Abstract
Objective:
To evaluate the financial consequences of using laparoscopic adjustable gastric banding (LAGB) in place of standard medical management (SMM) in obese patients with type 2 diabetes from a UK healthcare payer perspective.
Design and methods:
A budget impact model was constructed to evaluate the budgetary implications of LAGB in obese patients with type 2 diabetes in the UK. For patients undergoing LAGB, the model captured pre-, peri-, and post-operative costs including consultations with physicians, psychologists, nurses, and dieticians, the cost of surgery, and costs associated with post-surgical complications. The model also captured costs associated with medication for diabetes, asthma, hypertension, and hyperlipidemia, costs of diabetes complications, sleep apnea, and asthma, and costs of diagnostic tests. The SMM arm also captured costs associated with very low calorie diet products. Costs were modeled in a simulated UK cohort of 100 obese patients with newly-diagnosed diabetes. Future costs were discounted at 3.5% per annum and all costs were reported in 2010 pounds sterling.
Results:
Over the 5-year time horizon, the cohort of 100 patients who underwent LAGB incurred costs £91,287 lower than an equivalent cohort receiving SMM (£818,668 and £909,955, respectively). Costs of surgery and post-surgical complications (£254,000 and £40,981, respectively) were more than offset by savings arising from reduced diabetes, asthma, and sleep apnea medication costs, reduced incidence of diabetes complications, and fewer healthcare professional contacts. Sensitivity analysis (SA) showed that the model was most sensitive to assumptions around diabetes medication use, although none of the SA findings showed LAGB to be more costly than SMM.
Limitations:
In order to capture the diverse resource use and medical care costs arising in obese patients with type 2 diabetes, the analysis made use of a range of heterogeneous data sources. While the vast majority of data were applicable to obese patients with recently-diagnosed diabetes in the UK setting, some surrogate data (e.g. from different geographies) were used in cases where data in the target population were unavailable. Additionally, given the largely uncharacterized long-term risk profile in patients with remission of type 2 diabetes, remission was captured using a transparent and highly conservative approach.
Conclusions:
Based on the findings of the present analysis, the high initial costs of performing LAGB are offset within 5 years after surgery when compared with SMM in a population of obese patients with type 2 diabetes. The high up-front costs associated with surgery should not therefore be a barrier to its reimbursement in this patient group.
Introduction
The prevalence of type 2 diabetes continues to rise in the UK, with a recent estimate placing the number of patients with diagnosed diabetes in England at 2.3 million in 2010, representing 5.6% of the populationCitation1. Based on data from the 2006 Health Survey for England, the same study estimated that a further 0.8 million had undiagnosed diabetes, taking the estimated prevalence to 7.4%. Moreover, the study projected that the prevalence of all diabetes would increase to 9.5% by 2030, representing 4.6 million patients. The situation is similar in Scotland, with one recent study estimating that the 2008 prevalence of all diabetes was 9.4% compared with just 5.2% in 2003Citation2. Whilst these estimates do not distinguish between type 1 and 2 diabetes, type 2 diabetes accounts for ∼90% of all diabetes casesCitation3,Citation4. Type 2 diabetes is associated with numerous microvascular and macrovascular complications, which in turn frequently lead to premature mortality and morbidity and reduced quality-of-life. The increasing prevalence of diabetes has, therefore, become a major global issue clinically, economically, and socially.
Whilst the causes of type 2 diabetes have yet to be fully characterized, obesity is considered to be the primary risk factorCitation5. Indeed, studies have estimated that the risk of developing type 2 diabetes in severely obese patients is 93-times higher in women and 42-times higher in men, relative to patients of healthy weightCitation6,Citation7. More importantly, for overweight or obese patients who already have type 2 diabetes, even modest weight loss has been associated with improvements in glycemic control, hypertension, and dyslipidemiaCitation8. However, findings from the recent Swedish Obese Subjects (SOS) study and meta-analyses of randomized controlled trials have shown that weight loss achieved through diet and exercise alone or in combination with pharmacological intervention is modest and rarely sustained in obese patientsCitation9–11. Conversely, there is an increasing body of evidence to suggest that obese patients with type 2 diabetes can benefit significantly from bariatric surgery in terms of improved metabolic control and reduced risk of complications of diabetesCitation9,Citation12.
