Abstract
Objective:
To analyze the achievements, issues and policy recommendations for implementing essential medicine system in China after a 3-year effort.
Methods:
Policy documents analysis and Literature reviews are conducted.
Results:
From 2009–2011, a series of national essential medicine (EM) policies has been established which contain EM list, organizing production, quality assurance, pricing, tendering and procurement, distribution, rational use, monitoring and evaluation, etc. About 98.8% government-run primary healthcare institutions and 41.5% village health posts are conducting zero-mark-up policy while buying EMs. The average cost per visit, per admission, and per description in outpatient and inpatient departments has declined. The Issues with the national EM list cannot meet the requirements of clinical practice at the local level, all provinces have to increase additional 64–455 EMs in their local supplementary list; the limitation of EML in primary healthcare institutions causes patients to transfer directly to secondary or tertiary hospitals to search appropriate treatment; there is no defined regulation or legislation regarding the responsibility and accountability of government to compensate for the financial loss after implementing a zero mark-up policy in primary healthcare institutions. In the future, some innovative reform should be taken into account, such as revising EML, quality assurance, control margins within the distribution system, differential pricing and internal reference-based pricing, waive taxes and import duties of EMs, and separation between prescribing and dispensing in public hospital reform.
Conclusions:
Establishing a national essential medicine system is a difficult task to accomplish. The role of the zero-mark-up policy of EMs is to cut off the economic profit chain among different stakeholders. Using pharmaceutical profit to subsidize hospital revenue will be gradually eliminated in China.
Introduction
In April 2009, the Central Committee of the Chinese Communist Party and State Council issued a set of guidelines on medical and pharmaceutical system reformCitation1 together with a notice from the State Council on the implementation plan based on priorities of reform during 2009–2011Citation2. Five key reform areas were raised: (1) acceleration of setting up of a basic health insurance system; (2) preliminarily establishment of a national essential medicines system; (3) optimization of service for primary healthcare; (4) steady improvement in access to fundamental public health services; and (5) further advancement of public hospital services.
The establishment of a national essential medicine system is one of the most important components of China healthcare reform. Consequently, the Chinese government and related sectors issued a series of complementary policy documents on formulating a national essential medicine list, hospital formulary, drug procurement, tendering, supervision and quality assurance. A brief description of the essential medicine policy, and its pros and cons, are discussed here.
The main contents of the essential medicine policy framework in China include ensuring the selection, production, and distribution of essential medicines; maintaining quality standards; and setting reasonable prices, conducting a zero-mark-up policy on sales, promoting rational use and reimbursement, as well as monitoring and evaluation. The aim of the policy is to ensure access to, and the safety and provision of, adequate amounts of essential medicines to all who need them. The preliminary establishment of the national essential medicine system was accomplished in 2011, and a comprehensive national essential medicine system will be fully implemented in urban and rural China by 2020.
To some extent, China’s essential medical system is specific to Chinese medicine, which is different from the policies of other developed countries. Within this context, the essential medicine policy also has the role of eliminating drug margins as a compensation mechanism in hospitals. There are three parallel policies being carried out aimed at expanding the national essential medicines coverage: (1) All urban community health centres and rural township health centres are allowed to store and prescribe essential medicines; (2) The implementation of a zero-mark-up policy for essential medicines; and (3) The eradication of the 15–20% drug margins between wholesale and retail in hospitals in lieu of local government subsidies, or through the separation of revenue and expenditure accounts in comprehensive public hospital reform. The objective is to reduce the economic burden on patients when in need of medical care.
Achievements of the essential medicine system in China
After a 3-year effort, from 2009–2011, the essential medicine system has been established in China. It contains an essential medicine list, organizing production, quality assurance, pricing, tendering and procurement, distribution, rational use, monitoring and evaluation, etc. In those implemented areas, the number of clinical visits and hospitalizations is continuously increasing, and the average cost per visit, per admission, and per description in outpatient and inpatient departments is declining. The prices of essential medicines are significantly reduced. In 2011, all government-run grassroots health institutions implemented the policy; and the whole process of electronically monitoring quality is being conducted in the production and distribution system.
