Abstract
Objectives:
Two anti-cancer drugs are currently approved for the treatment of HER2-positive metastatic breast cancer (MBC): trastuzumab-based therapy (TBT) administered intravenously as first line therapy until disease progression and lapatinib, an oral self-administered dual therapy with capecitabine (L+C) as second intention for patients who continue to progress despite TBT. In current practice, TBT is still prescribed beyond disease progression. In addition to medical reasons, the difficulty to switch eligible patients to oral drugs may also be explained by economic reasons. Thus, we aimed at comparing the budgetary impact of TBT and L+C for progressing HER2+MBC after TBT from the French Health Insurance perspective.
Methods:
A budget impact analysis was performed on a 3-year time horizon (2012–2014) to simulate a dynamic cohort of 4182 HER2-positive patients with a progressing MBC treated with TBT (73%) and L + C (27%). The model was adjusted on progression-free survival (PFS). Office visits, clinical evaluations, drug acquisition, administration costs, and transportation costs obtained from the literature and published databases were considered.
Results:
In the base case analysis (2012), the annual treatment cost per patient for TBT (€36,077) was 2-times higher than that of L + C (€17,165). Using L + C for all patients (n = 4182) would avoid €34.8 million of drug administration and transportation costs. Hospital costs represented 1% vs 88%, while community costs represented 99% vs 12% of L + C and TBT treatment costs, respectively. The lack of direct comparison PFS and treatment dosage modification data were the main limitations. However, no major changes from baseline results were observed from sensitivity analyses.
Conclusions:
Despite a slightly higher acquisition cost, the treatment cost of L + C remains lower than that of TBT, and it is the only approved anti-HER2 treatment for HER2-positive patients with progressing MBC. Based on this, it seems important to consider the potential savings for Health Insurance with the use of oral drug due to the reduction of outpatient hospitalizations. Such reductions may result in a subsequent budget reduction for hospitals, but may also provide those facing acute medical activity with opportunities to better manage other diseases whose treatment cannot be externalized.
Introduction
Breast cancer (BC) is the most frequent tumor in women. Worldwide, 1.38 million new cases of BC occur each year, with more than 400,000 deathsCitation1. France registers one of the highest incidence rates of BC in Europe, with more than 50,000 new cases per year and with a 22% mortality rateCitation2. Approximately 20% of patients with BC over-express the Human Epidermal growth factor Receptor 2 (HER2-positive)Citation3, a protein causing higher tumor aggressiveness and risk of developing metastasesCitation4.
Two drugs are currently approved for the treatment of HER2-positive metastatic breast cancer (MBC). Tratuzumab (Herceptin; a registered trademark of Roche, Roche Registration Limited, UK), a monoclonal antibody administered by intravenous route was approved by the Food and Drug Administration (FDA, 1998) and the European Medicines Agency (EMA, 2000) as a monotherapy or in combination with cytotoxic agents (i.e., paclitaxel, docetaxel) or an aromatase inhibitorCitation5. It has been observed that trastuzumab continues to be widely used by clinicians even after disease progression as off-label drug useCitation6,Citation7, and has been adopted as a standard of care by many cliniciansCitation8. Some studies have shown that continuing with trastuzumab-based therapy (TBT) beyond progression may improve survival without substantial increase in cardiotoxicityCitation7,Citation9,Citation10. Ten years after and from the EGF100151 trial resultsCitation11, the FDA (2007) and EMA (2008) granted market authorizations for lapatinib (Tyverb; a registered trademark of GlaxoSmithKline, Glaxo Group Ltd, UK), an oral small-molecule inhibitor of the HER2/ErbB2 tyrosine kinase receptor for use in combination with capecitabine (Xeloda; a registered trademark of Roche) (L + C) in patients with metastatic disease progression who have received prior therapy including an anthracycline, a taxane, or trastuzumab. According to guidelines, upon disease progression on first line therapy, metastatic patients should be switched to lapatinibCitation12.
Since lapatinib became available, it may be suggested that other reasons than the clinical efficacy contribute to explain the current dilemma of switching to lapatinib or continuing with trastuzumab. This issue highlights the current debate on the competitive access of drugs available both in oral and intravenous forms and which can be explained by medical as well as economical reasonsCitation13. Despite patients’ preference for the oral route of chemotherapy administrationCitation14, there are several barriers to the use of oral anti-cancer drugs (OAD) for patients as well as for healthcare providers. Explanation of therapeutic protocol, control of treatment adherence, and monitoring of side-effects, therapeutic education, and phone monitoring to assist patients in the self-management of their disease require additional time which is currently not taken into consideration in the per-case payment of hospital activities in most countriesCitation15. In the current French healthcare system, the prescription of OAD is based on physician fees for outpatient visits, while the administration of intravenous anti-cancer drugs is performed through outpatient hospitalizations that generate medical activities for hospitals and therefore a higher income. The per-case payment system of medical activities and the organization of cancer care centered on hospitals may actively contribute to reducing the patient access to OAD. In the US, beneficiaries of the Medicare insurance program (MIP) may also face issues regarding their access to OAD, since there is no full parity between oral and intravenous reimbursement. The reimbursement of OAD is limited to a restrictive list of OAD with intravenous equivalence covered by the Medicare standard insurance. Since the adoption of the Medicare Prescription Drug Improvement And Modernization Act (2003), the beneficiaries of the MIP are provided with an annual extra-coverage ($2850 covered with a 5% copay) provided by private insurers in order to better cover the cost of drugs. Despite this measure, patients still have to face various out-of-pocket expenses depending on their private insurances which prevent those with low income to be treated with OADCitation16,Citation17. As in the French healthcare system, the US healthcare plan does not compensate additional time spent to manage compliance and adverse side-effects for OAD which are self-administered in a community setting. This may contribute to explain the difficulty for hospitals to switch eligible patients from an intravenous to an oral route of chemotherapy administration.