In the context of the rising prevalence and incidence of type 2 diabetes and obesity and the mounting evidence supporting the use of bariatric surgery in patients who have these frequently co-morbid conditions, it is becoming increasingly important for healthcare payers to evaluate the budgetary implications of all available treatment options, in addition to the clinical- and cost-effectiveness. With this need in mind, an increasing number of regulatory agencies and managed care organizations now require a budget impact analysis to accompany the cost-effectiveness analysis as part of the health economic evaluation of new healthcare technologiesCitation13. While there is a relative abundance of analyses that estimate the total economic burden of diabetes (either within a given country or globally), there is a paucity of studies reporting findings of budget impact analyses (i.e. studies that assess changes in budget resulting from the introduction of new healthcare interventions). A literature review recently published by Orlewska and Gulácsi.Citation14 found that, between January 2000 and November 2008, only 34 budget impact analyses were published in peer-reviewed journals across all therapeutic areas. Surprisingly, considering the financial consequences of the condition, only one of these focused exclusively on patients with diabetesCitation15.
The aim of the present study was to build a budget impact model to evaluate the financial consequences of introducing laparoscopic adjustable gastric banding (LAGB) into a cohort of obese patients with type 2 diabetes currently receiving obesity treatment in the form of standard medical management in the UK.
Methods
Model
A Microsoft Excel-based budget impact model (BIM) was designed to evaluate the costs of LAGB compared with standard medical management of obesity in patients with type 2 diabetes. (Microsoft and Excel are registered trademarks of Microsoft Corporation, Redmond, WA.) The BIM was designed to capture costs incurred by the respective obesity treatments, but also a number of cost offsets including costs of diabetes medications and costs associated with the treatment of diabetes complications, sleep apnea, and asthma.
The incidence of seven diabetes complications (myocardial infarction, congestive heart failure, ischemic heart disease, stroke, end-stage renal disease, blindness, and amputation) was modeled using equations from the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model, a full description of which has been previously published by Clarke et al.Citation16. For the present analysis, the equations were modified to allow cohort-level, rather than patient-level simulation. The regression analyses that were performed in the derivation of the UKPDS Outcomes Model equations identified a number of binary variables that would be set to either zero or one to represent, for example, whether a patient had a history of a specific complication. To facilitate a cohort-level simulation, these variables were substituted with the proportion of patients in the cohort who had a history of the complication in question. Furthermore, the panel regression equation to model changes in the proportion of smokers was also not incorporated into the present model, with the proportion of smokers instead being held constant over the time horizon of the analysis. The estimated cumulative incidence of diabetes complications was validated against the figures in the UKPDS Outcomes Model manuscript by overlaying projections in the UKPDS baseline cohort over a 15-year time horizon.
The BIM also incorporated a model of obstructive sleep apnea (OSA) based on data from the Sleep AHEAD study, a randomized trial investigating the effects of weight loss on OSA in obese patients with type 2 diabetesCitation17. Specifically, the prevalence-based model was predicated on the finding that, independently of other variables, a 1 kg/m2 increase in BMI was associated with a 10% increase in the predicted odds of severe OSA (95% confidence interval [CI] = 0–20%). The proportion of patients with OSA at baseline was also taken from Sleep AHEAD, in which OSA was defined as an apnea-hypopnea index (AHI) greater than five events per hour. At baseline, 86% of the patients enrolled in Sleep AHEAD met this criterionCitation17. Finally, whilst the prevalence of asthma was not assumed to change with either LAGB or standard medical management, the changes in costs associated with asthma treatment were captured through a medication-use model based on the findings of a 2010 study by Reddy et al.Citation18 in patients undergoing bariatric surgery, 36% of whom had diabetes. One year after surgery, the mean age of the Reddy et al.Citation18 cohort was 48 years and mean BMI was 35 kg/m2.
Target population
The characteristics of the baseline cohort were based primarily on data from a randomized controlled trial, published by Dixon et al.Citation19 in 2008, comparing LAGB with standard medical management in 60 obese patients with recently-diagnosed type 2 diabetes (). In brief, the cohort was 46.5% male with a mean baseline age of 46.9 years (standard deviation [SD] = 8.7 years). Mean baseline BMI was 37.1 kg/m2 (SD = 2.7 kg/m2), mean HbA1c was 7.7% (SD = 1.4%), and duration of diabetes was <2 years in all patients. In line with the Dixon et al.Citation19 study, the proportions of patients with a history of congestive heart failure, peripheral vascular disease, myocardial infarction, and stroke were set to zero (personal correspondence with John Dixon). The baseline prevalence of asthma was based on the proportion of patients taking asthma medications at baseline (18.6% or 2562 of 13,057 patients) in a 2010 retrospective study into changes in asthma medication use after bariatric surgeryCitation18.
