Abstract
Objective:
To compare hospitalizations and incidence of relapses among patients with schizophrenia initiating long-acting injectable (LAI) antipsychotics vs oral antipsychotics.
Methods:
Patients with schizophrenia initiating LAI antipsychotics or oral antipsychotics (index events) were identified from large databases (MarketScan; Truven Health Analytics, CA), containing commercial and Medicare healthcare claims and their pre-index (12-month baseline period) and post-index (12-month follow-up period) hospitalizations and relapse rates were compared. Descriptive and bivariate statistics were utilized to compare demographics, clinical characteristics, and hospital resource usage among cohorts. Multivariate analysis was used to evaluate the impact of initiating LAI vs oral antipsychotics on differences in the number of hospitalizations and length of stay (LOS) between follow-up and baseline periods.
Results:
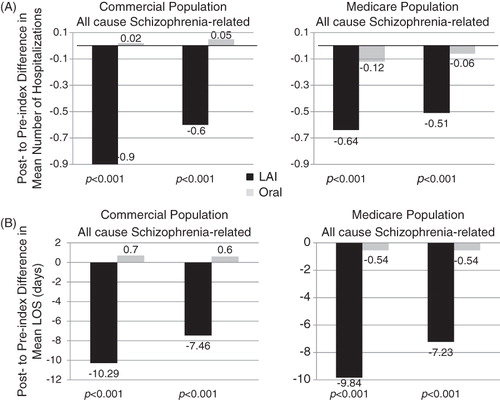
Commercially insured patients initiating LAI antipsychotics (n = 394) had significant reductions in inpatient healthcare usage after initiating antipsychotic therapy: mean number (±standard deviation) of all cause hospitalizations (1.60 ± 1.66 vs 0.70 ± 1.20, p < 0.001), LOS (16.9 ± 20.7 vs 6.6 ± 14.4 days, p < 0.001), schizophrenia-related hospitalizations (1.03 ± 1.26 vs 0.43 ± 0.86, p < 0.001), associated LOS (12.3 ± 17.7 vs 4.8 ± 12.8 days, p < 0.001). Patients initiating LAI vs oral antipsychotics (n = 2610) had significantly greater reductions during the follow-up period vs baseline period in the mean number of all cause hospitalizations (−0.90 ± 1.77 vs 0.02 ± 1.49, p < 0.001), LOS (−10.3 ± 23.2 vs 0.7 ± 16.7 days, p < 0.001), schizophrenia-related hospitalizations (−0.60 ± 1.37 vs 0.05 ± 0.99, p < 0.001) and associated LOS (−7.5 ± 20.7 vs 0.6 ± 12.5 days, p < 0.001). These results were further supported by multivariate analyses in which patient characteristics were taken into consideration.
Limitations:
This study attempted to minimize the impact of differences in patient characteristics by having patients serve as their own controls in the before vs after comparison followed by multivariate regressions, however one still may not be able to account for all confounders in this non-randomized study population.
Conclusion:
Patients with schizophrenia who initiated LAI vs oral antipsychotics experienced reductions in hospitalizations and schizophrenia relapses after drug initiation, which may be indicative of improved disease management.
Introduction
Schizophrenia refers to a group of severe, chronic, and disabling brain disorders characterized by hallucinations, delusions, thought disorders, emotional negativity, and poor cognitive functioning. A systematic review of more than 50 different studies reported a median incidence of schizophrenia of 15.2 per 100,000 persons, with rates generally being higher in menCitation1. Although the incidence of schizophrenia is low, the prevalence is high, with a median lifetime prevalence of 4.0 per 1000 personsCitation2. The high prevalence is attributed to the disease generally having an onset in early adulthood and persistent symptoms, lasting throughout a person’s lifetime. The healthcare burden of schizophrenia is great and in 2002 the cost of schizophrenia in the US was estimated at $62.7 billion, with nearly one-third of this cost incurred as direct healthcare costsCitation3.
