Abstract
Objective:
This retrospective study evaluated iron chelating therapy (ICT) discontinuation and costs in Sickle cell disease (SCD) Medicaid recipients using healthcare claims from 2006–2010.
Methods:
Patients with ≥1 SCD diagnosis claim, ≥2 claims for deferoxamine (DFO) or deferosirox (DFX), and continuous enrollment ≥6 months prior to and 18 months following ICT initiation were included. Outcomes included treatment discontinuation, persistence (i.e., refill gaps ≥6 weeks), and total healthcare costs.
Results:
The average age among 404 SCD patients meeting study inclusion criteria was 18.7 (±11.0) years, with 45.8% being males and 66.7% being Blacks. Switches or combinations from DFO at index occurred in 124 (74.7%) patients compared to 10 (4.2%) with DFX at index. The Cox regression model that assessed long-term medication persistence indicated a 1.30-times higher likelihood of treatment discontinuation with DFO compared to DFX (95% CI: 1.06–1.61). Some 19.7% of patient remained on DFX relative to 4.8% on DFO. Both inpatient and total costs were similar in DFX and DFO treatment groups. Following 1 year of treatment, 37.4% remained on DFX compared to 15.7% on DFO. Meaningful differences in treatment discontinuation between the two treatment groups did not occur until 220+ days during the study period. At 18-months, treatment discontinuation rates were high in both groups; 95% for DFO and 80% for DFX.
Conclusion:
This study of SCD Medicaid patients found more therapeutic switches from DFO to DFX and a higher medication persistency rate with DFX than DFO. The conclusions are limited by the study’s retrospective nature, which depends on multivariate statistics to account for patient heterogeneity and risk factors.
Introduction
Sickle cell disease (SCD) is an inherited disorder of the blood that leads to the production of an abnormal sickle hemoglobin and manifests as hemolysis and red blood cell rigidity and endothelial adhesion, wherein abnormal red blood cells may agglomerate and block blood from flowing to organs and tissuesCitation1,Citation2. Within the US, SCD is uncommon and has been recently reported to affect between 88,494–89,664 persons, of which 80,151 were Black and 8928 were HispanicCitation3. The total fees (charges) for 50 years of life expectancy for SCD patients have been estimated to be as much as $8.7 million per patientCitation4.
SCD obtained its name because the red blood cells assume an abnormal, rigid, sickle shape. The rigid, oddly-shaped red blood cells can obstruct capillaries and block blood flow to body tissues causing ischemia and pain. The key clinical manifestations of SCD include recurrent acute painful crises, fatigue, hemolytic anemia, and chronic organ damage, especially to the heart, lung, spleen, kidneys, bones, brain, skin, muscle, liver, and biliary tractCitation1,Citation2,Citation5. Hence, persons with SCD are subject to an increased prevalence of stroke, occlusive disease, acute chest syndrome, and immunologic or infectious conditionsCitation1,Citation6. The prevention of early complications from vaso-occlusive crises remains central to the treatment of SCDCitation7,Citation8. Although red blood cell transfusion therapy is recommended in persons with SCD that express anemic signs and symptoms from damage to their red blood cells, multiple red blood cell transfusions may produce iron overload from the iron content of the red blood cells, potentially leading to cellular damage and organ failureCitation2,Citation7–9.
SCD patients requiring blood transfusions may require more frequent hospitalizations than those patients not requiring transfusionsCitation10. Hospitalizations in SCD patients could be multifactorial in nature, with iron overload related sequelae being one of the reasons. A study by Fung et al.Citation10 reported that adult SCD patients requiring transfusions were 1.4-times more likely to be hospitalized than pediatric SCD patientsCitation11.