Table 1. Baseline characteristics of the simulated patient cohort.
Treatment effects
The clinical effects of LAGB were taken from the same randomized controlled trial as the cohort characteristicsCitation19. The treatment effects applied in the base case are outlined in . In brief, LAGB resulted in a 1.81% reduction in HbA1c, a 6 mmHg reduction in systolic blood pressure, and a 7.4 kg/m2 reduction in BMI over the 2-year time horizon, compared with 0.38%, 1.7 mmHg, and 0.35 kg/m2 reductions with SMM, respectively. The model also allowed a proportion of patients to experience remission of type 2 diabetes. Based on the findings of the Dixon et al.Citation19 study, 73% of patients undergoing LAGB and 13% of patients receiving standard medical management experienced remission of diabetes. In the model, patients with remission accrued no further costs for diabetes medications, and the calculation of mortality in this patient group was switched from the UKPDS Outcomes Model mortality regression equations to UK life tables20. The incidence of diabetes complications in the ‘remission’ group conservatively followed the Weibull regression equations from the UKPDS Outcomes Model (i.e. the base case assumed no additional reduction in the incidence of diabetes complications beyond that effected by the changes in clinical parameters observed in the Dixon et al.Citation19 study).
Table 2. Treatment effects used in the modeling analysis.
Costs and resource use
The costs captured by the BIM are defined in . Costs of diabetes complications in the year of onset and in subsequent years were taken from a 2007 UK cost-effectiveness study by Ray et al.Citation21.The cost of LAGB was taken from the 2010–2011 National Health Service (NHS) tariff, assuming healthcare resource group FZ05B (major stomach or duodenum procedures without complications)Citation22. The costs of surgical complications were then calculated separately in the model based on the incidence of complications reported in a 2008 cost-effectiveness analysis of LAGB vs non-operative weight loss by Salem et al.Citation23. Complications captured included band removal, revisional surgery, minor and major wound infection, deep vein thrombosis, non-fatal pulmonary embolism, laparoscopic cholecystectomy, and incisional hernia repair. Unit costs for these complications were also taken from the NHS tariff and distributed evenly over the 3 years following surgery. Other costs were taken from UK-specific health technology assessments (HTAs) and economic evaluations, NHS reference costs, and the 2010 Department of Health Prescription Cost Analysis ()Citation24–33. All costs were expressed in 2010 pounds sterling, using the consumer price index to inflate values where necessaryCitation34.
Table 3. Cost items captured in the bariatric surgery budget impact model.
Table 4. Unit costs used in the base case analysis.
Resource use for the treatment of OSA was based on a 2009 UK HTA by McDaid et al.Citation27. Specifically, it was assumed that 81% of patients with diagnosed OSA would be in possession of an automatic positive airway pressure (APAP) device and the remaining 19% would use a continuous positive airway pressure (CPAP) device. In the base case analysis, the model amortized the costs of the devices over the projected device lifetimes, which were set to 5 and 7 years for APAP and CPAP devices, respectively. In the case of APAP devices, the cost of a dehumidifier was also capturedCitation27.
In patients not experiencing remission of diabetes, diabetes medication use (comprising insulin and oral anti-diabetic agents) was assumed to increase linearly over the duration of the analysis from the levels observed in the Dixon et al.Citation19 study at baseline, to those observed in a recent UK-study of obese patients with type 2 diabetes by Singhal et al.Citation35 after 5 years (the end of the model time horizon). The final mean insulin dose was taken to be 137.8 International Units (IUs) per day (SD = 93.5 IUs). In line with recommendations from the American Diabetes Association, it was assumed that all patients on insulin would be performing self-monitoring of blood glucose 3-times dailyCitation36. The proportion of patients using medications for conditions other than diabetes (anti-hypertensives, statins, analgesics, anti-depressants, and two asthma treatments: corticosteroids and long-acting β2 agonists) was derived from an analysis by Segal et al.Citation37 which specifically investigated the reduction in the use of medications for co-morbid conditions after bariatric surgery. Finally, assumptions around the frequency of contacts with healthcare professionals (including general practitioners, nurses, dietitians, and psychologists) in the years following LAGB surgery were taken from a 2009 HTA report by Picot et al.Citation24 on bariatric surgery. In the first 2 years, frequency of healthcare professional contacts in the standard medical management arm were taken from the non-surgical weight loss program outlined by O’Brien et al.Citation38 (and referenced by Picot et al.Citation24). In subsequent years, it was assumed that patients would have a monthly 30-min appointment with a community dietitian.
