Abstract
Objective:
To estimate the cost-effectiveness of ipilimumab (3 mg/kg) compared with best supportive care (BSC) in pre-treated advanced melanoma patients.
Methods:
The analysis was based on a US payer perspective and lifetime time horizon. A three-state Markov model was developed representing clinical outcomes, quality-of-life, and healthcare resource use of patients treated with ipilimumab and BSC. Transitions between states were modeled using overall and progression-free survival data from the MDX010-20 trial. Utility data were from a melanoma-specific study of the health state preferences of the general population. Disease management costs expressed in 2011 US Dollars were based on healthcare resource use observed in a US retrospective medical chart study. Uncertainty was analyzed using one-way and probabilistic sensitivity analyses.
Results:
The gain in life years and QALYs from introducing ipilimumab over BSC were 1.88 years (95% CI = 1.62–2.20) and 1.14 (95% CI = 1.01–1.34) QALYs, respectively, over the lifetime time horizon. The estimated incremental cost of treating with ipilimumab vs BSC was $146,716 (95% CI = $130,992–$164,025). The estimated incremental cost-effectiveness ratios were $78,218 per life year gained and $128,656 per QALY gained. Ipilimumab was 95% likely to be cost-effective at a willingness-to-pay of $146,000/QALY.
Limitations:
Ipilimumab’s method of action causes a tumor response pattern that differs from the Response Evaluation Criteria in Solid Tumors upon which the model is based, leading to a potential under-estimate of quality-of-life of ipilimumab patients. Survival and QALY gains were related to the time horizon of the analysis. Sensitivity analyses indicated that qualitative conclusions regarding the cost-effectiveness of ipilimumab were unchanged when the method of quality adjustment and the time horizon were varied.
Conclusion:
The analysis shows that the estimated cost-effectiveness of ipilimumab is within what has been shown to be acceptable to payers for oncology products in the US.
Keywords::
Background
The global incidence of advanced melanoma, which includes unresectable and metastatic melanoma, continues to grow, increasing the death rate for this cancer compared to other cancer typesCitation1,Citation2. According to the World Health Organization, in 2008 almost 200,000 patients were diagnosed with cutaneous melanoma and there were over 46,000 deaths from the diseaseCitation3. In the US, there were 70,230 new melanoma cases and 8790 deaths in 2011Citation4. Another recent study using US healthcare plan data projected that 10,000 patients are diagnosed with advanced melanoma in the US each yearCitation5; according to Jemal et al.Citation6, this number has been increasing, although the rate of increase has begun to level off. The incidence of melanoma is also increasing in the European Union (EU), Australia, and in South American countries such as BrazilCitation7–9.
Most patients (84%) are diagnosed at an early stage and consequently have an excellent prognosisCitation10. However, the treatment of patients with advanced melanoma remains a challenge for both healthcare providers and their patients; the 5-year survival rate for patients with advanced melanoma is 15.2% and the median survival for patients is less than 1 yearCitation2,Citation10.
Until recently treatment alternatives for patients with advanced melanoma have been few and of limited effectiveness. Prior to March 2011, only dacarbazine and high-dose interleukin-2 (IL-2) were approved for treatment of patients with advanced melanoma, yet neither treatment (nor any previous treatment) had demonstrated a prolongation of median survival in phase III trialsCitation11. A meta-analysis of 42 phase II Cooperative Group trials conducted from 1975–2008 showed that no agent had a statistically significant difference in overall survival from the other agentsCitation12. This analysis was recently updated to include more recently tested treatments, including the gp100 vaccine; but, again, no agent demonstrated a statistically significant difference in overall survivalCitation13,Citation14.
A recent retrospective medical chart review study of patients with advanced melanoma in the US found that the majority of patients diagnosed from 2004–2008 received systemic anti-cancer therapy. This study found that temozolomide and dacarbazine were the most used systemic therapies for patients with advanced melanoma in both the first- and second-line settingCitation15.
The Food and Drug Administration’s (FDA) recent approval of new therapies has improved the outlook for US patients with advanced melanoma. In March 2011, the FDA approved the use of the immune checkpoint inhibitor ipilimumab for patients with unresectable or metastatic melanoma. Ipilimumab is a recombinant, human monoclonal antibody that binds to the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and blocks its ability to inhibit cytotoxic T-cell function.