Although the first national essential medicine list was put into effect in China in 1982, which contained 278 chemical and biological medicines, it was not made a national policy, nor was it a comprehensive essential medicine system until 2009Citation3. The 2009 version of the essential medicine list contained 307 generic medicines, which included 205 chemical and biological medicines and 102 traditional Chinese medicines (TCM) and all herbal preparations (). The criteria for selection of an essential medicine are as follows:
Necessity for prevention and treatment;
Safety and clinical efficacy;
Reasonable price;
Convenient use; and
Balance between chemical, biological, and TCMs.
Table 1. The number of drugs in essential medicine list in China.
The selection of essential medicines is mainly based on several rounds of expert consultation and key opinion leader suggestions on best practice, rather than using health technical assessment and pharmacoeconomic evaluation.
There are three basic medical insurance schemes in China; namely, urban employee basic medical insurance (UEBMI), urban resident basic medical insurance (URBMI), and the new co-operative medical scheme (NCMS). Each scheme has its own drug reimbursement listCitation4, but UEBMI has a more extensive drug coverage. For example, in the latest version of UEBMI, a total 2151 drugs can be reimbursed, which include 1164 chemicals and biologics and 987 TCMs (). In other words, all 307 essential medicines are included in Class A in the UEBMI reimbursement list, resulting in a higher reimbursement ratio of essential medicines than non-essential medicines. However, patients self-pay 10–15% of the drug price if taking medicines in Class B.
Table 2. The comparison of number of medicines in the reimbursement drug list between 2004 and 2009 in UEBMI.
The method of procurement for essential medicines is bulk purchasing. The approaches used in the bidding process are the ‘Two Envelope Selective Tender System’Citation5, combined with price–volume agreement and procurement. The period of financial repayment to the manufacturers is less than 3 months. A head procurement agency has been established in 31 provinces and municipalities. It is a government-led, non-profit agency to help bid the tenders for the manufacturers through the internet to ensure the supply of essential medicines. This resulted in a significant reduction in the average price of essential medicines in different provinces by 25% ().
Table 3. The average price reduction of essential medicines after tendering (2010).
All government-run primary healthcare institutions in China, including urban community and rural township health centres, underwent comprehensive reform in 2011 regarding the use of essential medicines. Now, the essential medicine system is even adopted in village health posts in seven provinces and municipals (Shanghai, Chongqing, Yunnan, Xizang, Gansu, Qinghai, and Ningxia). About 40% of village health posts are conducting zero-mark-up policy in essential medicines (). In addition, 13 provinces, such as Henan, Zhejiang, and Anhui, have regulated the volume of essential medicines in urban tertiary and secondary hospitals to 15–25% and 25–35%, respectively.
Table 4. The evaluation of national essential medicine policy in China.
After implementing the zero-mark-up policy in the essential medicine system, the average cost per clinical visit and drug cost per prescription has decreased. The economic burden of population has been released significantly. The number of ambulatory visits and admission to the hospitals is increasing in all regions. A satisfaction study in 1639 patients from 15 cities in five provinces showed that 66.5% patients felt the price of essential medicines was significantly reduced and that the economic burden on the patient was reducedCitation6.
Issues with the essential medicine system in China
The first issue faced by primary healthcare institutions is that 307 essential medicines are not enough to meet the needs of clinical utilization. Because of the variation in local socio-economic conditions and the reality of drug utilization in different provinces, provincial and local governments are able to adjust the national essential medicine list and select non-national essential medicines into the provincial supplementary essential medicine list. The number of pharmaceutical products, quantity and dosages selected is based on the characteristics of clinical use, requirements in medical settings, and reference to international experience.
All provinces have now increased the number of essential medicines in their local supplementary list ()—varying from an additional 64–455 medicines—which indicates that the national essential medicine list has lost its authority. A study on the utilization of essential medicines in 5812 grassroots healthcare institutions showed that only 213 essential medicines (49%) in the provincial supplementary essential medicine list are consistent with the national essential medicines list. Many low-efficiency medicines are present in provincial supplementary lists. On the one hand, this illustrates that nationally designated essential medicines cannot meet the requirements of clinical practice at the local level; and, on the other hand, it shows a lack of unified standards in the selection of additional essential medicines. Therefore, the criteria for drug selection at the provincial level needs further studyCitation7.
Figure 1. The number of essential medicines added in the provincial supplementary essential medicine list. Source: IBM.