Time horizon, discounting, and perspective
In line with recommendations on the methods for systematic review and economic evaluation published by the NHS Research and Development Health Technology Assessment (HTA) Programme, the model reported budget impact outcomes over a 5-year time horizon with the ability to report cumulative or per-year outcomes in each year of the analysisCitation39. In line with guidelines from the National Institute for Health and Clinical Excellence (NICE), a 3.5% annual discount rate was applied to all future costs, which were accounted from a UK healthcare payer perspective (i.e. the NHS) and reported in 2010 pounds sterlingCitation40.
Base case analysis and sensitivity analyses
The base case analysis investigated the scenario comparing a cohort of patients receiving standard medical management with an equivalent cohort all undergoing LAGB. The base case was run as a probabilistic analysis, capturing uncertainty around the baseline cohort characteristics and treatment effects by sampling 5000 times from distributions around these input parameters. Distributions were assumed to be normal (truncated at 0 where necessary) and were based on mean values and standard errors calculated from the Dixon et al.Citation19 trial.
A series of one-way and multi-way sensitivity analyses were performed around a number of model input parameters to establish the magnitude of their influence on model outcomes. As discounting is not required by the ISPOR budget impact modeling guidance, sensitivity analyses were performed in which the discount rate was set to 0% and 6% (in line with NICE guidance)Citation40. In terms of cohort characteristics, the baseline BMI was increased to 42.4 kg/m2 (the mean BMI in the surgical arm of the SOS study) to establish the budget impact of introducing LAGB to a more obese populationCitation9. The change in BMI was also altered to that observed in SOS (8.5 kg/m2, derived from the mean baseline height and weight of 1.69 m and 121.0 kg and a 20% decrease in weight after LAGB). A further analysis was run in which BMI was set to increase by 1.49 kg/m2 per year after LAGB. This increment was designed to completely abolish the BMI decrease observed in the first year after surgery by the fifth year of the simulation (i.e. 7.43/5 kg/m2).
A number of analyses were performed around the unit costs used in the model. First, all unit costs were increased and decreased by 10%. Additional analyses were then performed to investigate the effect of varying groups of costs in the same way. Diabetes medication costs, diabetes complication costs, surgical complication costs, and costs of healthcare professional contacts were all increased by 10% and decreased by 10%. Assumptions around diabetes treatment were investigated by holding the insulin dose constant at 40 IUs per day (in the same proportion of patients as in the base case) and accounting no costs of self-monitoring of blood glucose. Finally, two analyses were performed around other treatment: one in which the prevalence-based sleep apnea sub-model was switched off and one in which 5% of patients undergoing LAGB were assumed to subsequently undergo abdominoplasty funded by the healthcare payer.
Results
The base case scenario showed cost savings of GBP 91,287 over 5 years in a closed cohort of 100 patients undergoing LAGB, compared with an equivalent cohort receiving standard medical management for obesity (equivalent to savings of GBP 913 per patient; ). Cumulative costs for the two cohorts were GBP 818,668 (95% CI = GBP 808,260–833,593) and GBP 909,955 (95% CI = GBP 899,857–923,158), respectively (). Relative to the standard medical management arm, cost savings were observed in spite of a 0.5% relative reduction in mortality in the LAGB arm (driven by the UKPDS Outcomes Model mortality risk equations and patients with remission of diabetes switching to mortality risks derived from UK life tables). Initial costs of surgery were GBP 254,000, with treatment of surgical complications accounting for an additional GBP 40,981. However, these high upfront costs were more than offset by savings arising from reduced diabetes, asthma, and sleep apnea medication costs, reduced incidence of diabetes complications, and reduced contact with healthcare professionals. The ‘breakeven’ point (i.e. the time in the analysis at which the incremental costs of LAGB relative to SMM were GBP 0) was 4.0 years after surgery.
Figure 1. Cumulative incremental cost of LAGB relative to standard medical management. CI, confidence interval; GBP, 2010 pounds sterling; LAGB, laparoscopic adjustable gastric band.