In the MDX010-20 trial, patients with previously treated advanced melanoma who received ipilimumab survived a median of 3.7 months longer than patients treated with the gp100 vaccineCitation2. There were 20.3 (45.6% versus 25.3%), 16.9 (33.2% versus 16.3%), and 9.8 (23.5% versus 13.7%) percentage points more patients alive in the ipilimumab arm than in the gp100 arm at 12, 18, and 24 months, respectively. A subsequent trial demonstrated overall survival benefit (hazard ratio for death, 0.72; p < 0.001) for the combination of ipilimumab and dacarbazine relative to dacarbazine alone in previously untreated patients with advanced melanomaCitation16. This trial showed higher survival rates in the ipilimumab–dacarbazine group at 1 year (47.3% vs 36.3%), 2 years (28.5% vs 17.9%), and 3 years (20.8% vs 12.2%). More recently, a trial reported survival benefit for vemurafenib, a selective inhibitor of mutant BRAF kinase, relative to dacarbazine, in patients with previously untreated melanoma containing a V600 mutation in BRAF. Together these two agents represent the only therapies to show a survival benefit in phase III trials of patients with advanced melanoma. In March 2011, the FDA approved the first of these two novel therapies, ipilimumab, for treatment of unresectable or metastatic melanoma. The cost-effectiveness of ipilimumab vs the prior standard of care for advanced melanoma has not been previously described.
Objective
The objective of this study was to evaluate the cost-effectiveness of ipilimumab compared to best supportive care (BSC) in previously-treated patients with advanced (unresectable or metastatic) melanoma in the US.
Methods
Model structure
A Markov economic model containing three health states—stable disease, progression, and death—was constructed in Microsoft Excel™. Response was modeled as a sub-state of the stable disease state in which patients have a different quality-of-life but an equivalent cost of management. The proportion of patients in each health-state at each model cycle was calculated based on the relationship between the progression-free survival (PFS) and overall survival (OS) data from the phase III trial MDX010-20Citation2.
Model parameters
This model focused on patients with advanced melanoma with an average age of 55 years, and used a lifetime time horizon to ensure the costs and benefits of each intervention were fully captured. The analysis was conducted from a US third-party payer perspective; therefore, no other societal costs—such as lost productivity, patient time costs, or transport costs—were taken into account. Discount rates were set at 3% for both costs and outcomes, following the recommendations of the US Public Health Service Panel on Cost-Effectiveness in Health and MedicineCitation17.
Comparators
The clinical trial on which the model transitions were based compared ipilimumab to gp100, an experimental cancer vaccine. Although the gp100 vaccine has shown potential benefit in patients with melanoma, it remains an unapproved experimental treatment. For the purposes of the economic evaluation, an alternative therapy representative of the current standard of care was required. Given the absence of a gold standard therapy for patients with previously-treated advanced melanoma, and given the absence of a significant prolongation of survival from currently available therapies, best supportive care (BSC) was chosen as the base case comparator. BSC is defined as disease management without active chemotherapy. This choice of comparator could be considered conservative from a cost perspective, since in the US many patients with advanced melanoma commonly receive active second-line therapy such as dacarbazine, temozolomide, or paclitaxel + carboplatin. While these interventions have not demonstrated an OS benefit in randomized controlled trials, they impose substantial treatment costs.
Model analysis
The model estimated both the incremental cost per life year gained (ICER) and the incremental cost per QALY gained (ICUR). One-way sensitivity analyses, scenario analyses, and a probabilistic sensitivity analysis were also performed to test the strength of the assumptions made and to ascertain the impact of uncertainty. The scenario analyses included comparisons with other active therapies commonly used in the US, such as temozolomide, dacarbazine, and IL-2.
Model inputs: Clinical
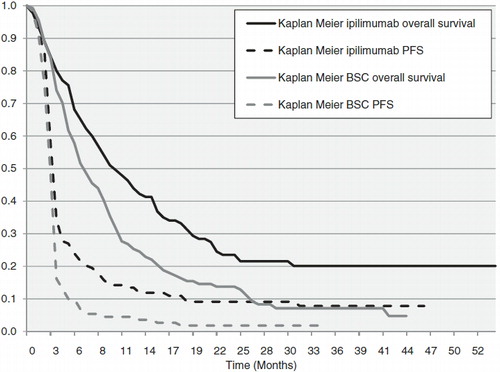
The main transition probabilities were derived from the MDX010-20 trialCitation2. In keeping with the listed product indications, this model compared only ipilimumab monotherapy with gp100, which was used as a proxy for BSC and other currently available therapies. As indicated by the Kaplan-Meier (KM) survival curves (), a significant proportion of patients (∼ 20%) were still alive at the end of the trial; the projection to lifetime survival therefore required extrapolation beyond the trial duration of 4.5 years to encompass a lifetime time horizon.