Iron-chelation therapies (ICTs) help to eliminate iron overload by binding with labile plasma iron to form non-toxic conjugates that can be safely excreted from the body. These treatments are often necessary in persons requiring long-term red-cell transfusions, including those with sickle cell disease, thalassemia major, or myelodysplastic syndromesCitation9. Specific guidelines and consensus statements for SCD explicitly address the need to use ICTs for transfusion-related iron overload and indicate that prophylactic therapy is generally preferred among those requiring chronic transfusionsCitation7–9. Deferoxamine (DFO) (Desferal®, Novartis) was approved by for use in the US in 1968 and is a parenterally-administered siderophore whose feroxamine complex is excreted primarily by the kidneys; it is most often administered via a portable pump for 8–10 h each day from 5–7 days per weekCitation9,Citation12. Approved in 2005, deferasirox (DFX) (Exjade®, Novartis) is an orally-administered chelator that forms a complex with plasma iron that is excreted in the bileCitation12. Due in part to its once-a-day dosing and oral administration route, research has found that DFX is associated with improved health-related quality-of-life (HRQoL), medication adherence, and patient satisfactionCitation13–15. A third chelating agent, deferiprone (Ferriprox®, Apotex; Kelfer®, Cipla), was recently approved in the US in 2011 for thalassemia major patients when the therapeutic response to current chelation therapy is inadequateCitation9,Citation16.
Limited empirical research has sought to investigate the long-term persistency of ICTs, and many initial investigations relied upon subjective measures of medication adherenceCitation15. Furthermore, little attention has focused in the scientific literature upon actual, real-world expenditures associated with both DFO and DFXCitation12,Citation15,Citation17–22. Given that patients with SCD that receive chronic blood transfusions require ICTs, good adherence (i.e., taking medications day-to-day as prescribed) is necessary to avoid morbidity and mortality associated with iron overload. As such, the purpose of this study was to evaluate ICT persistency (i.e., continuing treatment for the prescribed duration) and costs in Medicaid beneficiaries across 10 states in the US from 2006–2010.
Methods
Data
De-identified health claim records of Medicaid recipients from 2006–2010 that were present within the Thomson Reuters Medicaid Marketscan database were used in this studyCitation23. These multiyear longitudinal data, collected across 10 states in the US, include comprehensive hospital inpatient and outpatient medical claims, outpatient prescription drug claims, and Medicaid enrollment and eligibility information for ∼6 million beneficiaries.
Study sample
Patients included in this study had one or more diagnosis of SCD as identified by an International Classification of Disease, 9th edition, Clinical Manifestation (ICD-9-CM) code of 282.6x, two or more claims for ICT medications (i.e., DFO or DFX), and continuous enrollment for at least 6 months prior to ICT initiation through 18 months follow-up periodCitation24. Thus, the study’s index date was defined to correspond with first ICT prescription claim and the 6-month pre-index period was employed to measure relevant baseline patient characteristics. Although the study’s entire time frame spanned 5 years, subjects were included only if they were continuously eligible to receive Medicaid benefits for the 24 months study period (i.e., 6-months pre-index and 18-months post-index). Based on ICT exposure at the index date, patients were classified into two groups: patients treated with DFO (i.e., DFO use) and patients treated with DFX (i.e., DFX use).
Outcomes assessed
The principal outcomes analyzed within the current study included: (1) total direct healthcare costs associated with SCD-related complications from the perspective of the payer for the 18-month time period following ICT treatment initiation. Filtering of the data to identify patients with a list of specific SCD-related complications by ICD-9 codes for these complications was carried out; (2) persistence with ICTs was defined as time to discontinuation of treatment according to a 6-week or greater medication refill gap. Persistence was calculated by determining when ICT refill gap of 6 weeks or more occurred. To determine refill gaps, a medication possession ratio (MPR) was first measured according to Steiner and ProchazkaCitation25, defined as the total days of drug supply for each ICT, divided by the number of days between the first and last dispensing of the medication plus the days’ supply of last dispensing. The utilization of ICTs was captured using National Drug Classification codes (NDC) across both cohorts of DFO and DFX use; procedural codes (i.e., Current Procedural Terminology (CPT), Healthcare Common Procedure Coding System (HCPCS)) were also employed for DFO given its route of administrationCitation26. Other variables measured were age on the index date (including an age categorization of 18 years or older vs less than 18 years of age), gender, race/ethnicity (i.e., Black, Hispanic, White, other), number of transfusion episodes based upon the number of transfusion days observed, capitated Medicaid coverage (i.e., being in a healthcare system in which a medical provider is given a set fee per patient regardless of treatment required), Deyo-Charlson Comorbidity Index as a validated measure of co-morbid disease severity, 6-month pre-index baseline total healthcare costs, and other cost categories (i.e., hospitalization, outpatient, medication)Citation27,Citation28. Notably, the Deyo-Charlson Comorbidity Index assesses overall disease comorbidities and was not necessarily specific to SCD. Multiple transfusion claims occurring on a single day were counted only once to calculate number of transfusion episodes for each patient. Therefore, the actual number of transfusions may be larger than the number of transfusion episodes. ICT medication switches from DFO to DFX and vice versa were also assessed during the study period.
