered conservative since they are likely to exceed the true variation. The PSA results were used to generate a cost-effectiveness scatterplot of incremental costs vs incremental effectiveness for 1000 iterations and an acceptability curve to show the percentage of points below a range of willingness to pay thresholds for the German setting.
Table 3. Summary of distributions used in the probabilistic sensitivity analysis.
A series of one-way sensitivity analyses was performed to identify key drivers of model outcomes. Discount rates on costs and clinical benefits were varied between 0–10% (3% in the base case). The time horizon was varied to 10, 20, and 40 years (30 years in the base case). In terms of costs, the total cost of chemotherapy (including adverse event costs) was varied ±20% and the cost of distant recurrence was varied by ±20%. To investigate the impact of sick leave associated with chemotherapy administration, the indirect costs associated with lost productivity were included in a sensitivity analysis. For this analysis, each day of lost productivity was assumed to cost €38.24 per patient for up to 42 days of sick leave (after which time the sick funds will reimburse lost productivity) and based on days off work from a recent cost study of adjuvant chemotherapyCitation24. To investigate the impact of the utility scores used on cost-effectiveness outcomes, the disutility associated with chemotherapy was set to −0.037 based on Conner-Spady et al.Citation26 and −0.5 based on Simes and CoatesCitation30 (base case values was −0.07). In addition, the health utility associated with 1 year in the recurrence state was set to 0.51 based on the value on chemotherapy reported by Milne et al.Citation27 (base case value was 0.6). To investigate the impact of variation in clinical assumptions in the model, the following sensitivity analyses were run: the duration of post-recurrence survival was varied by ±50%; the risk of recurrence in the low RS group was set to 1.1% (0 in the base case) based on data from Paik et al.Citation11; the 10-year risks of recurrence were varied from the base case values of 3.20% (low RS), 9.10% (intermediate RS), and 39.50% (high RS) to values of 4.43%, 13.24%, and 27.26%, respectively, derived from data from the TransATAC studyCitation12. No sensitivity analyses were performed to investigate structural uncertainty as part of the analysis, although an internal validation study was performed.
Results
Base case analysis
The 21-gene assay was associated with a notable change in treatment recommendations based on the data reported by Eiermann et al.Citation9 (). Approximately one-third of all patients originally recommended chemotherapy were recommended endocrine therapy only after the 21-gene assay. In the group of patients originally prescribed endocrine therapy only, the addition of chemotherapy was recommended in ∼25.5% of patients. In 12.3% of cases patients did not accept their physician’s recommendation after the 21-gene assay, ultimately resulting in a reduction in overall chemotherapy use from 57.1% to 38.3%. Based on these data, optimized assignment of chemotherapy with the 21-gene assay was projected to improve life expectancy by ∼0.06 years per patient (). Taking quality-of-life into account, the analysis showed that the 21-gene assay was associated with an improvement of approximately 0.06 QALYs vs standard of care. Estimation of direct medical costs associated with chemotherapy and distant recurrence showed that these substantial clinical benefits were accompanied by cost savings of ∼€561. As a result, the 21-gene assay was considered dominant (cost and life saving) to standard of care.
Table 4. Summary of cost-effectiveness outcomes for the base case analysis of 21-gene assy testing vs standard care.
Probabilistic sensitivity analysis
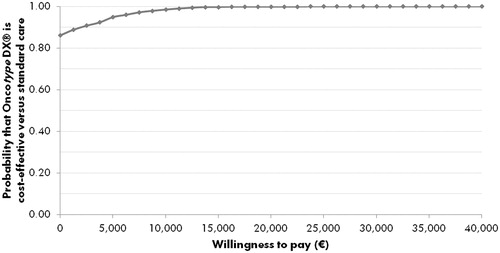
PSA was performed, in which the results of 1000 iterations of the model were averaged to give mean cost and effectiveness outcomes, to provide an estimate of the likely variance around the base case results (). All iterations showed an increase in quality-adjusted life expectancy with the 21-gene assay (i.e. points in the right half of the cost-effectiveness plane). In terms of incremental costs, ∼87% of iterations indicated that the 21-gene assay was cost saving vs standard of care. Mean PSA values showed an improvement of 0.062 QALYs (95% credibility interval (CI) = 0.061–0.063) with the 21-gene assay and a cost saving of €574 (95% CI = €544–€606) vs standard of care. Acceptability curve analysis indicated that, at a willingness to pay threshold of €20,000 per QALY gained, there would be a 100% probability of the 21-gene assay being cost-effective vs standard of care (). Assuming a willingness to pay threshold of €5000 per QALY gained, this probability was 96%.