For both the acute and maintenance phases of schizophrenia antipsychotic therapy remains the cornerstone of disease management. When antipsychotic therapy is continuously adhered to it can notably improve the symptoms of schizophrenia; however, with oral antipsychotic medications, discontinuation and/or irregular or intermittent use are widespread, significantly compromising successful management of the diseaseCitation4,Citation5. West et al.Citation6 reported that 37% of adult patients with schizophrenia in the US experience problems with treatment adherence. Other studies report the frequency of treatment non-adherence among patients with schizophrenia is greater than 50%, with partial adherence approaching 90%Citation5. Non-adherence to anti-psychotic therapy among patients with schizophrenia is a strong predictor of relapses and rehospitalizations, which for just Medicaid insured patients the associated healthcare costs were estimated at nearly $1.5 billion in 2005Citation7.
Long-acting injectable (LAI) formulations of antipsychotics, commonly referred to as ‘depot’ antipsychotics were developed with the primary intent of improving adherence to antipsychotic therapy. Results from clinical trials suggest that LAI formulations reduce the incidence of relapses, although randomized controlled trials comparing LAI antipsychotics with oral formulations have yielded inconclusive results regarding adherence and patient outcomesCitation8. Arguably, clinical trial patients are not representative of the patient population with schizophrenia seen in routine clinical practice as they are much more likely to adhere to antipsychotic therapy, making treatment differences difficult to discern.
A recently conducted observational study of 435 schizophrenia patients in the real world reported that, among schizophrenia patients who initiated LAI therapy with risperidone, hospitalizations for any reason decreased by 56% and schizophrenia-related hospitalizations decreased by 40%Citation9. Also in 2011, Ren et al.Citation10 reported that, among schizophrenia patients within the Veterans Health Administration, inpatient healthcare resource use and schizophrenia relapses significantly declined after initiating LAI risperidone therapy. These observational studies provide evidence that, in the real world, LAI risperidone therapy significantly improves the management of schizophrenia among certain populations of patients with schizophreniaCitation9,Citation10. In this study we evaluated hospital resource usage and schizophrenia relapses 12 months before and 12 months after initiation of any of the currently available LAI formulations in the US (fluphenazine, haloperidol decanoate, and risperidone) and compared it to that of patients with schizophrenia patients who initiated oral antipsychotic therapy in an effort to compare outcomes of the different treatment modalities. Additionally, we included both commercially insured schizophrenia patients and Medicare insured schizophrenia patients in our study population, to make the study population more generalizable to a large proportion of patients with schizophrenia in the real world.
Methods
Study design
This was a retrospective study of commercially insured and Medicare insured patients with schizophrenia in which cohorts were defined by the formulation of antipsychotic medication initiated: LAI vs Oral.
Study populations
Patients with schizophrenia who initiated the use of LAI and oral antipsychotics were identified from the research databases between January 1, 2005 and September 30, 2010. The databases consist of healthcare claims data from ∼100 different insurance companies and include inpatient and outpatient information, fully integrated health and productivity data, laboratory data, and detailed hospital drug data. The databases facilitate longitudinal studies and have been used to evaluate real-world outcomes of patients with schizophreniaCitation11,Citation12. Commercially insured patients with schizophrenia were identified and their data extracted from the Commercial Claims and Encounters Database that contains claims of employees and their dependents covered under a variety of fee-for-service and capitated health plans. Medicare insured patients with schizophrenia were identified and their data extracted from the Medicare Supplemental Databases which contain the healthcare claims of individuals with Medicare supplemental insurance paid for by employers. The date at which LAI or oral antipsychotic treatment was initiated was defined as the index event, with the associated date as the index date.