The shape of the survival curve for ipilimumab showed a clear plateau during the final 2 years of the trial, where no events occurred. This lack of events reduced the precision of the estimates and complicated the extrapolation. Initially standard parametric methods were used for survival extrapolation; however, given the poor fit of the resulting survival curves, an alternative non-parametric method was applied.
For the initial standard parametric extrapolation, four parametric models (exponential, log-logistic, log-normal, and Weibull) were fitted to both the OS and PFS curves in each arm using survival regression modeling. The best-fitting curves were selected using Akaike’s Information Criterion (AIC). The estimated parametric survival curves for the ipilimumab OS outputs are shown in . Since the best fitting parametric curve (log-normal) did not adequately represent the survival of ipilimumab patients, an alternative, non-parametric method, which assumed that patients died at a constant rate, was used. Three different non-parametric survival models were constructed using the observed hazard rate over three different periods of the trial (the entire trial, the last 3 years of the trial, and between the 2nd and 3rd years of the trial) to extrapolate mortality. A discussion of the relative merits of these parametric and non-parametric approaches to modeling survival in ipilimumab patients was provided in Annemans et al.Citation18 The hazard rate estimated over the last 3 years of the trial was chosen to model the overall survival of ipilimumab patients, due to its similarity to a hazard rate derived from Balch et al.’sCitation19 analysis of 15 years of real world survival data of patients with melanoma included in the American Joint Committee on Cancer (AJCC) registry. The hazard rate for patients with Stage IV disease was used for comparison, as the majority of patients in MDX010-20 had Stage IV disease upon entry. Because the historical data analyzed by Balch et al. are based on patients receiving previously available therapies with no demonstrable impact on overall survival, it is likely that this is a conservative assumption that under-estimates the benefit of ipilimumab. Also, since the KM curve of ipilimumab flattens considerably toward the end of the trial, the other two non-parametric methods considered—the hazard rate over the course of the whole trial, and the hazard rate between the 2nd and 3rd years—are even more conservative.
Figure 2. Comparison of the estimated log-normal parametric overall survival curves to the observed overall survival curves from MDX010-020.
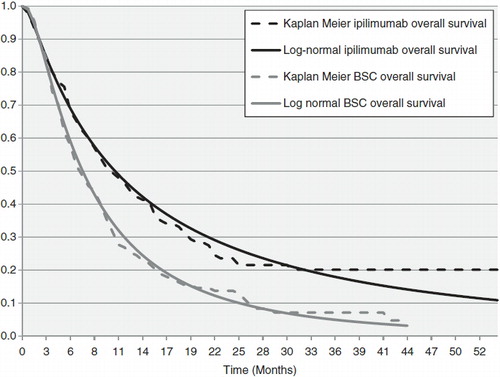
In the comparator arm, visual inspection revealed that the parametric distributions described the gp100 data well. The log-normal parametric function was chosen to represent GP100 overall survival as it had the lowest AIC value. Both PFS curves for ipilimumab and gp100 were also modeled using parametric functions.
The OS and PFS data from the gp100 arm of the MDX010-20 trial were used to represent the survival of patients receiving BSC and any other active comparators, since gp100 is the only therapy that has been directly compared with ipilimumab in a head-to-head setting. It is not possible to perform a network meta-analysis of all comparators, as a closed network of evidence cannot be formed with the available clinical trial data. However, as mentioned above, Korn et al.Citation12 demonstrated in a meta-analysis that there was no difference in survival between therapies (including BSC) tested in 42 Phase II studies from 1975–2008. As part of this analysis, Korn et al. developed an algorithm for calculating OS based on the characteristics of patients in the trials. This survival algorithm was applied subsequently in two meta-analyses of overall survival extended to include more recently tested treatments, including gp100; again, the gp100 control arm of the MDX010-20 trial, when corrected for prognostic factors, was not statistically different from overall survival in the trial arms that did not contain ipilimumabCitation13,Citation14.
In addition to the OS and PFS data, the following clinical parameters from the MDX010-20 trial were used in the model: the proportion of patients experiencing grade III or IV adverse events for both ipilimumab and gp100; the number of patients receiving a given number of doses; the proportion of patients who responded; and the proportion re-induced. A list of clinical, cost, and utility inputs to the cost-effectiveness model is given in . For the comparators considered in the sensitivity analysis, the following parameters were taken from the respective registration trial publications: overall survival; progression-free survival; the proportion of patients experiencing grade III/IV adverse events; and the proportion of patients who responded.
Table 1. Clinical, cost, and utility inputs used the cost-effectiveness model base case.