Furthermore, studies have drawn attention to the high acquisition cost of OADCitation18,Citation19. In a global context of rationalization of healthcare expenditures and optimization of healthcare organization due to higher economic constraints, it appears relevant to analyze the real impact of medical strategies. A budget impact model (BIM) is a useful tool routinely used by insurers as well as by providers to simulate the financial impact of introducing a new drug and contributing to the health technology assessment in pre-market authorization. Nonetheless, BIM may also be a relevant tool in post-market authorization in order to review the budgetary impact of health technologies and to highlight the economic issues related to their use in real-world medical practiceCitation20.
To our knowledge, no study about the budget impact of oral vs intravenous anti-cancer drugs in HER2-positive MBC based on the medical practice has been published. Therefore, we attempted to fill this gap by measuring the healthcare costs of L + C compared to TBT in the treatment of progressing HER2-positive MBC from the French Health Insurance perspective. As a secondary objective, we aimed at estimating the preventable hospitalization and transportation costs and the cost transfer from the hospital sector to the community (i.e., out-of hospital sector) induced by the use of L + C.
Methods
Purpose of the model
A budget impact model was developed to compare the cost of treatment with TBT beyond disease progression (scenario 1) to that of L + C (scenario 2) from the second-line therapy of HER2-positive MBC. We thought this comparison was appropriate since it reflects the current medical practice in France where most patients continue to be treated with TBT despite disease progression. The model framework was performed following the published guidelines on budget impact analysesCitation21 and was developed using an Excel spreadsheet. Guidelines on the management of breast cancer were analyzed and semi-directive interviews with healthcare professionals (oncologist, nurse, and pharmacist) have been conducted to describe the treatment pathways in real practice and to validate assumptions on healthcare resource use for each scenario.
Perspective and time horizon
The model was developed to analyze the 3-year projected budget impact (2012–2014) of TBT vs L + C from the perspective of the French National Health Insurance. A prospective perspective was considered relevant with regards to the evolution of important parameters such as the target population growth, the evolution of market share, and the hospital setting. This time horizon and not a longer one was considered relevant for the analysis regarding the fast evolution of anti-cancer drugs and treatment patterns in oncology.
Patient population
The baseline target population of the model was defined as all patients with a progressive HER2-positive MBC who were previously treated with chemotherapy or trastuzumab in the metastatic setting and who may initiate a second line therapy and more. We included in the analysis patients who required second line therapy and, of those, patients who had progression-free survival (PFS) without major side-effects. The drop-out rate was applied to the baseline target population and was determined from the use of progression-free survival data and toxicities described below. The prevalence of MBC was estimated to be 43,707 patients from a preliminary ad hoc analysis of the French national hospital database (PMSI database, 2010)Citation22. The proportion of HER2-over-expression was assumed to be 20% in the metastatic setting based on expert opinion. Thus, the prevalence of HER2-positive MBC was estimated to be 8741 patients, of whom 92% were assumed to be previously treated with chemotherapy and trastuzumab in the first metastatic line (CancerMPact®, Patient Metrics, KantarHealth, 2010). The distribution of these patients among first metastatic line and second metastatic line and more was estimated to be 48% and 52%, respectively, from expert opinion (French Transparency Committee Opinion on lapatinib, July 2008)Citation23. Therefore, the baseline target population of the model could be estimated to 4182 patients. Each year of the time horizon of the model simulates a cohort of new patients diagnosed and treated for their disease. We applied a 1.1% annual growth rate to the baseline target population of the model in order to take into account the growing incidence of cancer over the period 2012–2014 (i.e., 4182, 4228, and 4275 patients in 2012, 2013, and 2014, respectively) (CancerMPact®, Patient Metrics, KantarHealth 2010).
Structure of the model and general parameters
The model took into account the distribution of the target population among treatments (TBT vs L + C) and the type of hospital (public or private), the growth rate of the target population over 2012–2014 and the discontinuation rate between each cycle of chemotherapy due to treatment efficacy, toxicity, or death (i.e., length of treatment). The general parameters of the model are summarized in .
Table 1. Epidemiological parameters and healthcare resources use included in the model (base case value and Min–Max values used for sensitivity analyses).
Treatment modalities
Scenario 1 simulated a cohort of patients treated with TBT during a 1-day hospital stay which accounts for 91.5% of chemotherapy sessions (PMSI database, 2010). Scenario 2 simulated a cohort of patients treated with L + C based on a self-administration of treatment at home. The allotment of the target population between the two scenarios (73% and 27% for Scenario 1 and 2, respectively) was based on unpublished market data (KantarHealth). In the HERMINE study (n = 623), an observational study evaluating the use of trastuzumab beyond progression; trastuzumab was used in combination with chemotherapy in 94% of cases, mostly with paclitaxel, vinorelbine, and docetaxelCitation24. These drug combinations had no influence on the cost of treatment because the cost of paclitaxel, vinorelbine, and docetaxel (since March 1st 2012) is included in the cost of chemotherapy administration sessions.