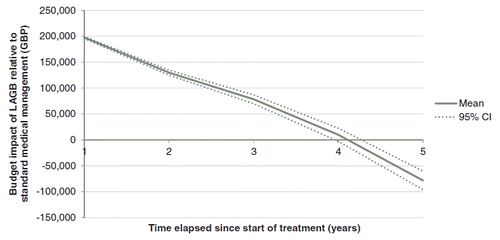
Figure 2. Total cost of treatment over 5 years with and without LAGB. GBP, 2010 pounds sterling; LAGB, laparoscopic adjustable gastric banding.
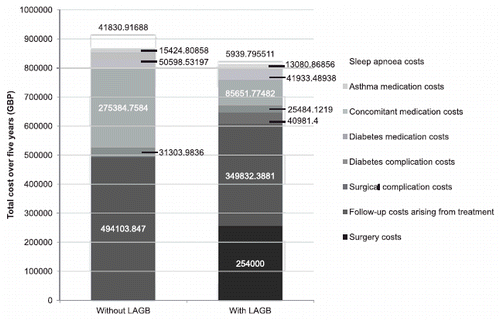
The largest cost saving in the LAGB arm was that arising from decreased use of diabetes medications (in patients experiencing remission of diabetes), which resulted in a saving of GBP 189,733 over 5 years. Other cost savings arose from reduced costs of sleep apnea treatment (GBP 35,891), reduced costs of non-diabetes medications (GBP 8665) and reduced costs of treating diabetes complications (GBP 5820).
Results of sensitivity analyses are presented in . Of the sensitivity analyses performed, none changed the finding of cost savings with LAGB relative to SMM. The daily insulin dose was the largest driver of incremental outcomes, with a 40 IU daily dose resulting in a reduced cost saving of GBP 10,131 with LAGB relative to SMM. Removing costs of self-monitoring of blood glucose also had a notable effect with cost savings reduced to GBP 51,231. The BMI sensitivity analyses, in which baseline BMI was increased to 42.4 kg/m2 and a post-surgical BMI creep was introduced had a modest effect on incremental outcomes yielding cost savings of GBP 91,817 and GBP 77,836, respectively. The discount rate had a substantial effect on incremental outcomes, with a 0% discount rate yielding an incremental saving of GBP 119,280 over 5 years and a 6% discount rate resulting in incremental cost savings of GBP 72,967 with LAGB relative to SMM. The analyses in which unit costs were varied by ±10% all had a relatively modest effect on incremental outcomes, with the exception of changes in diabetes medication costs, which resulted in cost savings of GBP 110,549 and GBP 72,737 with a 10% increase and 10% decrease, respectively. Finally, the analysis in which sleep apnea costs were not accounted decreased cost savings with LAGB to GBP 55,714 and the accounting of abdominoplasty costs in 5% of patients yielded cost savings of GBP 78,103 with LAGB relative to SMM.
Table 5. Sensitivity analysis results over a 5-year time horizon in a hypothetical cohort of 100 patients.
Discussion
Over a 5-year time horizon, the present analysis found LAGB to be cost saving when compared with SMM in the treatment of obese patients with type 2 diabetes in the UK. Specifically, the analysis found LAGB to yield a mean cost saving of GBP 91,287 (95% CI: GBP 73,007–GBP 108,848) in a cohort of 100 patients over 5 years, with the ‘breakeven’ point occurring 4.0 years after initiation of treatment. This was based on mean total costs of GBP 818,668 (95% CI = GBP 808,260–833,593) in the LAGB arm compared with GBP 909,955 (95% CI = GBP 899,857–923,158) in the arm with standard medical management.
The key strengths of the analysis include its broad coverage in terms of capturing the costs and cost offsets associated with obesity treatment and its extensive use of UK-specific data sources including HTAs, health economic analyses, and resource use assumptions. However, the analysis has a number of limitations that should be acknowledged. First, in attempting to capture the wide range of cost drivers associated with the treatment of obesity, type 2 diabetes, and its comorbidities, the present analysis utilized data from a variety of sources. Notably, data on the effectiveness of LAGB, prevalence of obstructive sleep apnea and asthma, incidence of post-surgical complications, and diabetes medication use in obese patients with type 2 diabetes in the UK were all sourced from different publications. Whilst such heterogeneity is not desirable in health economic analysis, it is also unavoidable, as no single study has captured a sufficiently broad array of economic and clinical end-points to evaluate all of the cost drivers included in the present analysis. In attempting to reduce the effect of such heterogeneity on the applicability of the study findings, the present analysis only utilized data specific to patients eligible to undergo bariatric surgery and, in the majority of cases, to patients with type 2 diabetes diagnosed in the past 5 years at baseline (asthma medication resource use was a notable exception to this criterion). Additionally, all costs used in the model were specific to the UK.