Model inputs: Costs
Three categories of cost data were used in the model: drug costs; disease management costs; and toxicity management costs. The first and last of these were only applied while patients were on treatment. Disease management costs incorporated the costs of monitoring patients and managing their symptoms, and were disease state- and treatment-specific. Disease management costs were taken from a recent US study on epidemiological, clinical, and demographic characteristics of patients with advanced melanomaCitation20.
The dosing regimen for ipilimumab was taken from the MDX010-20Citation2. The dosing regimens for dacarbazine and temozolomide were taken from the prescribing information and clinical guidelines, respectivelyCitation21,Citation22. Drug unit costs and administration costs were taken from the Thomson Healthcare RedbookCitation23 and the July 2010 Center for Medicare and Medicaid ServicesCitation24 data files, respectively.
Toxicity costs were based on a weighted average of the cost of treating toxicities in inpatient and outpatient settings as well as the medications and procedures required to treat them. Toxicities common to treatment with ipilimumab and comparator therapies were considered. These data were taken either from the literature or based on a survey of four US oncologists, who had treated advanced melanoma patients, on the resources required to treat these toxicitiesCitation25. The unit costs of treating toxicities as an inpatient were taken from Elting and ShihCitation26 and the Healthcare Cost & Utilization ProjectCitation27; in the outpatient setting the unit costs were based on CPT codes for individual procedures and NDC codes for individual drugs. The average cost of treating each toxicity is shown in .
Model inputs: Utility
Utilities in the model were taken from an advanced melanoma-specific preference elicitation project that reported the general population utility values for patients in response, stable disease, and progressive disease, as well as the quality-of-life decrement for patients who experienced adverse eventsCitation28.
In a sensitivity analysis, utilities derived from MDX010-20 patients were applied to assess the impact of general population vs patient-reported utilities on the outputs of the model. These included utilities derived from the SF-36 as well as the EORTC QLC C30 measuresCitation29. The former is a generic quality-of-life measure meaning that the number of QALYs generated may be compared with SF-36 based QALYs estimated from other disease areas; the latter is a cancer-specific questionnaire so is not comparable to other disease areas, but is more sensitive to the quality-of-life of a melanoma patient.
Results
The results were estimated over a lifetime time horizon (30 years) and represent the costs and outcomes accruing to the average patient with advanced melanoma over their remaining life.
Over the lifetime time horizon, the average ipilimumab patient was estimated to live for 2.88 years, 1.88 years longer than the average patient in the BSC arm. This estimate was the mean survival across a cohort of patients receiving ipilimumab and followed for their remaining lives. The average ipilimumab patient gained 1.76 QALYs, 1.14 more QALYs than the average BSC patient. The difference in QALYs was driven by the difference in life years gained, the difference in PFS, and the difference in the proportion of responders. Ipilimumab’s benefit in both life years and QALYs was statistically significant at 95% as the 95% CIs did not overlap, as shown in .
Table 2. Results of the cost-effectiveness analysis.
The model reported the average cost of therapy across the cohort of patients in each arm. These costs were disaggregated into the categories shown in . The total cost of ipilimumab therapy was higher than that of BSC, as shown in . The largest contributors to the difference between arms were the cost of ipilimumab and the cost of managing patients with progressed disease. The cost of progressive disease assumed that long-term survivors require the same intensity of management as those who did not demonstrate a durable response to treatment. This was a conservative assumption, but currently there is no quantitative data to support a lower cost of management for those who are long-term survivors. This assumption has been tested in the one-way sensitivity analysis described below.
Table 3. Disaggregated costs of treating advanced melanoma patients over the lifetime time horizon.
The base case ICER projected from the model was $78,000 per life-year gained, while the ICUR was estimated at $129,000 per QALY gained. Incremental results are summarized in Table 2.
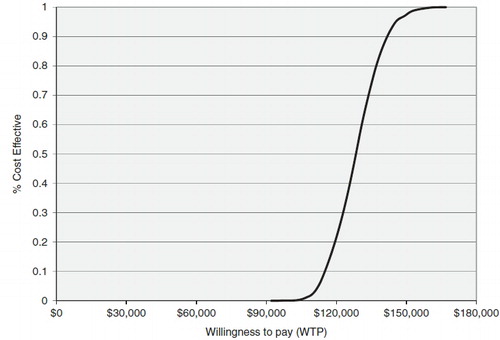
The results from the PSA confirmed that ipilimumab was more than 99% likely to be both more effective and more costly than BSC. shows a plot of the incremental cost and the incremental effectiveness results for each of the 10,000 simulations; all were in the north-east quadrant. is a cost-effectiveness acceptability curve, showing that ipilimumab was 6% likely to be cost-effective at a willingness-to-pay threshold of $113,000/QALY, and more than 95% likely to be cost-effective given a willingness-to-pay of $146,000/QALY.