We also introduced a variation regarding the evolution of the distribution of patients between the two scenarios, based on the assumption of an increasing number of patients treated with OAD, while the number of patients treated with intravenous chemotherapy may be expected to decrease over the time horizon of the model. This assumption was drawn from a report of the French National Cancer Institute which estimated that the sales associated with OAD increased from €16 million to €382 million over 2000–2008Citation2Citation5. This trend is not expected to fade away since experts estimated that 25% of anti-cancer drugs under development will be available in oral formCitation17. Finally, the growth of the target population was taken into account over the period 2012–2014 and based on the assumption of a 1.1% annual growth (CancerMPact®, Patient Metrics, KantarHealth 2010) which is consistent with the increasing incidence of breast cancer.
Hospital setting
The percentage of patients treated with chemotherapy during a 1-day hospital stay among public and private hospitals was introduced in the model to take into account the variability of costs between public and private sectors. The distribution rate was determined from the PMSI database analysis: 68% and 32% for public and private hospital, respectively. The evolution of the hospital setting over 2012–2014 was also considered, since the number of chemotherapy sessions is growing in the public sector, while it has been decreasing in the private sector over the period 2006–2010 (PMSI database, 2010). The evolution which was observed between 2006–2010 was extrapolated to the period 2010–2014 from the assumption of a linear trend.
Length of cycles and treatment discontinuation rate
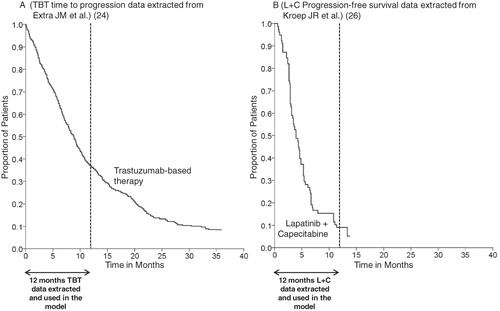
A total of 17 cycles of chemotherapy were considered over a year. The length of a chemotherapy cycle was set to 21 days corresponding to one-cycle of L + C and to three doses of TBT. The model was adjusted on drop-out rate between each cycle due to disease progression, toxicity inducing a change or cessation of therapeutic protocol, and death (i.e., dynamic cohort). From the baseline target population, only patients with stable disease (i.e., PFS) were analyzed, considering that patients with disease progression, those who died, or those presenting major side-effects are no longer treated with the therapies under evaluation (i.e., TBT and L + C). We applied a discontinuation rate to the baseline target population which was calculated from the PFS data and toxicity. The indirect comparison of PFS data was drawn from published survival analysis on patients with HER2-positive advanced breast cancer treated with L + CCitation26 or TBTCitation24. We selected data from observational studies since they reflect the effectiveness of treatment in real-life practice contrary to clinical trials where efficacy, tolerance, and treatment adherence may be over-estimated compared to those in real-life settings, due to the enhanced monitoring of patients. The PFS data for TBT were extracted without any additional adjustment for toxicity, since Extra et al.Citation24 did not mention any unexpected toxicity in their study. The PFS data for L + C were extracted from Kroep et al.Citation26 and were adjusted for major toxicity because toxicity resulted in withdrawal in 4% of patients from the study. These withdrawals were in addition to those who developed disease progression. Hence, discontinuation due to toxicity was only applied to the L + C arm, and we assumed that discontinuation due to toxicity in the TBT arm was included in the progression discontinuation rate ().
Figure 1. Data extracted from literature and used in the model for the treatment discontinuation calculations of each treatment over one-year. Time to progression (TTP*) data for trastuzumab-based therapy (TBT) (A) and progression-free survival (PFS**) data for lapatinib in combination with capecitabine (L + C) (B) were respectively extracted from a French observational study (24) and a Dutch Expanded Access Program (26). The median TTP was 8.6 months for TBT (40 months follow-up) and the median PFS for L + C was 4.2 months (15 months follow-up) and are not directly comparable. * TTP was defined as time from treatment initiation to progression or disease-related death; ** PFS was defined as time from treatment initiation until disease progression or death from any cause.
