Abstract
Objectives:
The objective was to review the published literature on seasonal influenza to assess the differences between complications and mortality rates for those adults at high risk of influenza complications, including the resource use of those hospitalized with influenza complications.
Methods:
A targeted literature review was performed using electronic database keyword searches, specific inclusion criteria, quality rating of the reviewed full-text articles and abstraction of data to present published evidence on the incidence, complication rates and health service use associated with clinical influenza in different adult high-risk groups including those who are aged 65 years and older or those with different chronic underlying medical conditions.
Results:
Key findings for incidence rates of clinical influenza were that incidence rates are similar among people with chronic cardiovascular or respiratory comorbidity, and may be higher in those with allogeneic stem cell transplants compared to those with autologous transplants. Rates of hospitalization and/or pneumonia or lower respiratory tract infection for those with chronic conditions or those who are immunocompromised are substantially higher than those in people over age 65 but without additional high-risk factors. A person who is hospitalized and has a laboratory-confirmed influenza diagnosis has a probability of intensive care unit admission of between 11.8–28.6% and of death of between 2.9–14.3%.
Conclusions:
These findings indicate that although the burden of influenza varied across high-risk groups, it also varied widely across studies within a single high-risk group. A key finding was that those over 65 years of age but without additional high-risk factors had a low risk of influenza complications. A limitation of the review is that most of the studies of hospitalized patients did not present outcomes data separately by high-risk group and only limited data were identified on rates of hospitalization or lower respiratory tract infection for most high-risk groups. Information about influenza complication rates and resource use, including influenza vaccines, chemoprophylaxis and/or treatment strategies for different high-risk groups, is needed to evaluate new interventions.
Background
Influenza is a seasonal disease with a northern hemisphere and a southern hemisphere winter epidemic pattern seeded in some seasons by influenza virus circulating in Southeast AsiaCitation1. Influenza has characteristic symptoms of sudden onset of high fever, aching muscles, headache, severe fatigue, non-productive cough, sore throat, and runny noseCitation2. While most infected people recover within 1–2 weeks without requiring medical treatment, in the very young, the elderly, and those with other serious medical conditions, infection can lead to exacerbations of the underlying condition, as well as neurologic complications, pneumonia, and deathCitation2–5.
The groups at high-risk for influenza complications are defined by age, chronic conditions, immune status and behavioural/occupational factors. presents a listing of high-risk medical conditions for which influenza vaccination is recommendedCitation6–8. In addition to those recommended for vaccination, other groups that may have a high risk of complications include pre-term infants and all infants aged younger than 6 monthsCitation9 and hospitalized patients, especially those in intensive care units (ICUs)Citation10. Persons with multiple risk factors, such as those aged over 65 years with a co-morbid condition, may be at especially high risk of complicationsCitation11.
Table 1. People recommended for influenza vaccination because of high risk of complications.
Vaccination is generally considered to be the most effective method for preventing both cases and complications of influenzaCitation12. However, the vaccine coverage rates for the high-risk groups are generally not more than 50%, with the exception of those aged older than 65 years, who generally have vaccine coverage rates of at least 65%Citation7,Citation13. In addition, studies have shown that vaccination is less effective at promoting an immune response in the elderly and in those who are immunocompromised than in other groupsCitation14–16. A Cochrane systematic review of vaccination in the elderly concluded that the impact of vaccination on the rate of influenza complications could not be determined from the published literatureCitation17. A recently published systematic review of influenza vaccines in the US also concluded that evidence for protection in adults aged 65 years or older is lacking and protection by the vaccine for all groups is greatly reduced or absent in some seasonsCitation18.
Prophylaxis or treatment with antiviral drugs is also recommended for those at high risk of influenza complications. For example, in the US, antiviral prophylaxis or treatment with neuraminidase-inhibitors is recommended for those at high-risk of influenza complicationsCitation19. Nevertheless, there are only limited data, mostly from observational studies, on the impact of the neuraminidase inhibitors zanamivir, oseltamivir, or peramivir on influenza complication rates or deaths in high-risk groups. Because of the lack of large randomized clinical trials among those at high risk, there is currently no consensus on the value of current antiviral therapies for reducing the influenza complication and mortality rates in high-risk groupsCitation20,Citation21.