Figure 3. Scatter plot of the 1000 sampled incremental cost and QALY estimates from the probabilistic sensitivity analysis.
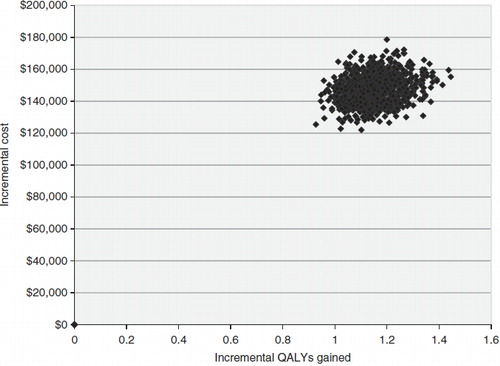
Figure 4. The cost effectiveness acceptability curve for the economic evaluation comparing ipilimumab and GP100.
A difference in the magnitude between the ICER and the ICUR was expected, given that the disease reduces quality-of-life. In this analysis, the difference may be exaggerated since the standard definition of disease progression used in the trial may over-state the rate of transition from the stable disease to progressive disease state for patients treated with ipilimumab. The progressive disease state carried a lower utility, resulting in a lower number of overall QALYs for ipilimumab.
One way sensitivity and scenario analyses
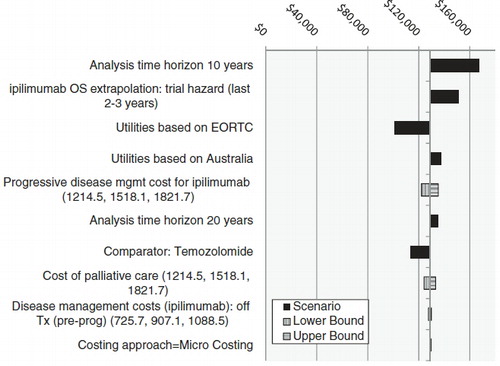
A set of one-way sensitivity analyses was performed to test the impact of changes in the assumptions and key parameter input values on the ICUR. The results are shown in . The 10 scenarios with the largest impact on the results are shown in . Reducing the time horizon resulted in the largest increase in the ICER (requiring a higher willingness-to-pay), and using the EORTC-based utilities resulted in the largest decrease. The sensitivity and scenario analysis also showed that the model was more sensitive to changes in assumptions such as time horizon and utility weights than input values such as disease management costs.
Temozolomide is widely used in the US to treat patients with melanoma but has shown no significant improvement in outcomes. Comparing ipilimumab therapy with temozolomide therapy led to an improvement in the cost-effectiveness of ipilimumab, driven by an increase in the cost of the comparator therapy but no corresponding increase in survival. This result was true of the other active comparators considered for scenario analyses.
Discussion
Ipilimumab is a novel therapy and was the first to demonstrate an OS benefit in patients with advanced melanoma in a phase III randomized controlled clinical trial, demonstrating an increase in median overall survival in the clinical trial (10.1 months vs 6 months). The model presented here estimates that, over a lifetime time horizon, the mean number of life years gained by treating a patient with ipilimumab instead of BSC would be 1.62–2.20. It also estimates that the mean number of QALYs gained per patient would be 1.01–1.34. Further, a durable long-term response/survival occurring in ∼20% of patients may be expected. The greater incremental improvement in mean survival, as opposed to median survival gained (1.88 years vs ∼4 months), reflects the long-term survival benefit observed in 20% of patients in this setting. From an economic perspective, the mean survival estimate is more relevant than the median, as emphasized in a recent paper on economic evaluation in oncology trialsCitation30.
The incremental cost of treating patients with ipilimumab was significantly higher than that of treating patients with BSC. This increase was statistically significant based on the 95% CI. This means that healthcare payers can expect a higher cost of therapy when treating patients with ipilimumab. Despite this, the substantial benefit in survival places the projected ICER below $100,000/LY gained (at $78,218/LY gained). The estimated base case ICUR was $128,656/QALY gained. This base case ICUR estimate was constructed to be conservative, and may represent an upper bound on the true ratio, given the difficulty of adapting our 3-state model to the dynamics of the response to ipilimumab by patients. Considering a less-conservative analysis where patient-rated utilities from the clinical trial—which take full account of the benefit of a durable response—were used to adjust survival, the incremental cost per QALY was reduced to $101,287/QALY; a sensitivity analysis incorporating both the clinical trial utilities and using temozolmide—a common systemic treatment in second line in the US—as the comparator, reduces the ICUR further, to $89,027/QALY.