Inclusion of claims from either study population required that patients be ≥13 years of age at the year of index date, that they had at least one inpatient or two outpatient visits on separate dates with a primary or secondary diagnosis of ICD-9-CM code 295.X prior to the index event, and that they had at least 12 months continuous medical and prescription drug coverage prior to the index event (baseline period) and after the index event (follow-up period). Each of the commercially insured and Medicare insured study populations were then separated into two study cohorts, one of patients who initiated LAI antipsychotics (LAI cohort) and the other of patients who initiated oral antipsychotics (Oral cohort). Since this study did not involve ‘identifiable human subjects’, it was exempt from Institutional Review Board overview under the Common Rule (45 CFR §46.101(b)(4))Citation13.
Baseline measurements
Baseline demographics consisting of age, geographic region, health plan type and index antipsychotic medication usage and baseline clinical characteristics, consisting of Deyo-Charlson Comorbidity Index (CCI), used to measure overall disease severity and comorbidities among patients and comorbid conditions, were evaluated for each LAI and Oral cohort within the study populations.
End-point measurements
Medication adherence, hospitalizations, and length of stay (LOS) were evaluated during the baseline and follow-up 12-month time periods. A patient’s medication adherence was described by a medication possession ratio (MPR). The MPR was calculated as the total number of days of drug supply during the baseline or follow-up period divided by the total number of days in the corresponding period and reported as the mean ± standard deviation. The mean number of hospitalizations and LOS for all cause and schizophrenia-related inpatient treatment during the baseline and follow-up periods were determined and compared among patient cohorts in order to assess the impact of initiating LAI or oral antipsychotics. The differences in hospitalizations and relapse incidence for the LAI and Oral cohorts between the follow-up period and the baseline period were also compared to one another in order to evaluate their relative effects on patient outcomes.
Statistical analyses
Descriptive and bivariate statistics were used to evaluate differences in patient demographics and clinical characteristics, with p-values provided by chi-square test and t-test when appropriate. Bivariate statistics were also used to evaluate the differences in the overall hospitalizations and relapse rates at the unadjusted data level by t-test. A generalized linear model was used to carry out multivariate regression to evaluate the impact of initiating LAI vs oral antipsychotics on the differences in the number of hospitalizations and LOS between follow-up and baseline periods. The analysis accounted for the following covariates: age, gender, region, health plan type, Deyo-Charlson Comorbidity Index, and index antipsychotic drug. A p-value of 0.05 was used to determine the level of statistical significance. All statistical analyses were carried out using SAS 9.2.
Results
Study populations
Of the 3004 patients with schizophrenia who met the inclusion criteria and who were commercially insured, 394 initiated LAI antipsychotics, while 2610 initiated oral antipsychotics. Of the 665 patients with schizophrenia who met the inclusion criteria and who were insured by Medicare, 147 initiated LAI antipsychotics while 518 patients initiated oral antipsychotics. Oral antipsychotic medications initiated included most frequently risperidone, aripiprazole, quetiapine, olanzapine, and to a much lesser extent haloperidol, paliperidone, clozapine, perphenazine, and fluphenazine. LAI antipsychotic medications initiated included risperidone, haloperidol, and fluphenazine.
Patient demographics
All evaluated demographics for both the commercially insured and Medicare insured study populations are reported in . Among patients with schizophrenia who were commercially insured, patients initiating LAI vs oral antipsychotics were older (41.7 vs 37.1 years of age, p < 0.001) and a greater proportion of them had comprehensive healthcare coverage (18.8% vs 9.8%, p < 0.001), while a smaller proportion had Health Maintenance Organization (17.8% vs 26.1%, p < 0.001) or Point-of-Service (9.6% vs 13.0%, p < 0.001) health plan coverage. A greater proportion of patients initiating LAI vs oral antipsychotics lived in the North Central US (47.0% vs 29.5%, p < 0.001), while more patients initiating oral antipsychotics were located in the Western US (24.9% vs 9.4%, p < 0.001).
Table 1. Baseline demographics of commercially and Medicare insured LAI and oral cohorts.
Among patients with schizophrenia who were insured by Medicare, those initiating oral vs LAI antipsychotics were older (73.2 vs 67.2 years of age, p < 0.001) and more often lived in the Western US (15.6% vs 2.7%, p < 0.001). A greater proportion of patients initiating LAI vs oral antipsychotics lived in the North Central US (66.7% vs 44.8%, p < 0.001).