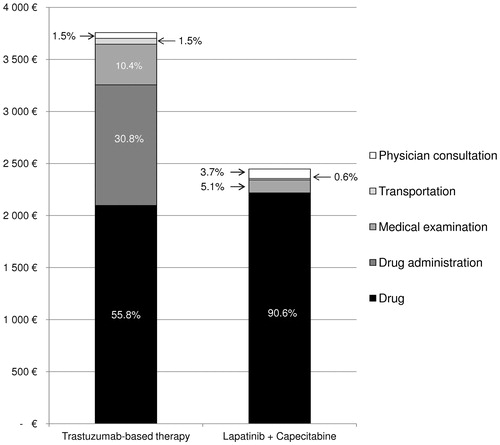
Healthcare resource use and costs estimation
The analysis included both direct medical costs and non-medical costs (i.e., transportation costs) (). The use of healthcare resources per cycle included a first specialist consultation for treatment prescription (i.e., outpatient physician consultation), specialist and general practitioner (GP) consultations for follow-up, clinical examinations before treatment initiation and for monitoring (i.e., complete blood cell count, hepatic function test, left ventricular ejection fraction, echocardiogram, blood ionogram), treatments, chemotherapy sessions, and medical transportation. Physician consultation fees were drawn from the published conventional feesCitation27,Citation28; clinical examination costs were estimated from the French Nomenclature of Medical Biology Procedures (NABM)Citation29 and the Common classification of medical acts (CCAM)Citation30. Treatment costs were calculated on the basis of unitary cost from the published drug database (BDM, Base des Médicaments et Informations Tarifaires)Citation31. The mean body surface area (BSA) reported in the literature was used for calculating the medication costs according to the recommended dosing schemesCitation32. In the base case analysis, the cost of TBT was based on trastuzumab given weekly with a first loading dose (4 mg/kg) and then subsequent weekly doses (2 mg/kg)Citation33. The cost for L + C was based on lapatinib (1250 mg/day) given continuously in combination with capecitabine (1000 mg/m2) given twice daily for 2 weeks in a 3-week cycle (21 days)Citation12. Cost for transportation was drawn from a publication presenting data from the Health Insurance database on the costs associated with chronic diseases in a French regionCitation34 and was up-dated to take into account the inflation rate over the period 2003–2012Citation3Citation5. Finally, the cost of managing the most frequent adverse events such as nausea and vomiting were not included in the analysis since they were deemed similar in both scenarios.
In this analysis, hospital costs included oncologist’ outpatient consultations (for public hospitals only), drugs, and outpatient hospitalizations for chemotherapy administration. Community costs included medical examinations (i.e., biological and physical exams were assumed to be performed in private laboratories), GP consultations, oncologists’ outpatient consultations (for private hospitals only), and drugs delivered by private pharmacies. Hospital costs are indirectly financed by the Health Insurance because they are firstly supported by the budget of hospitals and then reimbursed by the Health Insurance, while the community costs are directly reimbursed by the Health Insurance to the patient (anti-cancer drugs are fully reimbursed to the patient).
Sensitivity analyses
One-way deterministic sensitivity analyses were performed on the base-case analysis (for 2012) for parameters that could influence the results of the model, from ranges observed in the published literature or based on expert opinion: the total annual cost for the entire study population (i.e., rate of HER2-over-expression) and on the total annual cost of scenario 1 and scenario 2 (i.e., average weight and mean BSA, administration frequency of TBT, percentage of patients treated with L + C, and PFS data for patients treated with L + C). For the base case analysis, we used a rate of 20% of HER2-over-expression, but this proportion varies from 13–25% in the literature and may have an impact on the overall healthcare costsCitation3. The variation of BSA and weight may also have an impact since the dosage of drug is based on BSA and weight. Trastuzumab may be alternatively given every 3 weeks in a metastatic setting that may reduce outpatient hospitalization and transportation costs. The sensitivity analysis on progression-free survival for lapatinib-based therapy was carried out with clinical trial data from Geyer et al.Citation11 which reported a higher PFS, more comparable to data used for TBT, and that could result in a higher cost for the L + C scenario. Finally, we performed a sensitivity analysis on the percentage of patients treated with L + C since OAD are assumed to substantially reduce overall healthcare costs.
In light of current debates on alternative cancer care and valuation of hospital activities related to OAD, we also conducted exploratory sensitivity analyses. Such analysis was carried out on the cost of chemotherapy administration which varies according to the type of hospitalization (i.e., home hospitalization or cancer care network) (scenario 1). Chemotherapy sessions are mostly performed during 1-day hospital stays, but some are also performed during home hospitalizationCitation36 or in cancer care networksCitation37, and could potentially reduce the cost of the intravenous chemotherapy administration. This proportion can vary widely from one hospital to another depending on the policies applied in each institution. These types of care management could increase in the coming years. A second exploratory sensitivity analysis was performed on the 1-year cost of treatment of scenario 2 by adjusting the consultation fees to the longer time required for consultation (i.e., fictive consultation fees) and by integrating a fictive annual tariff for patient support provided by hospital staff members (i.e., therapeutic education, phone monitoring)Citation13. This analysis was assumed to be relevant with regards to the current debate on the lack of compensation of additional time spent to manage patients treated with OAD.
Results
Base case analysis
Budget impact of trastuzumab-based therapy vs lapatinib-based therapy
In the base case analysis (2012), on average the 1-year cost of treatment per patient, including administration and non-drug costs, was for TBT twice as much (€36,077) as that of L + C (€17,165). The total annual cost for patients treated with TBT (n = 3053) was €110,137,855, while it was estimated to €19,381,278 for patients treated with L + C (n = 1129) in 2012. Thus, the difference between the total cost of TBT (€110,137,855) and L + C (€19,381,278) was estimated to €90,756,577 and corresponds to the cost associated with the off-label use of TBT beyond disease progression in the population, since L + C is the only therapy currently approved for the treatment of progressing HER2-positive MBC after TBT in metastatic settings. Assuming that the market share of L + C increases linearly over the time horizon of the model, the total 1-year cost for the whole study population (n =4182) decreased from €129,519,134 to €116,790,315 over the period 2012–2014 (i.e., from €110,137,855 to €82,599,702 for TBT and from €19,381,278 to €34,190,613 for L + C). This pattern would represent a potential savings of €12,728,819 for the Health Insurance over the 3-year time horizon ().