Statistical analysis
Analyses were presented overall and according to the cohorts of DFO and DFX use at baseline, including switches or combinations. As appropriate, parametric or non-parametric univariate statistical approaches including the paired-sample t-test, independent groups t-test, chi-square, Mann-Whitney U, two-sample Kolmogorov-Smirnov, or Wilcoxon Signed Rank test were used to initially assess unadjusted group differences.
A Kaplan-Meier curve was employed to graphically present crude treatment discontinuations, with a log-rank test for equality of survivor functions employed to assess group differences between DFO and DFX. Subsequently, a Cox proportional hazards regression model with the Efron method for ties was used to evaluate the risk of treatment discontinuation associated with DFO and DFX after controlling for covariates including age (i.e., 18 years or older), gender, Deyo-Charlson Comorbidity Index, number of baseline transfusion episodes, and capitated Medicaid coverageCitation29,Citation30. The outcome of healthcare costs in the 18-month study period following ICT treatment initiation was assessed via generalized linear models (i.e., gamma distribution family with log link) using maximum likelihood estimation and controlling for the aforementioned predictor variables plus baseline pre-ICT healthcare costsCitation29,Citation31. A post-hoc sensitivity analysis was additionally conducted for this gamma regression to include the number of continuous days of ICT medication use as an offset variable to adjust for potentially differential exposures. Results of both the Cox and gamma regressions yielded relative risk measures, denoted a hazard ratio or exp(b), respectivelyCitation31. Residual diagnostics were conducted to assess model fit for both the Cox regression (e.g., Schoenfeld and Martingale residuals) and the gamma regression (e.g., Anscombe residuals); deviances were also assessedCitation31. Statistical analyses were conducted using SAS 9.2 (Cary, NC), Stata IC 11.2 (College Station, TX), and IBM SPSS 19 (Somers, NY). Statistical significance for all inferential analyses was assessed using an a priori alpha level equal to 0.05.
Results
Overall, 404 Medicaid enrollees met the study’s inclusion criteria; complete baseline descriptive information is presented in . Across all individuals, the average age was 18.7 (±11.0) years, with 44.8% (n = 181) being 18 years of age or older. Males comprised 45.8% (n = 185), and 66.7% (n = 269) were Blacks. A majority (62.4%, n = 252) were enrolled within capitated Medicaid coverage plans. The DFO use group comprised 41.1% (n = 166) of the study participants vs 58.9% (n = 238) for the DFX use group. Transfusion episodes were found to differ between the DFO and DFX groups. The monthly number of blood transfusion episodes in the baseline period was 0.61 (±0.53) and 0.45 (±0.55) for the DFO and DFX group, respectively (p = 0.003), while blood transfusion episodes in the post index period averaged 0.64 (±0.54) and 0.49 (±0.57) per month for the two groups, respectively (p = 0.009). Individuals whose therapy was switched over time and received either a new treatment or combination comprised 33.2% (n = 134) of the sample, with 124 switches or combinations occurring with DFO at index (74.7% of n = 166) and 10 occurring with DFX at index (4.2% of n = 238). The average treatment duration across an 18-month study period was 6.5 (±4.8) months for DFO and 7.6 (±6.5) months for DFX (p = 0.041). Compared to the pre-ICT period, the number of transfusion episodes increased from 0.45 to 0.50 per month in the post-index period for the DFX group (p = 0.030).
Table 1. Baseline descriptive characteristics.
Persistency and treatment discontinuation analysis
Overall, long-term persistency was low in all patients. shows the Kaplan-Meier graph of treatment discontinuations. Bivariate analysis showed that treatment discontinuation significantly differed between DFO and DFX (p = 0.004), and meaningful differences did not appear to occur until over 7 months into the study period. After 12 months, 15.7% remained on DFO compared to 37.4% on DFX, further decreasing to 4.8% for DFO and 19.7% for DFX at 18 months. The median time of treatment discontinuation across all subjects was 182 days (mean = 214) and, according to treatment groups: 171 days (mean = 194) for the DFO-use cohort and 211 days (mean = 228) for the DFX-use cohort (p < 0.001).