Patient clinical characteristics
All evaluated clinical characteristics for both the commercially insured and Medicare insured study populations are reported in . CCI scores were comparable between the commercially insured LAI and Oral cohorts. Of the commercially insured study population, a greater proportion of patients initiating LAI antipsychotics were diagnosed with diabetes (15.0% vs 9.7%, p = 0.001) or peripheral vascular disease (2.3% vs 1.0%, p = 0.026) in comparison to patients initiating oral antipsychotics. The most common comorbid conditions among commercially insured LAI and Oral cohorts were diabetes and chronic pulmonary disease (12.2% vs 11.5%).
Table 2. Baseline clinical characteristics of commercially and Medicare insured LAI and oral cohorts.
Of the Medicare insured study population, patients initiating oral antipsychotics had higher CCI scores (1.83 vs 1.24, p < 0.001), indicating a higher level of overall comorbidity. Also, higher proportions of patients in the Oral cohort were diagnosed with peripheral vascular disease (11.6% vs 5.4%, p = 0.030), cerebrovascular disease (24.5% vs 12.9%, p = 0.003), dementia (19.7% vs 11.6%, p = 0.023), and cancer (9.1% vs 2.7%, p = 0.011). Similar to the commercially insured study population, the most common co-morbid conditions among Medicare insured LAI and Oral cohort patients were diabetes, 27.9% and 26.1%, respectively, and chronic pulmonary disease, 21.8% and 23.8%, respectively.
Medication adherence
Medication adherence was significantly greater among patients of the LAI cohort during the follow-up period in comparison to before initiating LAI antipsychotic therapy for both the commercially insured (0.67 ± 0.34 vs 0.40 ± 0.37, p < 0.001) and Medicare insured (0.68 ± 0.34 vs 0.42 ± 0.39, p < 0.001) study populations (). During the follow-up period, among the commercially insured and Medicare insured study populations, the LAI cohort exhibited greater medication adherence in comparison to the Oral cohort (Commercial: 0.67 ± 0.34 vs 0.56 ± 0.35, p < 0.001; Medicare: 0.68 ± 0.34 vs 0.59 ± 0.36, p = 0.005).
Table 3. Medication adherence, hospitalizations, and relapses of commercially and Medicare insured LAI and oral cohorts during the baseline and follow-up periods.
Healthcare resource usage during baseline and follow-up periods
additionally contains the mean number of hospitalizations and mean LOS, all cause and schizophrenia-related during the baseline and follow-up periods for the LAI and Oral cohorts of the study populations. After initiation of antipsychotic medications, there was a significant reduction in the mean number (±standard deviation) of all cause hospitalizations (1.60 ± 1.66 vs 0.70 ± 1.20, p < 0.001) and mean LOS (16.93 ± 20.68 vs 6.64 ± 14.37 days, p < 0.001) for commercially insured LAI patients compared to baseline, a trend that was also observed among LAI patients insured by Medicare. Similarly, after initiation of antipsychotic medications, there was a significant reduction in the mean number of schizophrenia-related hospitalizations (1.03 ± 1.26 vs 0.43 ± 0.86, p < 0.001) and mean LOS (12.28 ± 17.74 vs 4.82 ± 12.80 days, p < 0.001) for commercially insured LAI patients in comparison to baseline, a trend that was also observed among LAI patients insured by Medicare.
Differences in hospitalizations and LOS, all cause and schizophrenia-related between the baseline and follow-up periods for the LAI cohort vs the Oral cohort
Reported in are the differences in hospitalizations (a) and LOS (b) for the study populations between the baseline and follow-up time periods. Among the commercially insured population, patients initiating LAI vs oral antipsychotics had much greater reductions in hospitalizations for any cause (−0.90 ± 1.77 vs 0.02 ± 1.49, p < 0.001) and associated LOS (−10.29 ± 23.23 vs 0.70 ± 16.73, p < 0.001) between the baseline and follow-up periods. Moreover, the number of schizophrenia relapses requiring inpatient care (−0.60 ± 1.37 vs 0.05 ± 0.99, p < 0.001), as well as the associated LOS (−7.46 ± 20.68 vs 0.60 ± 12.49, p < 0.001) were more significantly reduced between the baseline and follow-up periods for patients initiating LAI antipsychotics in comparison to those initiating oral antipsychotics. These results were similar among patients insured by Medicare ().