Because of the controversy around the level of protection conferred by influenza vaccination and the value of current antiviral therapies for reducing complication and mortality rates in high-risk groups, an unmet need remains for new effective treatments and/or management strategies for influenza. However, this unmet need may differ for the high-risk group categories defined in . To better understand the unmet need in the different high-risk group categories, it is necessary to analyse the disease burden within each group separately. In this article, we present the results of a targeted literature review to evaluate for different high-risk groups the annual incidence rates for clinical influenza, clinical complication rates, and healthcare resource use. This information is of critical importance for the targeting of new therapies and prophylactic options as well as for their economic evaluation.
Table 2. Probability of a clinical case of influenza.
Methods
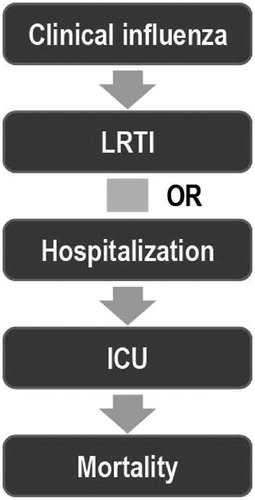
To characterize the burden of seasonal influenza complications in different high-risk adult groups, we followed the model of the disease progression shown in . Prior to initiating the targeted literature review, the methodology to be used for the searches, screening process (inclusion and exclusion criteria), and data extraction were defined as follows: The search focused on data published since 1990 to October 2011 in the MEDLINE database (using the PubMed platform). In addition, a bibliographic reference list of full-text articles identified with the electronic MEDLINE searches was reviewed to identify any relevant articles.
Figure 1. Framework for assessment of the burden of influenza complications. ICU, intensive care unit; LRTI, lower respiratory tract infection.
We performed two sets of electronic searches of the MEDLINE database. The first set of searches used title keywords relating to outcomes associated with influenza complications and included the following keyword combinations:
influenza*[ti] AND hospital*[ti] NOT (H1N1[ti] OR pandemic[ti] OR children[ti] OR pediatric[ti] OR infants[ti] OR workers[ti])
influenza*[ti] AND (death[ti] or mortality[ti]) NOT (H1N1[ti] OR pandemic[ti] OR children[ti] OR pediatric[ti] OR infants[ti] OR workers[ti])
influenza*[ti] AND pneumonia[ti] NOT (H1N1[ti] OR pandemic[ti] OR children[ti] OR pediatric[ti] OR infants[ti] OR workers[ti])
influenza*[ti] AND outcome*[ti] NOT (H1N1[ti] OR pandemic[ti] OR children[ti] OR pediatric[ti] OR infants[ti] OR workers[ti])
(influenza*[ti] OR ‘respiratory virus’[ti] OR ‘respiratory viruses’[ti]) AND prospective[ti] AND study[ti] NOT (H1N1[ti] OR pandemic[ti] OR children[ti] OR pediatric[ti] OR infants[ti] OR workers[ti])
The second set of searches included title searches for articles that included keywords for seasonal influenza and the following specific conditions in adults associated with a high risk for influenza complications: human immunodeficiency virus (HIV) infection, heart disease, renal disease, diabetes, multiple sclerosis, cystic fibrosis, stem cell or bone marrow transplant, solid organ transplant, asthma, chronic obstructive pulmonary disease (COPD), and the elderly.
The outcomes of interest in this review were (1) the annual incidence of clinical cases of seasonal influenza, (2) the probability of pneumonia or lower respiratory tract infection (LRTI) or hospitalization for those with a clinical case of influenza, (3) the probability of ICU admission and mechanical ventilation for those hospitalized with influenza, and (4) the probability of dying from influenza. In order to better understand the impact of seasonal influenza complications on medical resource use, other outcomes of interest included length of stay in the hospital and the ICU and duration on mechanical ventilation.