Figure 1. Kaplan-Meier graph for treatment persistency between Deferoxamine use and Deferasirox use. †Defined as the drug begun at the index date. Time at risk = 86,336; Log rank test for equality of survivor functions p = 0.004.
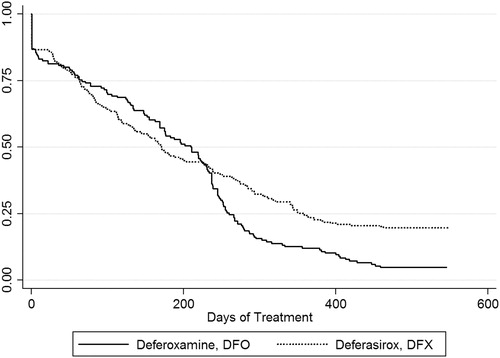
The Cox proportional hazards regression that assessed long-term treatment discontinuation after controlling for various predictors indicated a significant 1.305-times higher likelihood of treatment discontinuation with DFO compared to DFX (95% CI: 1.059–1.607, p = 0.012). Persons 18 years of age or older were significantly associated with a 1.304-times likelihood of treatment discontinuation (95% CI: 1.059–1.605, p = 0.013). Other predictors were not significantly associated with treatment discontinuation (i.e., number of transfusion episodes, male gender, capitated Medicaid coverage, Deyo-Charlson comorbidity index). The full results of the Cox regression are presented in .
Table 2. Predictors of treatment discontinuation.
Cost analysis
The average unadjusted total healthcare costs per member per month (PMPM) 6-month pre-ICT baseline and 18-month study period costs are presented in . Across the overall sample, average total pre-ICT costs were $4988 (±7592) PMPM, or $29,927 (±45,554) per patient during the 6-month baseline period. The average total study period costs were $6532 (±8575) PMPM, or $117,569 (±154,348) during the 18 month post-index study period. Compared to the pre-ICT period, mean monthly costs across the two cohorts during the 18-month treatment period increased, with the mean average PMPM difference being $554 in the DFO group (p = 0.010) and $2234 in the DFX group (p < 0.001). However, the unadjusted post-index PMPM costs between DFO and DFX of $5176 (±6219) and $7090 (±9812), respectively, did not statistically differ (p = 0.304).
Table 3. Baseline and study period per member per month (PMPM) unadjusted average costs according to treatment groups.
The gamma regression presented in found that the number of baseline pre-index transfusion episodes, aged 18 years or older, Deyo-Charlson comorbidity index, and baseline pre-ICT healthcare costs were significant predictors of total healthcare costs. The number of pre-index blood transfusion episodes was associated with higher costs by a factor of 1.028 (95% CI: 1.001–1.053, p = 0.021) higher costs. Age 18 years and above was associated with higher costs by a factor of 1.559 (95% CI: 1.308–1.859, p < 0.001). The Charlson Comorbidity Index, Deyo modification (a measure of comorbidities) was associated with higher costs by a factor of 1.100 (95% CI: 1.021–1.185, p = 0.012). Lastly, healthcare costs in the baseline period were associated with higher costs by a factor of 1.011 (95% CI: 1.001–1.014, p < 0.001). The use of different ICTs did not significantly impact overall costs. After controlling for transfusion episodes, age ≥18 years, gender, capitation, comorbidities, and baseline costs, the use of DFO vs DFX did not significantly differ with overall healthcare costs. Results of the post-hoc sensitivity analysis that controlled for the number of continuous days of ICT medication exposure across the 18-month study period as an offset variable yielded consistent results wherein no significant differences were noted in total healthcare costs between DFO vs DFX (exp(b) = 1.163, 95% CI: 0.632–2.138, p = 0.628). This post-hoc analysis was conducted, in part, due to the earlier observation that the unadjusted average treatment durations of DFO vs DFX were statistically different and, hence, suggestive of different exposures which could have been associated with changes in total healthcare costs.
Table 4. Predictors of total healthcare costs.