Multivariate analysis
After adjustment for patient characteristics, usage of LAI antipsychotic medications vs oral was associated with a decline in the number of hospitalizations, all cause = −0.85 (Confidence Interval (CI): Lower: −0.65, Upper: −1.05; p < 0.0001) and schizophrenia-related = −0.61 (CI: −0.47, −0.74; p < 0.0001), and LOS, all cause = −10.3 days (CI: −8.0, −12.6; p < 0.0001) and schizophrenia-related = −7.5 days (CI: −5.7, −9.3; p < 0.0001) during the follow-up period in comparison to the baseline period. Similar results were observed among LAI and oral patient cohorts who were insured by Medicare ().
Table 4. Multivariate regression results for evaluation of initiating long-acting injectable antipsychotics vs oral on the differences in hospital resource usage between the baseline and follow-up periods.
Discussion
This study, involving more than 3600 patients, showed that commercially and Medicare insured patients with schizophrenia, who initiate LAI vs oral antipsychotics, have greater reductions in the number of hospitalizations for any cause and for schizophrenia relapses after treatment initiation compared to before treatment began. These results were further supported by multivariate analyses in which patient differences were adjusted. The substantial decline in hospital resource usage and relapses after initiation of LAI antipsychotics may be attributed to greater adherence to antipsychotic therapy.
Brissos et al.Citation14 reported that, among patients with schizophrenia, remission of symptoms significantly improves social functioning and patients’ quality-of-life. However, a large observational study, conducted in the US, reported that only 10% of patients with schizophrenia have a sustained favorable outcome, as predicted by symptom severity, level of functioning, and use of acute care servicesCitation15. These studies underscore the fact that it is definitely possible to treat this debilitating and burdensome disease, but better ways and/or adjustments to existing methods are required for treatment to be more efficacious for a greater number of patients with schizophrenia.
Currently, the predominant method of treating schizophrenia is oral antipsychotic therapy, which is effective in ameliorating symptoms and reducing relapses; however, its effectiveness is dependent on continuity of treatmentCitation16. A study that evaluated the relationship between oral antipsychotic medication adherence and relapses found that poorly adherent patients with schizophrenia are more than twice as likely to be admitted to the hospital and have longer hospital stays in comparison to patients who have better adherence to oral antipsychotic medicationsCitation17. Another study on a cohort of California Medicaid patients with schizophrenia reported that, as adherence to antipsychotic therapy decreased, the risk of hospitalization significantly increased, with a more than 30-day medication gap correlating with nearly a 4-fold increased risk of hospitalizationCitation18.
In our study commercially insured patients with schizophrenia who initiated LAI antipsychotic medications demonstrated greater adherence during the follow-up period and had approximately a 56% reduction in the number of hospitalizations for any cause and a 58% reduction in the number of hospitalizations for schizophrenia after LAI treatment initiation compared to before treatment began. Furthermore, the length of stay for hospitalizations, all cause and schizophrenia-related, declined by 60%. These results were similar for Medicare insured patients with schizophrenia. Our data is comparable to that of Crivera et al.Citation9, who reported a 41% reduction in all cause hospitalizations and a 56% reduction in hospitalizations for schizophrenia among patients with schizophrenia after initiating risperidone LAI therapy compared to before treatment began. In our study, patients with schizophrenia who began oral antipsychotic therapy did not have improved disease management, as neither the number of all cause hospitalizations nor schizophrenia-related hospitalizations were reduced after treatment compared with before initiation of treatment. In fact, hospitalizations for relapses increased slightly for the commercially insured cohort who took oral antipsychotic therapy. Randomized clinical trial results have not consistently supported that the usage of LAI antipsychotics instead of oral antipsychotics improves adherence or reduces relapse ratesCitation19. However, as mentioned, using clinical trial data to evaluate whether LAI agents provide a benefit for adherence or clinical outcomes over oral antipsychotic medications is difficult since patients involved in clinical trials are monitored to a much greater extent and are more likely to adhere to treatment.