This analysis aimed to project the incremental cost-effectiveness of ipilimumab. For these estimates to be useful to decision-makers, these results should be put into the context of the wider US cost-effectiveness landscape. Ubel et al.Citation31 noted that the US Panel on Cost-effectiveness provided guidance to standardize and raise the quality of cost-effectiveness studies without providing decision-makers with context on what level of cost-effectiveness was acceptableCitation17. They also noted that the $50,000 per QALY threshold often mentioned in the literature goes back at least to 1982 and has been cited in the intervening decades without adjustment for inflationCitation31,Citation32. A recent analysis showed that ‘the 1982 valuation is equivalent to $197,000 per QALY in 2007 dollars after adjusting for the 5.5% average 1982 to 2007 healthcare inflation, and $126,000 per QALY if the overall inflation rate is used’Citation33. In the absence of an explicit threshold, Ubel et al.Citation31 inferred the level of cost-effectiveness that was generally acceptable in the US context by reviewing the literature, including: (1) examples of cost-effectiveness ratios for interventions that received broad support, and (2) a review of evidence on the value of statistical life, converted to dollars per QALY, including both studies of actual behavior (e.g., willingness-to-pay for safety improvements) and surveys about the hypothetical trade-offs between money and riskCitation32. Based on this evidence, they concluded that the cost-effectiveness threshold that was acceptable at that time (2003) approached $200,000 or more per QALYCitation31. More recent theoretical and empirical evidence has suggested that acceptable thresholds in the end-of-life context should be higherCitation34–36. Compared to this body of evidence, the projected ICERs and ICURs presented here are well within what is considered cost-effective in the US.
This cost-effectiveness model was built using the three-state Markov model approach traditionally used in oncology cost-effectiveness modeling. Two aspects of the model could be challenged: the survival modeling and the disease progression criteria used to inform the transition from stable to progressive disease. The overall survival curve for ipilimumab reported in Hodi et al.Citation2 was characterized by a plateau over the last 2 years of study follow-up. Due to censoring, there were relatively few at-risk individuals during this period, leading to increased uncertainty around the survival estimates in the latter years of the MDX010-20 trial and potentially impacting the validity of the survival extrapolation. However, consideration of the totality of long-term survival data, including those from the ipilimumab + gp100 arm of MDX010-20 and other ipilimumab trials, supports the projection that ipilimumab leads to durable benefit in ∼20% of patients with advanced melanomaCitation2,Citation16,Citation37. This was further supported by data from an 8-year long trial of IL-2, which also revealed a plateau in OS in a group of immunotherapy-treated melanoma patients, suggesting that this may be a feature of active immunotherapy for patients with melanoma in generalCitation38.
Traditional oncology models extrapolate survival beyond the trial period using parametric regression models based on the survival data. However, given the plateau described above, the overall survival data from the ipilimumab arm of MDX010-20 were not well represented by any of the currently available regression models. Thus, alternative methods were explored. Annemans et al.Citation18 suggested that the extrapolation of OS data cannot be based on the entire trial population due to the different prognosis of patients who die ‘early’ in the trial from those patients who survive beyond a particular duration. This was supported by current clinical opinion and data from trials with other active immunotherapiesCitation39. The hazard rate calculated over the last 3 years (the base case extrapolation method) was representative of the mortality rate associated with patients who survive beyond a particular duration and thus better reflect the survival prognosis for patients remaining than one that includes data for those who died earlier in the trial. The absolute hazard rate used was shown to be consistent with survival of Stage IV melanoma patients in the AJCC registryCitation19.
The use of progression-free survival to capture transitions (decreases) in quality-of-life within the model may have caused an under-estimation of QALYs for patients treated with ipilimumab. Traditional methods, and indeed the methods used in this model, assume that, once a patient had progressed, it is not possible to experience any improvement in quality-of-life. Similarly, these models assume that patients require intensive physician management from the point of progression until death. Although these standard definitions of response are generally valid for traditional chemotherapies such as dacarbazine, ipilimumab can demonstrate a different tumor response pattern, whereby some patients demonstrate a long-term response even after an initial appearance of disease progression. This phenomenon has prompted the establishment of immune response criteria that are distinct from the standard Response Evaluation Criteria In Solid Tumors (RECIST) criteria, and are particularly applicable to patients treated with ipilimumabCitation40.