Table 2. Results of the 3-year budget impact for the whole study population in Euros (n = 4182).
Cost savings and costs transfer between the hospital and the community sectors
The use of oral chemotherapy instead of intravenous chemotherapy in the study population would avoid 25,357 outpatient hospitalizations for chemotherapy administration, representing cost savings of €9,056,050 in the base case analysis (2012). The use of oral chemotherapy would also avoid a cost of transportation of €351,815. Hospital costs and community costs accounted for 1% and 99% of the treatment cost of L + C, while they accounted for 88% and 12% of the treatment cost of TBT. The relative comparison of cost differences in cost components between each scenario shows that the acquisition cost of L + C was higher than that of TBT, but the total cost of L + C was offset by the absence of drug administration cost and by fewer transportation costs. The budget impact analysis of TBT compared to L + C highlights that TBT have a lower budget impact in terms of physician consultation and acquisition drug compared to L + C ().
Sensitivity analysis
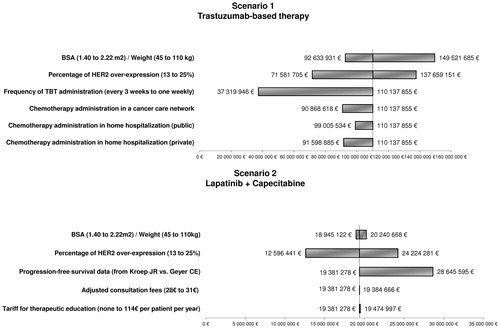
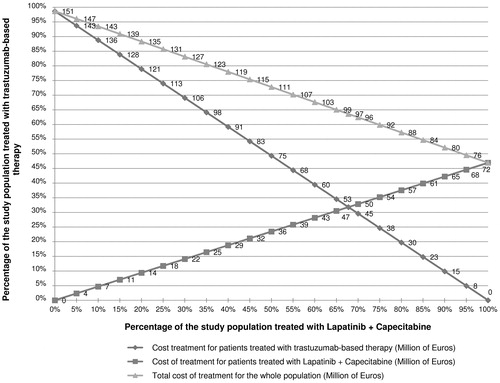
When the BSA (1.40–2.22) and weight (45–110) were altered, the total cost of TBT varied between €92,633,931 and €149,521,685, while the cost of L + C varied between €18,940,548 and €20,243,175. When the HER2-over-expression varied from 13–25%, the total cost of the whole population varied between from €84,178,146 to €161,883,432. The administration of trastuzumab every 3 weeks instead of a weekly administration would reduce the total treatment cost for TBT by 66%. The use of PFS data from Geyer et al.Citation11 showed that a better PFS would result in an increased total treatment cost for lapatinib-based therapy (€28,645,595 instead of €19,381,278). The exploratory sensitivity analyses showed that the total cost for TBT may vary between €99,005,534 and €91,598,885 and €90,868,618 when chemotherapy administration is performed during public or private home hospitalization or in a cancer care network, respectively. For scenario 2, adjusting the consultation fees and implementing a tariff to compensate hospital time required for therapeutic education would increase the total treatment cost of L + C by 0.5%, which would remain lower than that of TBT (). Finally, the analysis on the conversion from L + C to TBT showed that the higher the proportion of patients treated with L + C is, the higher the overall healthcare cost decreases. The optimal cost of treatment of the whole population is achieved when 100% of the population is treated with L + C. A neutral point is achieved when 67.8% of patients are treated with L + C while 32.2% are treated with TBT ().
Discussion
The objective of this study was to perform a budget impact analysis to compare the healthcare costs of trastuzumab-based therapy (TBT) vs lapatinib combined with capecitabine (L + C) in the treatment of progressing HER2-positive metastatic breast cancer after TBT and to quantify the potential saving of healthcare resources induced by the cost transfer from hospitals to the community setting.
This analysis determined that the cost of treatment with L + C is lower than that of TBT. The higher cost of oral drug is offset by the avoided cost of intravenous chemotherapy administration and medical transportation. Even if OAD may help to reduce the overall healthcare costs, we have to mention that the increasing resistance to anti-cancer drug therapies would also lead to the increasing concomitant use of anti-cancer drug therapies and, thus, to a potential increasing cost of cancer treatments. These results were especially sensitive to the percentage of HER2-over-expression among MBC that remains difficult to estimate with regards to heterogeneous data in the literature. The analysis of costs components showed that the cost of medical examinations was higher for TBT due to the monitoring of cardiotoxicity, and that the cost of physician consultation was higher for L + C due to additional GPs’ consultations fees for the follow-up of patients. The use of L + C modifies also the allocation of healthcare resources use between hospital and community. OAD allows avoiding outpatient hospitalizations costs for the Health insurance but it also induces a loss of revenue for hospitals. This cost transfer could contribute to explain the dilemma to switch from an intravenous to oral route of chemotherapy administration. As a consequence, OAD also have an impact on the role of healthcare providers. GPs as well as nurses and pharmacists may be more involved in the monitoring of patients. Finally, OAD also induces a shift of treatment responsibility from physicians to patients, resulting in a smaller control of adherence which may alter the treatment effectiveness and tolerability if patients are not compliant to their treatment and which may result in increasing costs to manage adverse events and changes of treatment protocol not taken into account in the model. This point would suggest that, in addition to economic considerations, the selection of suitable patients to OAD may also play a significant role since not all patients are eligible to these therapies. Age, performance status, quality-of-life, co-morbidities, patient’ preferences and socio-economic environment, complexity of therapeutic protocol, type, and stage of cancer may also influence the choice for OADCitation13.