In clinical practice, LAI antipsychotic medications provide both an assurance that treatment is delivered and a mechanism for monitoring treatment adherence. Despite these advantages of LAI antipsychotic medications, according to studies conducted in the US, they were only prescribed to between 19–30% of patients, who had demonstrated recent medication non-adherenceCitation20,Citation21. In our study, only 13% of the commercially insured and 22% of the Medicare insured patient populations were prescribed LAI antipsychotics. Therefore, there appears to be a reluctance to prescribe and/or use LAI antipsychotics. Additionally, new atypical antipsychotics are not widely available as LAI formulations; in fact risperidone LAI was the only atypical represented in our study. Thus, any benefits of the newer atypical agents as LAI formulations were not captured in our study.
We additionally found that patients who initiated LAI antipsychotics have a much greater disease severity initially, as indicated by their higher number of schizophrenia-related hospitalizations in comparison to those who began oral antipsychotic treatment, a point that has been reported elsewhereCitation22. In current clinical practice, patients with schizophrenia are commonly started on oral antipsychotic medications and are only switched to an LAI agent after chronic non-adherence is recognized. This therapeutic sequence potentially selects the most treatment resistant and severely affected patients for LAI antipsychotic therapy, thus creating a bias in any real-world assessment of its effectiveness. Despite this bias, the greater response of patients to LAI antipsychotics during the follow-up period in our study suggests that earlier initiation of them may be beneficial given the observed scale of reductions in healthcare resource usage and relapses.
The LAI and Oral patient cohorts also differed in multiple other patient and clinical characteristics. Therefore, we took the approach of comparing pre- and post-index hospital resource usage, such that each patient essentially served as a control for the comparison of outcomes between the baseline and follow-up time periods. In addition to the likelihood of having a direct impact on hospital costs of care for schizophrenia patients, the marked reduction in the incidence of relapses and hospital length of stay will likely allow for patients using LAI antipsychotics to function better socially, maintain a job with less absenteeism, abuse substances to a lesser degree, and be involved in less violence, all of which could potentially contribute towards a large reduction in the indirect costs of schizophrenia, which in the US in 2002 were estimated at $32.4 billionCitation23.
Limitations
While our study provides valuable insight into the healthcare impact of initiating LAI vs oral antipsychotics among patients with schizophrenia in routine clinical practice, as with all studies utilizing healthcare databases, there are inherent limitations. The databases used consist of claims submitted by healthcare providers to insurance companies for reimbursement on behalf of individuals employed by various companies, and such claims are subject to possible coding errors, coding for the purpose of rule-out rather than actual disease, and under-coding, either by the healthcare provider or due to limitations imposed by the databases. Changes in employment status or employer can limit the amount of continuous data available and, consequently, constrain the study sample sizes available for analysis. In addition, the claims databases used are based on a large convenience sample and, because the sample is not random, it may contain biases or fail to generalize well to other populations, particularly those who have alternate healthcare coverage such as those provided by the government (Medicaid, Medi-Gap, etc.). Additionally, the demographics and clinical characteristics of the patients recorded in the claims databases used in this study may not match with the overall US population. Also, of the LAI antipsychotics initiated a greater proportion were typical antipsychotics, which may have different risk benefit profiles than atypical antipsychotics and, therefore, limit the results of this study.