Figure 2. Cost-effectiveness scatterplot of the probabilistic sensitivity analysis. The cost-effectiveness scatterplot shows incremental costs vs incremental effectiveness expressed in quality-adjusted life years (QALYs) for the comparison of 21-gene assy with standard care. Each point represents one iteration of the probabilistic sensitivity analysis (with data based on sampling from distributions around clinical and cost parameters).
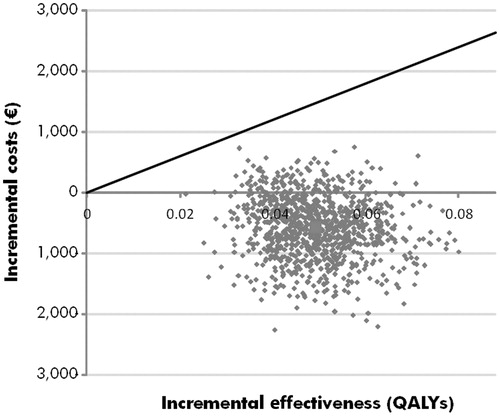
Figure 3. Cost-effectiveness acceptability curve for 21-gene assay vs standard care from the probabilistic sensitivity analysis. The acceptability curve shows the percentage of points from the probabilistic sensitivity analysis (see ) that fall below a range of willingness-to-pay values from €0 to €40,000 per quality-adjusted life year gained.
Sub-group and deterministic sensitivity analysis
The exploratory sub-group analysis in patients with ER+, node-positive, early-stage breast cancer showed that the 21-gene assay was projected to be dominant to current clinical practice (with benefits in quality-adjusted life expectancy and lower costs) (). Overall, quality-adjusted life expectancy was notably lower in this sub-group and costs were higher compared to the base case analysis due largely to the higher risk of recurrence in these patients (allied to a higher cost of chemotherapy in these patients). In node-negative patients, the 21-gene assay was cost-effective with an incremental cost-effectiveness ratio of €16,667 per QALY gained vs standard of care. A lower cost of chemotherapy in these patients was a main reason for the difference in results in this sub-group compared to the base case analysis.
Table 5. Summary of sub-group and sensitivity analysis outcomes for 21-gene assay testing vs standard care.
Deterministic sensitivity analysis showed that the base case outcomes were relatively robust to variation in a range of key input parameters. Outcomes were most sensitive to variation in chemotherapy costs and variation in the net changes in chemotherapy use (). Increasing and decreasing chemotherapy costs led to corresponding increases and decreases in the ICER (as would be expected). Including the costs associated with lost productivity during chemotherapy, the cost saving (from a societal perspective) was ∼€1446 per patient with the 21-gene assay, compared to €561 in the base case analysis.
Discussion
In this modeling analysis, using the 21-gene assay was projected to be both cost and life saving (dominant) vs the current standard of care in Germany over 30 years in a scenario where all patients receive the test. The 21-gene assay was associated with a notable change in treatment recommendations based on the data reported by Eiermann et al.Citation9, with over one-third of all patients originally prescribed chemotherapy recommended endocrine therapy only after the 21-gene assay. Based on these changes, and modeling the long-term risk of distant recurrence based on published trial data, including the 21-gene assay as a treatment decision tool was projected to improve quality-adjusted life expectancy by 0.06 QALYs per patient. A substantial part of this benefit was driven by the addition of chemotherapy to high RS patients likely to benefit from it that are not identified by classical parameters as high risk patients. The cost savings from a sick fund perspective associated with the 21-gene assay were estimated to be ∼€561 per patient. PSA showed that there was an 87% probability that the 21-gene assay would be cost saving and a 100% probability that it would be highly cost-effective based on commonly quoted willingness to pay thresholds (e.g. €30,000 per QALY gained). Sensitivity analysis showed that the cost-effectiveness of the 21-gene assay was most sensitive to variations in the cost of chemotherapy and net changes in chemotherapy use across all three RS groups (low, intermediate, and high).