Our results also provided the first estimation of chemotherapy administration in home hospitalization (tariffs on home hospitalization were first published in December 2011)Citation38. Chemotherapy administration through a cancer care network or home hospitalization may be a less expensive alternative to outpatient hospitalization. In addition, adjusting consultation fees to a longer consultation time and adding a tariff to compensate additional time spent to manage compliance and adverse side-events for OAD would only increase the cost of patients management with L + C by 0.5%, which would remain lower than that of TBT. Nevertheless, this simulation was limited to the impact for the Health insurance to fulfil the perspective adopted in this study. It does not take into account the cost to hospitals for training medical staff and the cost of healthcare re-organization to provide patients with this therapeutic education. No published data are available on the cost of therapeutic education in France, which limits the exploratory analyses on this topic.
The comparison of our results to those of the literature remains difficult since, to our knowledge, no other budget impact model has been published on this topic. Nonetheless, several studies comparing the cost of capecitabine administered orally and intravenously for the treatment of colorectal cancer have confirmed the cost savings induced by the conversion from intravenous to oral anti-cancer drugsCitation39,Citation40,Citation41 Lau et al.Citation42 performed a 1-year budget impact analysis on the cost saved with conversion from intravenous to oral chemotherapy on four targeted intravenous drugs, and reported a potential saving of €1,666,759 in the year 2010 within a US teaching hospital. Only two cost-effectiveness and cost-utility studies with lapatinib and capecitabine have been published. The authors have concluded to a non-acceptable cost-effectiveness or cost-utility ratio of the L + C arm vs the C arm from the US and UK health plan perspective. Our results may not be extrapolated to all types of cancer. The issue of competitive access to oral and intravenous drugs depends also on the availability of OAD per type of cancer. In some disease areas, intravenous chemotherapy administration is still the dominant form.
Budget impact analysis is routinely used to estimate the financial impact of new health technologies. However, this method leads to a partial evaluation of the impact, since efficacy criteria are usually not included in the analyses. To address this classical limit, we indirectly took into account the treatment efficacy of TBT and L + C, through an indirect comparison, by introducing discontinuation rates in the model with PFS data drawn from the literature. Although treatment adherence is a key clinical and an economic issue for OAD, the lack of data prevented us from conducting sensitivity analyses on the level of adherence. We attempted to fill this gap by using PFS data from an observational study that may reflect real-world treatment use including adherence and its impact on treatment efficacy. The sensitivity analyses performed on PFS data showed that a higher PFS resulted in a higher number of patients treated and, thus, in a higher total cost of treatment in Scenario 2, resulting in reduced cost difference between TBT and L + C. We must notice the limit of the indirect comparison of PFS between TBT and L + C since patients are not fully comparable in terms of clinical characteristics. This underlines the classical limitation of the use of indirect comparisons. The modifications of dosage during the course of treatment were not considered in the analysis due to lack of data. Other limitations to our study should be noted. First, the cost of compensation for lost wages was not included due to a lack of data on the professional activities of this sub-set of patients and on the proportion of patients benefiting from compensation for sick leave. We could assume that patients treated with L + C are less concerned with lost wages than patients treated with TBT due to lower hospitalizations. Second, these treatments may also present a risk of cardiac failure and a risk of infection of the injection site (TBT), which was not considered in the analysis. In the same way, administration of oral treatments to non-adherent patients could alter both efficacy and tolerability of treatment, resulting in additional costs (outpatient consultations, change of protocol, and emergency unit visits), but they were not considered in this analysis. This may result in an under-estimation of the budget impact of lapatinib-based therapy. Despite this limitation, sensitivity analyses showed that the direction of the results does not change since the cost of TBT remains higher than that of L + C. The higher cost of TBT within the first cycle (100% of the population treated in each arm) highlights this evidence (). Finally, the evolution of drug prices and cost of drug administration over the period 2012–2015 were not included in the analysis because this depends on pricing policies adopted annually.
Conclusion
Despite the slightly higher drug acquisition cost, the overall treatment cost of L + C remains lower than that of TBT, since it is compensated by the avoided cost of hospitalizations for drug administration and medical transportation. A widespread use of L + C for eligible patients would allow decreasing the overall healthcare cost of treatment of HER2-positive MBC, a point which is important to consider given the increasing constraints on the Health Insurance budget. Nonetheless, the conversion from intravenous to oral chemotherapy induces a cost transfer from hospital budget to community budget generating a loss of revenue for hospitals which are also facing economic constraints. However, it may also provide them with new opportunities to optimize their healthcare resources use to manage other diseases which cannot be externalized, especially for hospitals which are dealing with acute medical activity. Finally, adjusting the consultation fees and implementing a tariff to compensate hospital time required for therapeutic education would remain financially sustainable compared to the use of intravenous anti-cancer drug. Further data on the efficacy of oral agents in real life would also be relevant to generate in order to fulfil a complete health technology assessment.