Conclusions
Patients, insured commercially or by Medicare, who initiated LAI vs oral antipsychotics, had a much greater reduction in the number of hospitalizations and schizophrenia relapses occurring after treatment initiation compared with before treatment began. The fact that patients just starting to use LAI antipsychotics have fewer relapses and use healthcare resources less after treatment initiation implies that an earlier introduction to them may provide a considerable benefit to patient outcome and potentially reduce the burden on healthcare resources.
Transparency
Declaration of funding
This research was supported by Otsuka America Pharmaceutical, Inc.
Declaration of financial/other relationships
Steve Offord and Dario Mirski are employees of Otsuka America Pharmaceutical, Inc. Bruce Wong is a paid consultant for Otsuka America Pharmaceutical, Inc. in connection with conducting this study and development of this manuscript. Ross Baker is an employee of Otsuka Pharmaceutical Development and Commercialization, Inc. Jay Lin is an employee of Novosys Health, which has received research funds from Otsuka America Pharmaceutical, Inc. in connection with conducting this study and development of this manuscript.
Acknowledgments
We would like to acknowledge Melissa Lingohr-Smith from Novosys Health in the editorial support and review of this manuscript.
References
- McGrath J, Saha S, Welham J, et al. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med 2004;2:13
- Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med 2005;2:e141
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122-9
- Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002;63:892-909
- Lambert T, Olivares JM, Peuskens J, et al. Effectiveness of injectable risperidone long-acting therapy for schizophrenia: data from the US, Spain, Australia, and Belgium. Ann Gen Psychiatry 2011;10:10
- West JC, Wilk JE, Olfson M, et al. Patterns and quality of treatment for patients with schizophrenia in routine psychiatric practice. Psychiatr Serv 2005;56:283-91
- Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin 2007;23:2305-12
- Adams CE, Fenton MK, Quraishi S, et al. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br J Psychiatry 2001;179:290-9
- Crivera C, DeSouza C, Kozma CM, et al. Resource utilization in patients with schizophrenia who initiated risperidone long-acting therapy: results from the Schizophrenia Outcomes Utilization Relapse and Clinical Evaluation (SOURCE). BMC Psychiatry 2011;11:168
- Ren XS, Crivera C, Sikirica M, et al. Evaluation of health services use following the initiation of risperidone long-acting therapy among schizophrenia patients in the veterans health administration. J Clin Pharm Ther 2011;36:383-9
- Citrome L, Reist C, Palmer L, et al. Impact of real-world ziprasidone dosing on treatment discontinuation rates in patients with schizophrenia or bipolar disorder. Schizophr Res 2009;115:115-20
- Crown WH, Neslusan C, Russo PA, et al. Hospitalization and total medical costs for privately insured persons with schizophrenia. Admin Policy Ment Health 2001;28:335-51
- US Department of Health and Human Services. Code of Federal Regulations. Human Subjects Research (45 CFR 46). US Department of Health and Human Services. Washington, DC. 20201.
- Brissos S, Dias VV, Balanzá-Martinez V, et al. Symptomatic remission in schizophrenia patients: relationship with social functioning, quality of life, and neurocognitive performance. Schizophr Res 2011;129:133-6
- Cuyun Carter GB, Milton DR, Ascher-Svanum H, et al. Sustained favorable long-term outcome in the treatment of schizophrenia: a 3-year prospective observational study. BMC Psychiatry 2011;11:143
- Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004;30:193-217
- Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care 2002;40:630-9
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004;55:886-91
- Schooler NR. Relapse and rehospitalization: comparing oral and depot antipsychotics. J Clin Psychiatry 2003;64(1 Suppl):14-17
- West JC, Marcus SC, Wilk J, et al. Use of depot antipsychotic medications for medication nonadherence in schizophrenia. Schizophr Bull 2008;34:995-1001
- Valenstein M, Copeland LA, Owen R, et al. Adherence assessments and the use of depot antipsychotics in patients with schizophrenia. J Clin Psychiatry 2001;62:545-51
- Shi L, Ascher-Svanum H, Zhu B, et al. Characteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophrenia. Psychiatr Serv 2007;58:482-8
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122-9