Discussion
Using a national database of Medicaid enrollees across 10 states in the US, this study assessed the 18-month persistency and direct costs associated with the use of DFO and DFX in the treatment of SCD. For the 404 beneficiaries that met the study’s inclusion criteria between 2006–2010, treatment discontinuation was high and statistically different at 95.2% for DFO vs 80.3% for DFX (p = 0.004). After controlling for several predictors (i.e., age category, transfusion episodes, gender, capitated Medicaid coverage, Deyo-Charlson comorbidity index), a Cox proportional hazard regression found DFO use to be significantly associated with a 1.305-times higher likelihood of treatment discontinuation relative to DFX (p = 0.012). These findings become particularly relevant when also considering results of the multivariate cost analysis, which indicated that no significant difference was present in total healthcare costs between DFO and DFX after controlling for various predictors. Rather, significant associations with total healthcare costs were found with baseline transfusion episodes, age category, baseline costs, and case-mix comorbid risk adjustment.
Although other authors have broadly investigated adherence to ICTs and costs of SCD based across various settings and populations, the current study extends these findings by assessing the comparative, real-world patterns of care surrounding the persistence and costs specifically associated with parenteral vs oral ICTs. Grosse et al.Citation32 articulated the importance of using large administrative datasets in addressing health services research for hemoglobinopathies, including SCD, particularly in the objective measurement of cost and resource utilization outcomes. To the authors’ knowledge, the current study represents the first investigation to also comprehensively evaluate real-world direct medical costs according to either DFO or DFX utilization.
Early investigations of ICT adherence were conducted using subjective physician observations or patient self-assessments. Therein, Gabutti and PigaCitation33 reported an average adherence of 64% for DFO over a 30-year time horizon, while Weissman et al.Citation34 reported that 62% of persons with thalassemia or SCD were adherent to DFO treatment. In more closely-related work comparing patterns of use with DFO and DFX, Trachtenberg et al.Citation35 measured adherence through patient self-reports and medical chart reviews from 2007–2009 among 265 individuals on DFO or DFX. Results for the short-term non-adherence at 1 month were low, at 8% for DFO and 3% for DFX, although these values worsened with age; comparative results for treatment discontinuations in the current study using claims data were 17% for DFO and 15% for DFX. Alvarez et al.Citation36 reported via pill counts and patient self-reports that 29% of 21 children with SCD using DFX were non-adherent with therapy after 1 year, while Raphael et al.Citation37 reported 24% non-adherence based upon a retrospective chart review of 59 children; the current study found high percentages of discontinuation at 84% for DFO and 63% for DFX after 1 year that continued to decrease by month 18. Based exclusively upon objective claims data, Jordan et al.Citation15 investigated Medicaid beneficiaries within three states and found median times to treatment discontinuation ranged from 86 days (any DFO patients) to 253 days (deferasirox switchers). Comparatively, the current study found the median time of treatment discontinuation across all subjects to be 182 days (mean = 214) and, according to treatment groups: 171 days (mean = 194) for the DFO-use cohort and 211 days (mean = 228) for DFX-use cohort (p < 0.001). Multivariate analyses within both Jordan et al.Citation15 and the current investigation consistently found that DFO was associated with statistically higher likelihood of treatment discontinuation relative to DFX after controlling for other predictors.
Several studies have investigated overall costs associated with SCD at both the national and state levelCitation4,Citation17,Citation38–40. Pertaining to medication use, however, Delea et al.Citation20 studied DFO use alone for chelation due to iron overload from repeated blood transfusions in 39 thalassemia and 106 SCD patients (which included 17 Medicaid enrollees) using a large US health insurance claims database of 40 million members in over 70 health plans from 1997–2004. Average annual total medical costs in SCD patients were reported to be $59,233 ($4936 per month) ($90,044, $7504 per month, USD 2011), with DFO plus additional costs for ICT administration averaging $19,621 per year ($1635 per month) ($29,827, $2486 per month, USD 2011); no multivariate analyses were presented by Delea et al.Citation20 to ascertain the association of DFO with costs after controlling for other predictors. Mvundura et al.Citation41 and Amendah et al.Citation42 reported that medication claims among children enrolled in Medicaid were higher than those enrolled in commercial insurance plans at $1049 vs $531 ($1288 vs $652, USD 2011), although average total expenditures were lower in Medicaid at $11,075 vs $14,722 ($13,601 vs $18,081, USD 2011), despite a similar number of outpatient blood transfusionsCitation41,Citation42. In descriptive terms, the current study found annualized total overall medical costs of $78,384 ($6532 per month) ($85,839, $7153 per month, USD 2011). Therein, combined annualized medication plus outpatient costs were $23,640 ($1970 per month) ($25,888, $2157 per month, USD 2011) for the DFO-only cohort vs $36,240 ($3020 per month) ($39,687, $3307 per month, USD 2011) for the DFX-only cohort. Although the lower descriptive cost observation for DFO vs DFX in the current investigation may have been driven by its significantly higher treatment discontinuation rate, no significant difference in total cost was ultimately found between the two ICTs after controlling for various predictors in the multivariate analysis.