The cost-effectiveness analysis can be considered conservative in some regards, as it did not capture some of the long-term adverse effects of chemotherapy treatment (including cardiotoxicity, secondary leukemia, or effects on cognitive impairment). Local recurrence was not captured in the modeling analysis and, given the purported benefits in terms of treatment recommendations with the 21-gene assay, this may also have led to an under-estimation of the clinical benefit of the 21-gene assay. Moreover, recent data from the Canadian setting has provided evidence that breast cancer patients experience difficulties returning to work after chemotherapy. The survey showed that almost one-fifth of respondents had rehabilitation and ergonomic adjustments to their workplace to accommodate their new situationCitation31. Others, who did not have a gradual return to work, reported distress, pain, fatigue, and, in certain cases, having to leave full-time employment. In addition, the study showed that those women who received chemotherapy had the greatest drop in family income, took more time off work, and were more likely to leave the work force. Recently, Peugniez et al.Citation32 reported comparable findings from the French setting, with chemotherapy patients taking a median time of 14.3 months to return to employment, emphasizing the dramatic impact of breast cancer on costs from a societal perspective.
A potential weakness of the analysis is that, in the absence of German data, it relied on long-term clinical data from the US to estimate the risk of distant recurrence in patients with low, intermediate, and high RS. However, a sensitivity analysis using risk of distant recurrence from the TransATAC study performed in the UK confirmed the robustness of the base case results. Similarly, quality-of-life utility scores were not Germany-specific. However, sensitivity analysis indicated that the utility scores used were not substantial drivers of cost-effectiveness (it seems unlikely that German utility scores would notably change the outcome of the analysis). Information on variance around many of the model inputs were limited and, therefore, assumptions have been made regarding the shape and, in certain cases, the parameters defining distributions for PSA. In most cases, these assumptions have been conservative (over-estimating the likely variance). Although sensitivity analyses have not been performed to investigate the impact of changing these assumptions, it is unlikely that any changes within plausible limits would notably alter the findings of the analysis (although specific results and estimates of variance may be influenced). One further confounding factor with regard to variance was that no data are currently available on how the parameters in the model are likely to co-vary (e.g. the distribution of patients by risk category, risk of recurrence with and without chemotherapy are not likely to be conditionally independent). As a result, conditional independence has not been captured in the analysis, which could also contribute to an over-estimation of variance in the PSA.
Other aspects of the modeling assumptions may have had an influence on outcomes. For example, in the absence of appropriate data, it was assumed that time between distant recurrence and death was the same in low, intermediate, and high RS patients. It is plausible that high risk patients may have a reduced time from recurrence to death, which could influence the life expectancy estimates projected by the model. The use of data from Paik et al.Citation6 from the NSABP B-20 cohort to estimate chemotherapy benefit in the model may also be a potential limitation. Patients in the trial received tamoxifen only, whereas patients in modern clinical practice tend to receive tamoxifen plus an aromatase inhibitor. Data from the TransATAC study has demonstrated that the Recurrence Score is prognostic both in patients treated with tamoxifen and aromatase inhibitorCitation12. Moreover, patients in the NSABP B-20 trial received CMF (cyclophosphamide, methotrexate and 5-fluorouracil) or MF (methotrexate and 5-fluorouracil), rather than the newer and more effective regimens reported in the present analysis. It is not clear how this assumption is likely to influence the impact of chemotherapy on distant recurrence in the modeling analysis.
The clinical findings of the decision impact study from our group that formed the basis of this modeling study are consistent with published data from other settingsCitation9. Hornberger and ChienCitation15 published a meta-analysis of nine published studies with a total of 1154 eligible patients, which showed that the impact of the 21-gene assay on chemotherapy decision-making has been largely and consistent across studies and countries. The analysis showed that the 21-gene assay was associated with a reduction in the proportion of patients recommended chemotherapy from 58% of patients before testing to 34% after testing (i.e. a 41% relative reduction). In patients originally recommended for chemotherapy, 51% were reassigned to endocrine therapy only. Overall, the RS was reported to have changed more than one-third of treatment decisions: 30% of the overall recommendations changed from endocrine therapy plus chemotherapy to endocrine therapy only, and 5% of the overall recommendations changed from endocrine therapy only to endocrine therapy plus chemotherapy. Reassuringly, the results of the present cost-effectiveness analysis were generally consistent with those observed in other settings: the published studies to date have indicated that the 21-gene assay is likely to be cost-effective or cost saving in all published analyses to dateCitation22,Citation33–38. Observed differences between published cost-effectiveness analyses are due to setting-specific factors such as the cost of chemotherapy, which can vary substantially between different countries, and local practice differences (e.g. approach to dealing with patients with intermediate RS). The inclusion or exclusion of indirect costs is also an important factor in creating differences between country-specific cost-effectiveness analyses.