Taking this response pattern into account, it may be reasonable to assume that it was possible for patients treated with ipilimumab (and similar active immunotherapies) to experience an improvement or stability in quality-of-life following an initial increase in tumor burden. Further, it may also be reasonable to assume that these patients will require less frequent monitoring and be less costly to manage on an annual basis. Neither of these possibilities was incorporated in the base case for two reasons. First, there is not yet a consensus among regulatory audiences (such as the FDA) supporting the immune response criteria; and, second, there was insufficient long-term data to demonstrate the quality-of-life and management cost of patients who have been treated with immunotherapies like ipilimumab. Further research is needed to resolve these issues, which may have an important impact on cost-effectiveness estimates for ipilimumab. For example, the sensitivity analysis using the patient-rated EORTC-based utilities resulted in a decrease of ∼$30,000 in the estimated ICUR.
Advanced melanoma, once a disease with limited treatment options, now has several promising agents on the horizon. Ipilimumab is the first of these therapies to produce a long-term OS benefit. For vemurafenib, the next agent to receive approval for treatment of advanced melanoma, interim results and a further update have been reported; however, the median follow-up time and maximum follow-up durations reported to date are insufficient for purposes of robust economic evaluationCitation41,Citation42. As data on these other agents mature, cost-effectiveness estimates for these agents and potential combinations of agents will provide important evidence for healthcare decision-makers.
The analysis presented here applied both standard and innovative methods to develop estimates of the cost-effectiveness of ipilimumab, an agent with a novel method of action and benefit profile, in the treatment of patients with advanced melanoma. In summary, the estimates indicate that the projected cost-effectiveness of ipilimumab is within what has been shown to be acceptable to payers in the US. Further research extending the evidence on the long-term benefit of ipilimumab and other comparators will provide useful support to decision-makers as they consider the value of therapeutic options for advanced melanoma patients.
Transparency
Declaration of funding
This study was funded by Bristol Myers Squibb, Wallingford, CT.
Declaration of financial/other relationships
Srividya Kotapati and John R Penrod have disclosed that they were employees of Bristol Myers Squibb. Srividya Kotapati and John R Penrod own shares in Bristol Myers Squibb. Yumi Asukai and Victor Barzey have disclosed they were employees of IMS Health, a company that was paid consultancy fees by the sponsor of this study. Michael Atkins was a Professor at Georgetown University Medical School and a Deputy Director of the Georgetown-Lombardi Comprehensive Cancer Center. Dr Atkins received an honorarium payment for his support. Louis Garrison was a Professor at the School of Pharmacy, an Adjunct Professor in Global Health, and an Adjunct Professor in Health Services within the Pharmaceutical Outcomes Research & Policy Program at the University of Washington School of Pharmacy. Professor Garrison also received an honorarium payment for his support.
Author contributions
Victor Barzey and Yumi Asukai built the model. Srividya Kotapati and John Penrod provided data inputs and conceptual support. Dr Atkins provided validation of the clinical assumptions. Professor Garrison provided validation of the economic assumptions. All authors contributed to the drafting and reviewing of the manuscript.
Notice of Correction
The version of this article published online ahead of print on 31 October 2012 contained an error on page 1. The sentence “Ipilimumab was 95% likely to be cost-effective at a willingness-to-pay of £146,000/QALY.” should have read “Ipilimumab was 95% likely to be cost-effective at a willingness-to-pay of $146,000/QALY.” The error has been corrected for this version.
Acknowledgment
The authors would like to acknowledge the work of Sonja Sorensen, MPH, and Feng Pan, PhD, from United BioSource Corporation for their initial contribution to the development of the cost-effectiveness model.