Transparency
Declaration of funding
Funding for the study was provided by GSK and had no influence on the study design, execution, and publication of results.
Ethics
The study was conducted according to international guidelines on budget impact analysis. Data included in the model were drawn from the published literature, when available, and no data on specific patients or hospitals centers were included. No specific consultation or authorization from ethic committees was required. GlaxoSmithKline (GSK) is the purveyor of lapatinib, a small-molecule inhibitor of the HER2/ErbB2 tyrosine kinase receptor for use in the treatment of HER2-positive advanced or metastatic breast cancer.
Declaration of financial/other interests
LB is a doctoral fellow whose researches are financed by the ANRT (Association Nationale pour la Recherche et la Technologie, Paris, France) and GSK. LB is also an employee of GSK. VB, CR, and GVT were members of the steering committee (SC) of the study and perceived fees for their participation to the SC. MI and BF are employees of PharmIdeas, a Contract Research Organization.
Authors’ contributions
LB was responsible for the study conception and monitoring, literature review, data collection, face-to-face interviews with healthcare professionals, construction of the model framework, cost analysis, interpretation of the results, and manuscript writing. VB, CR, and GVT helped in the study design, results interpretation, data reviewing, and manuscript review. BF and MI helped in the model development, created a user-friendly interactive analytic interface, and contributed to interpretation of the results. All authors reviewed and approved the manuscript.
Notice of Correction
The version of this article published online ahead of print on 26th September 2012 contained a single graph (Figure 1) on page 6. To clarify the presentation of the data this figure has been presented as two separate graphs side by side. This has been corrected for this version.
Acknowledgments
The authors wish to thank all healthcare professionals for their time and help in the data collection and review of the model, especially Pr Jean-Pierre Durand (Département d’Oncologie, Hôpital Cochin, Paris, France) and the nurses in oncology of the outpatient hospital service of the Hôpital Paul Brousse (Villejuif, France), Vincent Navarro (PharmIdeas, Lyon, France) for assistance in the study coordination, and Gaëlle Nachbaur (GlaxoSmithKline, Marly le Roi, France) for support in the study conception and funding and for the reviewing of the manuscript.
References
- GLOBOCAN. International Agency for Research on Cancer. 2008. Available at http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900. Accessed 23 January 2012
- Belot A, Grosclaude P, Bossard N, et al. Cancer incidence and mortality in France over the period 1980-2005. Rev Epidemiol Sante Publique 2008;56:159-75
- Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009;14:320-68
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92
- EMA - European public assessment report on trastuzumab (EPAR). 2000. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000278/human_med_000818.jsp&mid=WC0b01ac058001d124. Accessed 29 September 2011.
- von MG, du BA, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol 2009;27:1999-2006
- von MG, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer 2011;47:2273-81
- Poncet B, Colin C, Bachelot T, et al. Treatment of metastatic breast cancer: a large observational study on adherence to French prescribing guidelines and financial cost of the anti-HER2 antibody trastuzumab. Am J Clin Oncol 2009;32:369-74
- Waddell T, Kotsori A, Constantinidou A, et al. Trastuzumab beyond progression in HER2-positive advanced breast cancer: the Royal Marsden experience. Br J Cancer 2011;104:1675-9
- Huober J, Baumann M, Rochlitz C, et al. Trastuzumab treatment beyond progression in advanced breast cancer: patterns of care in six Swiss breast cancer centers. Oncology 2011;81:160-6
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43
- EMA - European public report assessement (EPAR) on lapatinib. 2008. Available at http://www.ema.europa.eu/ma/index.jsp?curl=pages/medicines/human/medicines/000795/human_med_001120.jsp&mid=WC0b01ac058001d124. Accessed 29 September 2011
- Benjamin L, Cotte FE, Philippe C, et al. Physicians' preferences for prescribing oral and intravenous anticancer drugs: A Discrete Choice Experiment. Eur J Cancer 2011;48:912-20
- Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 1997;15:110-5
- Vergnenègre A. Prescription de la chimiothérapie orale: comment valoriser cet acte spécifique du cancérologue avec le nouveau mode de financement T2A?. Le Nouveau Cancérologue 2008;1:125-33
- Johnson PE. Changes in reimbursement rates and rules associated with the Medicare Prescription Drug Improvement and Modernization Act. Introduction. Am J Health Syst Pharm 2006;63(21 Suppl 7):S2-S6
- Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw 2008 Mar;6(Suppl 3):S1-S14
- Le QA, Hay JW. Cost-effectiveness analysis of lapatinib in HER-2-positive advanced breast cancer. Cancer 2009;115:489-98