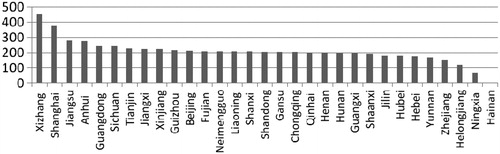
The second issue is the impact on patient flow since implementation of the essential medicine policy. The limitation of essential medicines in primary healthcare institutions causes patients to transfer directly to secondary or tertiary hospitals to get appropriate treatment. This has a negative impact on the patient, as medical care is not available at the first place of contact. Sometimes it also weakens clinical performance and leads to a financial deficit and brain drain in community healthcare centres.
The third issue is the feasibility of financial subsidies from local government for implementing the zero mark-up policy. The main hurdle is that there is no defined regulation or legislation regarding the responsibility and accountability of government to compensate for financial loss after implementing a zero mark-up policy in primary healthcare institutions.
China has more than 8000 pharmaceutical manufacturers and 20,000 distribution firms; most of which have GMP and GSP accreditation approved by the National Food and Drug Administration Bureau and the Bureau of Quality Control. In the ‘Two Envelope Selective Tender System’, the content of first envelope, which reflects technical indicators such as capacity of production, clinical efficiency, modern logistics facilities, and economic scale, is actually not meaningful. The only criterion in the second envelope is the price of medicine; the lower the price, the higher the likelihood of a successful tender. In that case, the bidding manufacturers are encouraged to lower quality to save cost. If the bidding price is much lower than the actual cost, it would not make the provision of essential medicines in the market sustainable.
Usually, the process of tendering and procurement is separate. The tendering function is run by the government-led, non-profit agency, but there is no market power to purchase drugs from the manufacturers at the same time. The real purchasing power is from the individual hospital. The hospital will negotiate with manufacturers again to get bigger margins before purchasing the essential medicines. The authority of government procurement is therefore rendered unfeasible. Another phenomenon is that some large-scale pharmaceutical manufacturers with high-quality products are unable to obtain market share because the price is not low enough.
The Price Bureau of the National Development and Reform Commission manages price setting in China. They set all drug prices on the basis of maximum retail price, but sometimes the bidding price of essential medicines is higher than the actual price due to market competition.
At present, tendering has used a system of sole supplier, price–volume agreement, and has preferred to use the cost-plus approach to pricing essential medicines. In other words, the price of tendering is dependent on the volume of procurement and the period of payback to the pharmaceutical manufacturers. However, the sole supplier for each medicine causes a market monopoly. Once the required supply of medicines cannot be achieved, then a shortage of certain medicines will occur.
The future challenges of the essential medicine system in China
Strengthening the essential medicine system is one of three prioritized healthcare reform measures in the next 5-year plan in ChinaCitation8.
Dynamic adjustment of the essential medicine list
The China national essential medicine list will be revised every 3 years. The present adjustment of the essential medicine list will be announced at the end of 2012 in order to meet the needs of basic healthcare, which includes appropriate increasing the number of essential medicines, standardization of products, dosages, and package size, adding more elderly and children medicines, and chronic patient care.
Before working on revising the national essential medicine list, the criteria and standards of selection should be reconsidered. The impact of the fourth hurdle, including access to more cost-effective drugs, evidence of clinical values, and budget impact analysis, will be emphasized again. Even if the provincial supplementary essential medicine list is maintained, empowerment should not be extended to the city, district, or county level.
Expanding coverage of the essential medicine system
In 2011, 98.8% of government-run grassroots healthcare institutions had adopted the essential medicine system and zero-mark-up policy. The next step will be to consolidate the implementation in all government-run grassroots healthcare institutions and to gradually expand to all village health posts. However, an appropriate government subsidy policy to solve the remuneration and pension system of the village doctors is required.
In addition, coverage of the essential medicine system is also required to be conducted in non-government (private) grassroots healthcare institutions. It is, however, difficult to adopt the policy unless medical services are purchased by the government.
Integration of the essential medicine system into the three-tier hospital system
It is necessary to encourage secondary and tertiary hospitals to use essential medicines so as to favour the transfer of patients between all level hospitals. To guide the patient flow to the grassroots health institution, increasing the number of pharmaceuticals on the essential medicine list is required, especially for the use in children, elderly, and chronic care of hypertension and diabetes.