The substantial clinical, economic, and humanistic burden of breast cancer in Germany is a major healthcare challenge. In recent years advances in patient management, particularly in terms of endocrine and chemotherapy, have been accompanied by notable improvements in survival. However, it is well recognized that there is a significant over-treatment (and select under-treatment) with chemotherapy in the adjuvant setting in patients with ER+ early-stage breast cancer based on a conventional care paradigm. Since the absolute clinical benefit of adjuvant chemotherapy in node-negative breast cancer is modest (estimated absolute benefit of 4% (92% with vs 88% without) in terms of 10-year distant recurrence in the NSABP B-20 trial), but the toxicity is significant, the selection of patients for adjuvant chemotherapy remains an important clinical issueCitation39. Based on the results of a recent decision impact study, the present modeling analysis demonstrates that guiding decision-making on adjuvant chemotherapy using the 21-gene assay breast cancer test would improve survival and quality-adjusted life expectancy and would reduce costs from a healthcare payer perspective in comparison with the current standard of care in women with ER+, HER2− early-stage breast cancer in Germany.
Transparency
Declaration of funding
This study was supported by a grant from Genomic Health International (GHI).
Declaration of finacial relationships
Jens-Uwe Blohmer and William Valentine received grant support from GHI to carry out this study. Jens-Uwe Blohmer, Thorsten Kühn, Kay Friedrichs, and Wolfgang Eiermann have acted as speakers on behalf of GHI. Sherko Kümmel and Jens-Uwe Blohmer have received speakers’ honoraria from GHI. Kay Friedrichs has received sponsorship from Roche. Mahdi Rezai, Thorsten Kühn, Mathias Warm, and Anja Benkow have no relevant financial relationships to disclose.
Notice of Correction The version of this article published online ahead of print on 12 September 2012 contained an error on page 4. An incorrect version of Table 2 was published. This does not affect any of the results of the analysis. The error has been corrected for this version.
Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- Katalinic A, Pritzkuleit R, Waldmann A. Recent trends in breast cancer incidence and mortality in Germany. Breast Care (Basel) 2009;4:75-80
- Statistisches Bundesamt. Gesundheit - Krankheitskosten, 2002, 2004 und 2006. 2008. Wiesbaden: Statistisches Bundesamt
- Conner-Spady BL, Cumming C, Nabholtz JM, Jacobs P, Stewart D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant 2005;36:251-9
- Canadian Breast Cancer Network. Breast cancer: Economic impact and labour force re-entry. May 2010. http://www.cbcn.ca/index.php?pageaction=content.page&id=2912&lang=en. Accessed November 17, 2011
- Peugniez C, Fantoni S, Leroyer A, et al. Return to work after treatment for breast cancer: single center experience in a cohort of 273 patients. Bull Cancer 2011;98:E69-79
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726-34
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687-717
- Hassett MJ, Hughes ME, Niland JC, et al. Chemotherapy use for hormone receptor–positive, lymph node–negative breast cancer. J Clin Oncol 2008;26:5553-60
- Eierman W, Rezai M, Kümmel S, et al. The 21-Gene Recurrence Score Assay Impacts Adjuvant Therapy Recommendations for ER-positive, Node-negative and Node-positive Early Breast Cancer Resulting in a Risk-adapted Change in Chemotherapy Use. Ann Oncol 2012, in press
- Blohmer JU, Kühn T, Rezai M, et al German multicentre decision impact study of Oncotype DX® Recurrence Score® (RS) on adjuvant treatment in estrogen receptor positive (ER+) node negative (N0) and node positive (N+) early breast cancer. Abstract #P206 presented at 2011 SG-BCC - 12th International St. Gallen Breast Cancer Conference, St. Gallen, Switzerland. 2011
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817-26
- Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with Anastrozole or Tamoxifen: A TransATAC study. J Clin Oncol 2010;28:1829-34
- Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25
- Albain KS, Barlow WE, Shak S, et al Prognostic and predictive value of the 21-gene recurrence score assay in a randomized trial of chemotherapy for postmenopausal, node-positive, estrogen receptor-positive breast cancer. Lancet Oncol 2010;11:55-65
- Hornberger J, Chien R. Meta-analysis of the decision impact of the 21-gene breast cancer recurrence score in clinical practice. San Antonio Breast Cancer Symposium. 2010. Poster Presentation #P3-09-06