A second limitation pertains to the relatively straightforward manner in which the present model captured remission of type 2 diabetes. The use of remission rates from the Dixon et al.Citation19 study (73% in patients undergoing LAGB and 13% in patients receiving SMM) is perhaps realistic in a recently-diagnosed cohort, but may not be applicable in cohorts with more advanced diabetes. Even putting aside the issue of applicability to different cohorts, the cardiovascular disease risk profile of patients who have experienced diabetes remission is largely uncharacterized. Whilst long-term prospective studies such as SOS are starting to provide a picture of the longer-term clinical profile of patients who have undergone bariatric surgery, accurately modeling the incidence of complications in patients who have experienced remission of diabetes is challenging. We believe that the approach used in the present analysis is both transparent (with mortality taken from UK life tables, microvascular and macrovascular complication incidence taken from the UKPDS Outcomes model, and no diabetes medication costs) and conservative.
Finally, the analysis did not capture costs associated with hypoglycemia. The Dixon et al.Citation19 study (on which the diabetes-related effectiveness assumptions were based) reported only one instance of minor hypoglycemia in the surgical arm and no instances of major hypoglycemia (i.e. a hypoglycemic episode requiring third-party assistance) in either arm. Given the reduction in diabetes medication use observed in patients who have undergone bariatric surgery, and the potential costs associated with hypoglycemia in patients using insulin, this assumption was highly conservative.
In comparing the findings of the present study with other budget impact analyses of bariatric surgery, only one UK-specific analysis was apparent in the literature. The study published by Ackroyd et al.Citation15 in 2006, presented the findings of a budget impact analysis of laparoscopic Roux-en-Y gastric bypass (LRYGB) and LAGB vs SMM in Germany, France, and the UK. Whilst the findings in France and Germany were that LAGB was cost-saving relative to SMM (with savings of EUR 3586 and EUR 4480 per patient, respectively), the UK analysis showed an increase in cost of GBP 1984 per patient over 5 years (compared with a cost saving of GBP 913 in the present study). The absolute cumulative costs reported by Ackroyd et al.Citation15 were GBP 9072 per LAGB patient vs GBP 7088 per patient receiving SMM, compared with GBP 8187 and GBP 9100, respectively, in the present study. Although the studies are separated by 5 years, the LAGB cost estimates are remarkably similar (less than GBP 900 difference over a 5-year time horizon). However, the SMM cost estimates differed by just over GBP 2000 per patient over 5 years. The difference can likely be explained by the assumptions made around diabetes medication use. The final mean insulin dose of 137.8 IUs in the present study was taken from the recent UK-specific Singhal et al.Citation35 study, in which patients had a mean duration of diabetes of 7 years (range 1 month to 35 years). The assumption that 87% of SMM patients would titrate to this dose (compared with 13% of surgical patients) is a key driver of the high costs in the SMM arm. This was explored in a sensitivity analysis in which all patients on insulin (defined by a linear increase to 100% of diabetes patients over 5 years) received a 40 IU daily dose. The analysis showed that LAGB would still be cost saving over 5 years, but the breakeven point was pushed back to 4.9 years after initiation of treatment. Unfortunately, neither the assumed duration of diabetes nor the diabetes medication costing methodology employed by Ackroyd et al.Citation15 were detailed, which complicates the identification of the underlying factors driving the cost difference between the studies.