Another issue is whether having some non-essential medicines in grassroots healthcare institutions is allowed or not. From the continuous healthcare point of view, if a patient is transferred and periodically consulted by a higher-level hospital, then the patient could get some non-essential medicines at grassroots healthcare institutions under the supervision. Eliminating the need to go to higher-level hospitals frequently will save costs. Some pilot studies have been conducted in China in which the poor can dispense some essential medicines free of charge, with the cost covered by the government.
Price setting for essential medicines
After running the tendering system for 3 years, there are now many sole suppliers. To ensure the quality of essential medicines and to offer price reductions, some deferred tenders could be selected to produce chemical entities purchased by government.
Some innovative reform of the essential medicine system should be taken into account, such as quality assurance, volume–price agreement, control margins within the distribution system, differential pricing and internal reference-based pricing, waive taxes and import duties in essential medicines, etc.
Separation between prescribing and dispensing
In China, 40% of national health accounts are from pharmaceutical expenditure. Essential medicines cost ∼25% of total pharmaceutical expenditure. The separation between prescribing and dispensing is a future direction of public hospital reform. The purpose is to delink hospital revenue from margins on drug sales so as to avoid over-use of medicines.
To cut the economic chain between pharmaceutical expenditure and hospital medical service, government should provide more financial subsidies to hospitals, including basic investment in buildings and equipment, human resources, and technical development. The operation costs of a hospital will be borne by medical insurance schemes through purchasing health services and price negotiation.
In the grassroots healthcare institution, the financial policy will be conducted through separation of revenue and expenditure accounts. Government will subsidize financial losses after implementing a zero mark-up policy in essential medicines and some recurrent costs. Expanded medical insurance coverage and improving benefit packages and integration of hospitals and primary healthcare institutions to form a multi-hospital system will be the future of healthcare reform in China.
Conclusion
In sum, establishing a national essential medicine system is not only the priority of heath system reform in China, but is also a difficult task to accomplish. The role of the zero mark-up policy of essential medicines is to cut off the economic profit chain among different stakeholders, such as government financial departments, third-party payers, pharmaceutical manufacturers and distributors, hospitals, doctors, and patients. In the recent public hospital reform, using pharmaceutical profit to subsidize hospital revenue will be gradually eliminated.
Transparency
Declaration of funding
The author has received no funding in preparation of this manuscript.
Declaration of financial/other relationships
The author declares no conflicts of interest.
Acknowledgements
English language editing was provided by JME Editor-in-Chief, Professor Kenneth Lee, of Monash University and Mr Phil Garner, employed by Informa Business Information, the same parent company as Informa Healthcare.
References
- CPC Central Committee and the State Council. The opinions of deepening health care system reform. 2009. Published by China CPC Office. No. 6, Beijing
- China State Council. An implementation plan of recent priority areas in the health care system reform (2009–2011). 2009. Published by State Council No. 12, Beijing
- Hu SL. Financing, pricing, and utilization of pharmaceuticals in China: the road to reform. China Health Policy Notes. The World Bank, Health, Population and Nutrition. East Asia and Pacific Region. Washington DC 20433 USA. 2010
- Yip W, Hsiao WC, Chen W, et al. Early appraisal of China’s huge and complex health-care reforms. The Lancet 2012;379:833-42
- Chaudhury RR. Implementation of a programme of rational use of drugs in Delhi State. International experience in rational use of drugs. Bangkok, Thailand: Chulalongkorn University, 1995. p 108–23
- Fan WJ, Jiang HL, Chen W, et al. A study on satisfaction of doctors and patients for National essential medicine system. Chin J Health Inform Man 2011;9:36-9
- Jiang HL, Chen MS, Chen W, et al. The preliminary effectiveness and problem analysis in the implementation of national essential medicine system. Chin J Health Inform Man 2011;9:40-3
- China State Council. Deepening health care reform in twelfth-five year period and its implementation plan. State Council, Beijing. 2012
- Health Statistics & Information Center. The monitoring and evaluation of healthcare system reform in China. Ministry of Health. November. 2011. (Unpublished)
- IMS China Institute for Healthcare Informatics. Personal communication