- Delea TE, Tappenden P, Sofrygin O, et al. Cost-effectiveness of lapatinib plus capecitabine in women with HER2+ metastatic breast cancer who have received prior therapy with trastuzumab. Eur J Health Econ 2011;48:721-8
- Dee A, Hutchinson M, De La Harpe D. A budget impact analysis of natalizumab use in Ireland. Ir J Med Sci 2011;181:199-204
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health 2007;10:336-47
- Benjamin L, Cotte FE, Mercier F, et al. Burden of breast cancer with brain metastasis: a French national hospital database analysis. J Med Econ 2012;15:493-9
- Haute Autorité de Santé. Avis de la Commission de Transparence du 16 juillet 2008. Tyverb. Available at http://www.has-sante.fr/portail/upload/docs/application/pdf/2008-08/ct-5358_tyverb.pdf. HAS. Accessed 29 September 2011
- Extra JM, Antoine EC, Vincent-Salomon A, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist 2010;15:799-809
- INCa. Situation de la chimiothérapie des cancers en 2010. Collection Rapports & synthèses, ouvrage collectif édité par l'INCa, Boulogne-Billancourt. 2010. Available at http://www.e-cancer.fr/toutes-les-actualites/360/4615-un-rapport-inca-sur-la-situation-de-la-chimiotherapie-en-2010. Accessed 25 July 2011
- Kroep JR, Linn SC, Boven E, et al. Lapatinib: clinical benefit in patients with HER 2-positive advanced breast cancer. Neth J Med 2010;68:371-6
- Ameli.fr. Tarifs conventionnels des médecins généralistes en France métropolitaine. Available at http://www.ameli.fr/professionnels-de-sante/medecins/votre-convention/tarifs/tarifs-conventionnels-des-medecins-generalistes/tarifs-des-medecins-generalistes-en-metropole.php. Accessed 3 November 2011
- Ameli.fr. Tarifs conventionnels des médecins spécialistes. Available at http://www.ameli.fr/professionnels-de-sante/medecins/votre-convention/tarifs/tarifs-conventionnels-des-medecins-specialistes/les-tarifs-des-medecins-specialistes/tarifs-des-medecins-specialistes-en-metropole.php. Accessed 3 November 2011
- CNAMTS. Codage des actes de biologie (NABM). Available at www.codage.ext.cnamts.fr. CNAMTS. Accessed 3 November 2011
- Ameli.fr. Codage des actes médicaux (CCAM). Available at http://www.ameli.fr/professionnels-de-sante/directeurs-d-etablissements-de-sante/codage/codage-des-actes-medicaux-ccam.php. Accessed 3 November 2011
- CNAMTS. Base des médicaments et informations tarifaires (BDM). Available at http://www.ameli.fr/professionnels-de-sante/directeurs-d-etablissements-de-sante/codage/medicaments/base-des-medicaments-et-informations-tarifaires.php. Accessed 3 November 2011
- Rezai K, Urien S, Isambert N, et al. Pharmacokinetic evaluation of the vinorelbine-lapatinib combination in the treatment of breast cancer patients. Cancer Chemother Pharmacol 2011;68:1529-36
- Agence Française de sécurié sanitaire des produits de santé (Afssaps). Avis de la Commission de Transparence du 28 mars 2001. Herceptin. Available at http://www.has-sante.fr/portail/jcms/c_398959/herceptin-150-mg-poudre-pour-solution-a-diluer-pour-perfusion-boite-de-1?xtmc=&xtcr=37. Accessed 29 September 2011
- Weill A, Chinaud F, Vallier N, et al. Frequency and cost of the thirty long-term disorders in the Midi-Pyrénées region in 2003. Rev Med Ass Maladie 2005;36:237-287
- Commission Européenne. Eurostat: évolution des taux d'inflation. Available at http://epp.eurostat.ec.europa/inflation_dashboard/#. Accessed 5 December 2011
- Agence technique de l'information sur l'hospitalisation (ATIH). Statistiques en ligne issues de la base PMSI en HAD. Available at www.atih.sante.fr. Accessed 5 December 2011
- Buthion V, Lagrange T, Fanidi A. La chimiothérapie à domicile: complémentarité ou concurrence dans la stratégie des structures hospitalières?. Journal d'Economie Médicale 2011;29:19-36
- Agence technique de l'information sur l'hospitalisation (ATIH). Valeurs nationales de coûts HAD 2009. Available at www.atih.sante.fr. Accessed 5 December 2011
- Twelves C, Boyer M, Findlay M, . (Xeloda Colorectal Cancer Study Group). Capecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer 2001;37:597-604
- Jansman FG, Postma MJ, van Hartskamp D, et al. Cost-benefit analysis of capecitabine versus 5-fluorouracil/leucovorin in the treatment of colorectal cancer in the Netherlands. Clin Ther 2004;26:579-589
- Cassidy J, Douillard JY, Twelves C, et al. Pharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes' C colon cancer: the X-ACT trial. Br J Cancer 2006;94:1122-1129
- Lau BD, Pinto BL, Thiemann DR, et al. Budget impact analysis of conversion from intravenous to oral medication when clinically eligible for oral intake. Clin Ther 2011;33:1792-6
- Purdie CA BLAA. Increased mortality in HER2 positive, oestrogen receptor positive invasive breast cancer: a population-based study. Br J Cancer 2010;4:475-81
- Journal officiel de la République française (JORF). Arrêté du 1er mars 2011 fixant pour l'année 2011 les éléments tarifaires mentionnés aux I et IV de l'article L. 162-22-10 du code de la sécurité sociale et aux IV et V de l'article 33 modifié de la loi de financement de la sécurité sociale pour 2004. 2011. Available at www.legifrance.gouv.fr. Accessed 25 July 2011