References
- American Cancer Society. Cancer Facts and Figures 2011. Atlanta, GA: American Cancer Society, 2011
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917
- Seidler AM, Pennie ML, Veledar E, et al. Economic burden of melanoma in the elderly population: population-based analysis of the Surveillance, Epidemiology, and End Results (SEER)–Medicare data. Arch Dermatol 2010;146:249-56
- Taneja C, Edelsberg J, Penrod JR, et al. Epidemiology of advanced melanoma: findings from a large US health insurance database. Presented at Perspectives in Melanoma XV, September 16–17, 2011; New York, NY, USA
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010;46:765-81
- Melanoma information page. Cancer Council Australia, 2012. http://www.cancer.org.au/aboutcancer/cancertypes/melanoma.htm. Accessed 27 January 27, 2012
- Bakos LÃ. Melanoma cutâneo: estudos de base populacional no Brasil. Anais Brasileiros de Dermatologia 2006;81:402
- Surveillance, Epidemiology and End Results (SEER) Stat Fact Sheets: Melanoma of the Skin. National Cancer Institute, Bethesda, 2012. http://seer.cancer.gov/statfacts/html/melan.html. Accessed July 13, 2012
- Coit DG, Andtbacka R, Anker CJ, et al. Melanoma. J Natl Compr Canc Netw 2012;10:366-400
- Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527-34
- Dequen P, Lorigan P, Jansen JP, et al. Systematic Review and Network Meta-Analysis of Overall Survival Comparing 3 mg/kg Ipilimumab with Alternative Therapies in the Management of Pretreated Patients with Unresectable Stage III or IV Melanoma. Oncologist. 2012 Oct 10. [Epub ahead of print]
- Wada R, Feng Y, Zhang N, et al. Meta-analysis of Kaplan-Meier overall survival curves from selected randomized controlled phase II/III trials in advanced melanoma. Presented at the American Conference on Pharmacometrics, 3–6 April 2011; San Diego, California, USA
- Macahilig C, Botteman M, Prinz GM, et al. Patterns of care and healthcare utilization of US advanced melanoma patients: evidence from a retrospective medical chart review. Presented at Perspectives in Melanoma XV, September 16–17, 2011; New York, NY, USA
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26
- Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996
- Annemans L, Asukai Y, Barzey V, et al. MO2 Extrapolation in oncology modelling: novel methods for novel compounds. Value Health 2011;14:A242-3
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009 December 20;27(36):6199-206
- Bristol-Myers Squibb. The epidemiology and clinical and demographic characteristics of advanced melanoma patients in the US: evidence from a retrospective medical chart review. [Data on file, 2011]
- Dacarbazine for Injection, USP. Hospira is based in Lake Forest, Illinois, 2007. http://www.hospira.com/Products/dacarbazine.aspx. Accessed July 17, 2012
- Quirt I, Verma S, Petrella T, et al. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist 2007;12:1114-23
- RED BOOK Drug References. Thomson Reuters Micromedex Clinical Evidence Solutions [Internet], 2010. http://thomsonreuters.com/products_services/healthcare/healthcare_products/clinical_deci_support/micromedex_clinical_evidence_sols/med_safety_solutions/red_book/
- ASP Drug Pricing Files. Centers for Medicare & Medicaid Services, 2010. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/01a19_2010aspfiles.html. Accessed October 19, 2012
- Stokes ME, Muehlenbein CE, Marciniak MD, et al. Neutropenia-related costs in patients treated with first-line chemotherapy for advanced non-small cell lung cancer. J Manag Care Pharm 2009;15:669-82
- Elting LS, Shih YC. The economic burden of supportive care of cancer patients. Support Care Cancer 2004;12:219-26
- AHRQ. Health Care Cost & Utilization Project: Nationwide Inpatient Sample (NIS). 2005
- Beusterien KM, Szabo SM, Kotapati S, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer 2009;101:387-9
- Batty AJ, Fisher D, Winn B, et al. PCN148 estimating quality of life in advanced melanoma; a comparison of standard gamble, SF-36 mapped, and Eortc QLQ-C30 mapped utilities Value in Health. 2011;14:7 p A461-2
- Davies A, Briggs A, Schneider J, et al. The ends justify the mean: outcome measures for estimating the value of new cancer therapies. Health Outcomes Research in Medicine February 2012;3(1):e25-e36
- Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med 2003;163:1637-41
- Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making 2000;20:332-42
- Hillner BE, Smith TJ. Efficacy does not necessarily translate to cost effectiveness: a case study in the challenges associated with 21st-century cancer drug pricing. J Clin Oncol 2009;27:2111-3
- Becker G, Murphy K, Philipson T. The value of life near its end and terminal care. NBER Working Paper Series 2007; Working Paper 13333: published online July 13, 2012. http://www.nber.org/papers/w13333 accessed 22 October 2012
- Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist 2006;11:90-5
- Seabury SA, Goldman DP, Maclean JR, et al. Patients value metastatic cancer therapy more highly than is typically shown through traditional estimates. Health Aff (Millwood) 2012;31:691-9
- Wolchok JD, Weber JS, Maio M, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase 2 trials. Pigment Cell Melanoma Res. 2011;24(5):1072
- Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364:2119-27
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16
- McArthur G, Hauschild A, Robert C, et al. Vemurafenib improves overall survival compared to dacarbazine in advanced BRAFV600E-mutated melanoma: updated Survival results from a Phase III randomized, open-label, multicentre trial. Presented at the European Society of Clinical Oncology Conference , 23--27 September 2011; Sweden, USA