Given the increasing prevalence of diabetes, obesity, and overweight in the UK, we believe the present analysis represents a timely evaluation of the costs associated with LAGB relative to SMMCitation41. Projections from the ‘Tackling Obesities: Future Choices’ report from the UK Government Office for ScienceCitation42 estimated that NHS expenditure attributable to obesity alone will reach GBP 5.3 billion by 2025. Although the report noted that advances in bariatric surgery have resulted in very low rates of operative morbidity and mortality and short post-operative hospital stays, the report noted that the impact of bariatric surgery on mean population weight was negligible, as the proportion of patients eligible for bariatric surgery remains low. In terms of clinical eligibility, NICE guidelines currently emphasize diet and physical activity for patients with a BMI lower than 35 kg/m2, but recommend obesity surgery in patients who have failed to lose weight (or maintain weight loss) by non-surgical measures and whose BMI is either greater than 40 kg/m2 or between 35–40 kg/m2 with comorbidities such as type 2 diabetes, hypertension, or cardiovascular disease. Finally, patients with a BMI > 50 kg/m2 are eligible for bariatric surgery as a first-line therapy for obesityCitation43. Despite these recommendations, only a fraction of eligible patients are considered for such intervention in the NHS, as corroborated by NICE data, which reported that only 6643 bariatric procedures (excluding band adjustments) were performed in England in 2010/2011Citation44. Given that 2007 NICE estimates placed the number of patients eligible and willing to undergo bariatric surgery at 257,000, there is currently a shortfall in the number of bariatric procedures being performedCitation45. While bariatric center throughput may be the limiting factor, the present study clearly shows that the costs of performing LAGB should not present a barrier to the use of LAGB in obese patients with type 2 diabetes.
It should be noted that, as demonstrated in the Ackroyd et al.Citation15 study, the findings of budget impact analyses are generally highly country-specific. The findings of the present analysis are specific to the UK setting, capturing UK costs and, in the vast majority of cases, UK-specific resource use estimates. While the model itself is readily adaptable to other geographies, the generalizability of the findings to other country settings may be limited, as differing surgical eligibility criteria, resource use estimates, and costs all have the potential to change the magnitude of the cost savings reported in the current analysis. However, our finding that costs are more than recovered 5 years after surgery is in line with the findings from the Ackroyd et al.Citation15 study in the French and German settings.
Conclusion
Key strengths of the present analysis include the comprehensive nature of the costs captured, the UK-specific nature of the costs and underlying diabetes model, and the use of effectiveness data from a randomized controlled trial of LAGB vs SMM. Based on the bespoke budget impact model and the resource use and cost assumptions outlined in the present study, the high initial costs of performing LAGB would be offset 4 years after surgery when compared with SMM in a population of obese patients with type 2 diabetes. The high initial costs associated with LAGB should not, therefore, restrict its use in this patient group in the UK setting. Based on the clinical findings of the recent randomized controlled trial by Dixon et al., any increase in the uptake of LAGB would be expected to significantly improve clinical outcomes in these patients, relative to the use of SMM.
Transparency
Declaration of funding
This study was sponsored by Allergan Ltd., Marlow, UK.
Declaration of financial/other relationships
RP and WJV have disclosed that they are full-time employees of Ossian Health Economics and Communications GmbH, which received consultancy fees from Allergan Ltd to construct the budget impact model, develop the simulation plan, and write the manuscript. JC is an employee of the University of Sheffield School of Health and Related Research, which received consultancy fees from Allergan Ltd to validate the budget impact model and develop the simulation plan. GM is a full-time employee of Allergan Ltd.
References
- Holman N, Forouhi NG, Goyder E, et al. The Association of Public Health Observatories (APHO) Diabetes Prevalence Model: estimates of total diabetes prevalence for England, 2010–2030. Diabet Med 2011;28:575-82
- Hamer M, Kengne AP, Batty GD, et al. Temporal trends in diabetes prevalence and key diabetes risk factors in Scotland, 2003--2008. Diabet Med 2011;28:595-8
- Lindström J, Louheranta A, Mannelin M, et al.; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230-6
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:s62-9
- Nathan DM, Buse JB, Davidson MB et al.; American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17-30
- Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481-6
- Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961-9
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374-83
- Sjöström L, Narbro K, Sjöström CD, et al.; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741-52
- Norris SL, Zhang X, Avenell A, et al. Long-term non-pharmacologic weight loss interventions for adults with type 2 diabetes. Cochrane Database Syst Rev 2005;preprint(2):CD004095
- Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005preprint;preprint(2):CD004096
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122(3):248-56
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health 2007;10:336-47
- Orlewska E, Gulácsi L. Budget-impact analyses: a critical review of published studies. Pharmacoeconomics 2009;27:807-27
- Ackroyd R, Mouiel J, Chevallier JM, et al. Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes Surg 2006;16:1488-503
- Clarke PM, Gray AM, Briggs A, et al.; UK Prospective Diabetes Study (UKDPS) Group. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59
- Foster GD, Sanders MH, Millman R, et al.; Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009;32:1017-9
- Reddy RC, Baptist AP, Fan Z, et al. The effects of bariatric surgery on asthma severity. Obes Surg 2011;21:200-6
- Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316-23
- Office for National Statistics. UK Interim Life Tables, 1980--82 to 2008--10. 2011. http://www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2008-2010/sum-ilt-2008-10.html. Accessed November 28, 2012
- Ray JA, Boye KS, Yurgin N, et al. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin 2007;23:609-22
- National Health Service. National Tariff 2010–2011. http://data.gov.uk/dataset/payment-by-results-2010-11-national-tariff-information. 2010. Accessed November 28, 2012
- Salem L, Devlin A, Sullivan SD, et al. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis 2008;4:26-32
- Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13:1-190, 215-357, iii-iv
- National Health Service. Electronic Drug Tariff. 2010. www.ppa.org.uk/ppa/edt_intro.htm. Accessed August 2, 2011
- Department of Health. Prescription Cost Analysis. 2010. http://www.ic.nhs.uk/webfiles/publications/007_Primary_Care/Prescribing/Prescription_Cost_Analysis_England_2010/Prescription_Cost_Analysis_2010.pdf. Accessed August 2, 2011
- McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess 2009;13:iii-iv, xi-xiv, 1-274
- National Institute for Health and Clinical Excellence. Inhaled corticosteroids for the treatment of chronic asthma in adults and in children aged 12 years and over. http://www.nice.org.uk/nicemedia/pdf/TA138Guidance.pdf. Accessed November 28, 2012
- National Institute for Health and Clinical Excellence. Preoperative tests: template to calculate costing implications. 2007. http://www.nice.org.uk/media/59D/32/Preoperative_tests_rec_reminders_2-3_costing.xls. November 28, 2012
- National Institute for Health and Clinical Excellence. Costs used in the York model. http://www.nice.org.uk/nicemedia/live/11966/47855/47855.pdf. Accessed November 28, 2012
- NHS reference costs 2009-2010. 2011. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123459. Accessed November 28, 2012.
- National Institute for Health and Clinical Excellence. Lipid modification costing report. 2008. http://guidance.nice.org.uk/nicemedia/live/11982/40776/40776.pdf. Accessed November 28, 2012
- National Institute for Health and Clinical Excellence. Hypertension in pregnancy costing report. 2010. http://www.nice.org.uk/nicemedia/live/13098/50503/50503.pdf. November 28, 2012
- Office for National Statistics. Consumer Price Indices - July 2011. 2011. http://www.ons.gov.uk/ons/rel/cpi/consumer-price-indices/july-2011/index.html. Accessed November 28, 2012
- Singhal R, Ahmed M, Krempic A, et al. Medium-term outcomes of patients with insulin-dependent diabetes after laparoscopic adjustable gastric banding. Surg Obes Relat Dis 2011. preprint http://www.ncbi.nlm.nih.gov/pubmed/21955740; http://www.soard.org/article/S1550-7289(11)00592-2/abstract
- American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl 1):S11-61
- Segal JB, Clark JM, Shore AD, et al. Prompt reduction in use of medications for comorbid conditions after bariatric surgery. Obes Surg 2009;19:1646-56
- O'Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 2006;144:625-33
- Clegg AJ, Colquitt J, Sidhu MK, et al. The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-153
- National Institute for Health and Clinical Excellence. Guide to methods of technology appraisal – NICE. 2008. http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf. Accessed October 22, 2012
- Howel D. Trends in the prevalence of abdominal obesity and overweight in English adults (1993–2008). Obesity (Silver Spring) 2012;20:1750-2
- Government Office for Science. Tackling obesities: future choices — Project report. 2007. http://www.bis.gov.uk/assets/foresight/docs/obesity/17.pdf. Accessed October 22, 2012
- National Institute for Health and Clinical Excellence. Obesity: guidance on prevention, identification, assessment and management of overweight and obesity in adults and children. Guideline 43. 2006. http://www.nice.org.uk/CG43. Accessed November 28, 2012
- National Institute for Health and Clinical Excellence Bariatric Surgical Service. Assumptions used in estimating a population benchmark. 2012. http://www.nice.org.uk/usingguidance/commissioningguides/bariatric/assumptions.jsp. Accessed November 28, 2012
- National Institute for Health and Clinical Excellence Bariatric Surgical Service for the Treatment of People with Severe Obesity. Commissioning guide: implementing NICE guidance. 2007. http://www.nice.org.uk/media/87F/65/BariatricSurgeryFINALPlusNewToolUpdates.pdf. Accessed November 28, 2012