- Holt S, Bertelli G, Brinkworth E, et al. Results from a prospective clinical study on the impact of Oncotype DX® on adjuvant treatment decision in a cohort of 142 UK patients. San Antonio Breast Cancer Symposium. 2011. Abstract Presentation #P5-14-26
- Yamauchi H, Nakagawa C, Yamashige S, et al. Decision impact and economic evaluation of the 21-gene Recurrence Score® assay for physicians and patients in Japan. Poster presented at the annual European Society for Medical Oncology Congress. 2011. Abstract Presentation
- De Boer RH, Baker C, Speakman, Mann B. Australian Decision Impact Study: The impact of Oncotype DX Recurrence Score (RS) on adjuvant treatment decisions in hormone receptor positive (HR+), node negative and node positive (N+9 early breast cancer (ESBC) in the multidisciplinary clinic. San Antonio Breast Cancer Symposium. 2011. Abstract Presentation #P4-09-18
- Semmel A, Coy P. Kostenersparnis durch die Anwendung von Oncotype DX. Asklepios Paulinen Klinik, Präsentation vom 4 August 2011
- Paepke S, Völkel P, Munte A, et al. Oncotype DX Mammakarzinom-Recurrence-Score in der klinischen Routinediagnostik – Benefi t für Patientin und Gesundheitssystem. Poster 7 Senology Conference Dresden. 2011
- Holt SDH, Bennett H, Bertelli G, et al. Cost-effectiveness of the Oncotype DX® breast cancer assay in clinical practice in the UK. Poster presented at the 34th Annual San Antonio Breast Cancer Symposium. December 6–10, 2011
- Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 2005;11:313-24
- Thomas RJ, Williams M, Marshall C, et al. The total hospital and community UK costs of managing patients with relapsed breast cancer. Br J Cancer 2009;100:598-600
- Eiermann W, Rezai M, Kümmel S, et al. The economic burden of adjuvant chemotherapy in Germany. Poster presented at the 14th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research in Madrid, Spain. November 5–8, 2011
- Lux MP, Wöckel A, Benedict A, et al. Cost-effectiveness analysis of anastrozole versus tamoxifen in adjuvant therapy for early-stage breast cancer—a health-economic analysis based on the 100-month analysis of the ATAC trial and the German health system. Onkologie 2010;33:155-66
- Conner-Spady BL, Cumming C, Nabholtz JM, et al. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant 2005;36:251-9
- Milne RJ, Heaton-Brown KH, Hansen P, et al. Quality-of-life valuations of advanced breast cancer by New Zealand women. Pharmacoeconomics 2006;24:281-92
- Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert Rev Pharmacoecon Outcomes Res 2010;10:553-66
- IQWIG Report. Methods for assessment of the relation of benefits to costs in the German statutory health care system. 2008. https://www.iqwig.de/download/08-10-14_Draft_Methods_of_the_Relation_of_Benefits_to_Costs_v_1_1.pdf. Accessed February 23, 2012
- Simes RJ, Coates AS. Patient preferences for adjuvant chemotherapy of early breast cancer: how much benefit is needed? J Natl Cancer Inst Monogr 2001;30:146–52
- Canadian Breast Cancer Network. Breast Cancer: Economic impact and labour force re-entry. May 2010. http://www.cbcn.ca/index.php?pageaction=content.page&id=2912&lang=en. Accessed November 17, 2011
- Peugniez C, Fantoni S, Leroyer A, et al. Return to work after treatment for breast cancer: single center experience in a cohort of 273 patients. Bull Cancer 2011;98:E69-79.
- Klang SH, Hammerman A, Liebermann N, et al. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 2010;13:381-7.
- Kondo M, Hoshi SL, Yamanaka T, et al. Economic evaluation of the 21-gene signature (Oncotype DX) in lymph node-negative/positive, hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03). Breast Cancer Res Treat 2011;127:739-49
- Tsoi DT, Inoue M, Kelly CM, et al. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 2010;15:457-65
- Madaras B, Rózsa P, Gerencsér Z, et al. Evaluation of the cost-effectiveness of the Oncotype DX® multigene assay in Hungary. Poster Presentation at the 12th St. Gallen International Breast Cancer Conference. 16–19 March, 2011. St. Gallen, Switzerland
- de Lima Lopez G, Chien R, Hornberger J. Cost-effectiveness analysis of the 21-gene Recurrence Score® for early stage breast cancer in Singapore. Poster Presentation at the 12th St. Gallen International Breast Cancer Conference. 16–19 March, 2011. St. Gallen, Switzerland
- Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer 2007;109:1011-8.
- Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat 2011;127:133-42.