The present investigation allowed for actual patterns of care to be analyzed, focusing upon naturalistic settings (i.e., effectiveness) rather than clinical trial settings (i.e., efficacy)Citation43. However, this study was retrospective in nature and patients were not randomized to either DFO or DFX like they are in clinical trials. Hence multivariate statistics were used to account for patient heterogeneity and risk factorsCitation29,Citation31. Despite this, several limitations must be noted. Foremost, severity of SCD could not be determined given the retrospective and administrative nature of the national database. While an 18-month treatment horizon was studied, this time frame may be insufficient to measure long-term effectiveness of either ICT or SCD complications. As the population being evaluated were Medicaid enrollees, any reimbursement restrictions that might have limited the use of ICT treatment from 2006–2010 were not assessed due to lack of specific location-related information for each patient. Furthermore, while total direct healthcare costs were measured from the perspective of Medicaid, results may not be generalizable other patient populations or health systems that utilize different coverage mechanisms. In addition, the results from this analysis may not be generalizable to other countries with different health system structures and costs. The medication costs presented in this analysis included all medication use and was not restricted to ICT alone. Finally, the use of a 6-month pre-index time period without ICT use may have introduced selection bias. While the strength of this approach was that it identified new starts or new restarts on ICT, it would have excluded patients that were already adherent with ICT. Another strength of this approach is that the 6-month pre-index period allowed baseline data to be collected that was then used to control for patient differences (e.g., baseline costs) in the multivariate analysis, which increased internal validity.
The implications of the current study are particularly important when considering the role of payers and providers in a public health contextCitation44,Citation45. The economic, clinical, and humanistic impact of iron overload may become substantial and poor adherence to ICTs may contribute to increased morbidity, health-related quality-of-life, and costsCitation37,Citation46–48. The long-term ramifications of non-adherence to ICTs in persons with iron overload may manifest in clinical complications, particularly hepatic failure, iron-induced cardiomyopathy, or pancreatic iron depositionCitation9. Based upon cost-effectiveness and cost-utility analyses, DFX has been reported to be cost-effective relative to DFO in the treatment of transfusional iron overload from the perspective of payers both in the US and UKCitation12,Citation17–22. These models, however, assumed high compliance across patient populations, which do not appear to correspond to actual patterns of careCitation12. It is also important to note that persons with SCD have reported significant barriers in access to healthcare, particularly those associated with delays in treatmentCitation49. Predictors of non-adherence in SCD remain complex and multifaceted and current suggestions state that an integrated team approach involving healthcare providers, family caregivers, and treatment centers be utilized to achieve optimal results with chronic therapiesCitation35,Citation48.
Overall, the current findings suggest that payers and providers should review the care of SCD patients with a goal of identifying those that may have prematurely discontinued ICT treatment. Approximately 5% remained on DFO vs 20% for DFX after 18 months in this investigation. Despite this, overall healthcare costs did not differ between DFO vs DFX. As persons with SCD may alter the utilization of their medications for several reasons, future research should be directed toward identifying and implementing multifaceted patient-centered programs to optimize long-term persistency and outcomes.
Conclusion
The investigation of real-world ICT utilization patterns among Medicare beneficiaries with SCD across 10 states from 2006–2010 in the US found that ∼95% of individuals discontinued use of DFO and 80% of individuals discontinued use of DFX 18-months following treatment initiation. After multivariate analyses controlled for various predictors, total healthcare costs did not differ between DFO and DFX, even though treatment discontinuation was 1.305-times higher with DFO. Public health concerns surrounding patient persistency with ICTs places a responsibility on payers and providers to evaluate drug utilization patterns in order to identify poor persistence and risk of discontinuation in this vulnerable population. The results from this analysis may not be generalizable to other countries or health systems.
Transparency
Declaration of funding
This study was funded by Novartis to Strategic Therapeutics, LLC.
Declaration of financial/other relationships
Drs Armstrong and Skrepnek are consultants to Novartis through Strategic Therapeutics, LLC. Drs Sasane and Snodgrass are employees of Novartis. Dr Ballas is a collaborator and a consultant for this study.
References
- Saunthararajah Y, Vichinsky EP. Sickle cell disease - clinical features and management. In: Hoffmann R, Benz EJ, Shattil SS, eds. Hematology: basic principles and practice. 5th ed. Philadelphia, PA: Elsevier Churchill Livingstone, 2008. p 577–601
- Roseff SD. Sickle cell disease: a review. Immunohematology 2009;25:67–74
- Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol 2010;85:77–8
- Ballas SK. The cost of health care for patients with sickle cell disease. Am J Hematol 2009;84:320–2
- Redding-Lallinger R, Knoll C. Sickle cell disease–pathophysiology and treatment. Curr Probl Pediatr Adolesc Health Care 2006;36:346–76
- Strouse JJ, Jordan LC, Lanzkron S, et al. The excess burden of stroke in hospitalized adults with sickle cell disease. Am J Hematol 2009;84:548–52
- Institute NHLaB. The management of sickle cell disease. 2002. http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf, accessed April 4, 2012
- Society SC. Standards for the clinical care of adjust with sickle cell disease in the UK. 2008. http://www.sicklecellsociety.org/app/webroot/files/files/CareBook.pdf, accessed April 4, 2012
- Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med 2011;364:146–56
- Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: a report from the multi-center study of iron overload. Am J Hematol 2007;82:255–65
- Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Semin Hematol 2001;38(1 Suppl):30–6
- Yang LP, Keam SJ, Keating GM. Deferasirox: a review of its use in the management of transfusional chronic iron overload. Drugs 2007;67:2211–30
- Cappellini MD, Bejaoui M, Agaoglu L, et al. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with beta-thalassemia. Clin Ther 2007;29:909–17
- Vichinsky E, Pakbaz Z, Onyekwere O, et al. Patient-reported outcomes of deferasirox (Exjade, ICL670) versus deferoxamine in sickle cell disease patients with transfusional hemosiderosis. Substudy of a randomized open-label phase II trial. Acta Haematol 2008;119:133–41
- Jordan LB, Vekeman F, Sengupta A, et al. Persistence and compliance of deferoxamine versus deferasirox in Medicaid patients with sickle-cell disease. J Clin Pharm Ther 2012;37:173–81
- Betito E. ApoPharma announces FDA advisory committee recommendation in favor of Ferriprox® (deferiprone) approval. 2011. http://www.apotex.com/global/about/press/20110914.asp, accessed April 4, 2012
- Zhang B, Donga PZ, Corral M, et al. Pharmacoeconomic considerations in treating iron overload in patients with beta-thalassaemia, sickle cell disease and myelodysplastic syndromes in the US: a literature review. Pharmacoeconomics 2011;29:461–74
- Imran F, Phatak P. Pharmacoeconomic benefits of deferasirox in the management of iron overload syndromes. Expert Rev Pharmacoecon Outcomes Res 2009;9:297–304
- Delea TE, Edelsberg J, Sofrygin O, et al. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. Transfusion 2007;47:1919–29
- Delea TE, Hagiwara M, Thomas SK, et al. Outcomes, utilization, and costs among thalassemia and sickle cell disease patients receiving deferoxamine therapy in the United States. Am J Hematol 2008;83:263–70
- Delea TE, Sofrygin O, Thomas SK, et al. Cost effectiveness of once-daily oral chelation therapy with deferasirox versus infusional deferoxamine in transfusion-dependent thalassaemia patients: US healthcare system perspective. Pharmacoeconomics 2007;25:329–42
- Kamon J, Akehurst RL, Jewitt K, et al. Cost utility analysis of deferasirox versus deferoxamine (Desferal) for patients requiring iron chelation therapy in the United Kingdom. Haematologica 2007;92(1 Suppl):222–3
- Reuters T. MarketScan research databases. 2012. http://thomsonreuters.com/products_services/healthcare/healthcare_products/a-z/marketscan_research_analytics, accessed July 2, 2012
- Software A. 2012 ICD-9-CM Diagnosis Code 282.6. 2012. http://www.icd9data.com/2012/Volume1/280-289/282/282.6.htm, accessed July 2, 2012
- Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol 1997;50:105–16
- Services CfMM. Healthcare Common Procedure Coding System (HCPCS). 2012. https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/index.html?redirect=/MedHCPCSGenInfo/, accessed July 2, 2012
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9
- Skrepnek GH, Olvey EL, Sahai A. Econometric approaches in evaluating costs and outcomes within pharmacoeconomic analyses. Pharm Policy Law 2012;14:105–22
- Hertz-Picciotto I, Rockhill B. Validity and efficiency of approximation methods for tied survival times in Cox regression. Biometrics 1997;53:1151–6
- Skrepnek GH. Regression methods in the empiric analysis of health care data. J Manag Care Pharm 2005;11:240–51
- Grosse SD, Boulet SL, Amendah DD, et al. Administrative data sets and health services research on hemoglobinopathies: a review of the literature. Am J Prev Med 2010;38(4 Suppl):S557–S67
- Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol 1996;95:26–36.
- Weissman L, Treadwell M, Vichinsky E. Evaluation of home desferal care in transfusion-dependent children with thalassemia and sickle cell disease (SCD). J Pediatr Hematol Oncol 1996;18:454–5
- Trachtenberg F, Vichinsky E, Haines D, et al. Iron chelation adherence to deferoxamine and deferasirox in thalassemia. Am J Hematol 2011;86:433–6
- Alvarez O, Rodriguez-Cortes H, Robinson N, et al. Adherence to deferasirox in children and adolescents with sickle cell disease during 1-year of therapy. J Pediatr Hematol Oncol 2009;31:739–44
- Raphael JL, Bernhardt MB, Mahoney DH, et al. Oral iron chelation and the treatment of iron overload in a pediatric hematology center. Pediatr Blood Cancer 2009;52:616–20
- Nietert PJ, Silverstein MD, Abboud MR. Sickle cell anaemia: epidemiology and cost of illness. Pharmacoeconomics 2002;20:357–66
- Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol 2009;84:323–7
- Lanzkron S, Haywood C, Jr Segal JB, et al. Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. Am J Hematol 2006;81:927–32
- Mvundura M, Amendah D, Kavanagh PL, et al. Health care utilization and expenditures for privately and publicly insured children with sickle cell disease in the United States. Pediatr Blood Cancer 2009;53:642–6
- Amendah DD, Mvundura M, Kavanagh PL, et al. Sickle cell disease-related pediatric medical expenditures in the U.S. Am J Prev Med 2010;38(4 Suppl):S550–6
- Skrepnek GH. Cost-effectiveness analysis. In: Bootman JL, Townsend RJ, McGhan WF, eds. Principles of Pharmacoeconomics. 3rd ed. Cincinnati, OH: Harvey Whitney Books Company, 2005. p 82–116
- Yusuf HR, Lloyd-Puryear MA, Grant AM, et al. Sickle cell disease: the need for a public health agenda. Am J Prev Med 2011;41(6 Suppl 4):S376–83
- Hulihan MM, Sayers CA, Grosse SD, et al. Iron overload: what is the role of public health? Am J Prev Med 2011;41(6 Suppl 4):S422–7
- Payne KA, Rofail D, Baladi JF, et al. Iron chelation therapy: clinical effectiveness, economic burden and quality of life in patients with iron overload. Adv Ther 2008;25:725–42
- Abetz L, Baladi JF, Jones P, et al. The impact of iron overload and its treatment on quality of life: results from a literature review. Health Qual Life Outcomes 2006;4:73
- Porter JB, Evangeli M, El-Beshlawy A. Challenges of adherence and persistence with iron chelation therapy. Int J Hematol 2011;94:453–60
- Boulet SL, Yanni EA, Creary MS, et al. Health status and healthcare use in a national sample of children with sickle cell disease. Am J Prev Med 2010;38(4 Suppl):S528–35