Titles and abstracts of the studies identified from the electronic database were screened, and full-text copies were obtained for those that appeared to present quantitative data on one of the outcomes of interest for the review. Data were abstracted from English-language articles that presented quantitative information on at least one of the following topics for those with clinical influenza for at least one of the high-risk groups: clinical influenza attack rates, probability of hospitalization or pneumonia/LRTI or mortality, ICU admission and mechanical ventilation use, and/or length of stay at each level of care. The abstracted data were presented in a set of tables presenting the outcomes by specific high-risk groups.
For each study included in the tables, information related to the following study characteristics was included in the tables as indicators of study quality: study design (retrospective cohort or database, prospective cohort or other); the number of sites included in the study (single or multiple); study sample representative of the high-risk population studied at the site(s) (yes, cannot tell, and no); the method used to identify a case of influenza in the study (influenza or influenza-like illness [ILI] diagnosis, influenza diagnosis, laboratory-confirmed influenza [LCI]); and the extent to which the outcome was clearly defined (yes, cannot tell, no). These quality ratings were adapted from questions 2, 3, 4, and 5 of the CASP checklist for cohort studiesCitation22. These questions were considered of greatest relevance for our study objectives, and this approach is recommended for reviews of observational studies by the University of York Center for Reviews and DisseminationCitation23.
Results
We reviewed 1845 titles and abstracts, and 121 full-text articles. Of these, we selected 31 articles that presented quantitative data on influenza attack rates, complication rates, or resource use in high-risk groups. Of the selected articles, the majority were from the US (19), with three from Canada, two from Spain, two from France, one from Italy, one from the UK, and three using data from multiple countries. Fourteen of the studies were prospective cohort studies, 14 were retrospective cohort studies or database studies, and three used other methods, meta-analysis of clinical trial data, or estimation of the excess risk of hospitalization or death. Seventeen of the studies were multi-site studies, and 14 were single-site studies. Twenty-two of the studies estimated the complications associated with LCI, while the other nine studied those with an influenza or ILI diagnosis in the medical record that was not confirmed in the laboratory.
presents a summary of studies that estimated the probability of individuals in different high-risk groups having a clinical case of influenza each year. The reviewed articles included many influenza seasons, from 1985–2003, and multiple countries. Data were limited and, in the articles reviewed, clinical influenza rates ranged from a low for ILI of 0.23% for those aged 65 years and older living in the community with a vaccination rate of 59–64% in Spain in 2002–2005Citation24 to a high for LCI of 7.2% among patients of all ages with congestive heart failure or chronic pulmonary disease in the US in the 2001–2002 influenza seasonCitation25. The Vila-Córcoles et al.Citation24 study only captured cases of ILI which resulted in a physician visit, while the Falsey et al.Citation25 study captured all symptomatic cases, whether or not they resulted in a physician visit. The Falsey et al.Citation25 study, conducted over four influenza seasons, found that rates of confirmed clinical influenza in otherwise healthy elderly (2.4–5.0%) were similar to the rates in those of all ages with congestive heart failure or chronic pulmonary diseases (2.4–7.2%). In another study in Spain in 1999–2003 in stem cell transplant patients, those with an allogeneic transplant had a greater probability of clinical influenza (6.55%) than those with an autologous transplant (3.05%)Citation26.
presents estimates of the probability of pneumonia or LRTI in high-risk groups by age and high-risk sub-group. The probability of pneumonia or LRTI in those with confirmed influenza ranged from 0% among those aged 65 or more years who were otherwise healthy and living in the community in the US in 1999–2003Citation25 to 80% among adults with leukemia in the US in 1993–1994Citation27. The rates were considerably higher in studies that included only hospitalized patients with an influenza diagnosis or LCI (27–48%) or that studied patients with hematologic disorders, stem cell or bone marrow transplants, and solid organ transplants who were not treated with antiviral drugs (26–83%). Two retrospective influenza cohort studies of people with hematological malignancies or stem cell transplants or both compared rates of pneumonia and LRTI in those treated with antiviral drugs and those not treated with these drugsCitation28,Citation29. Both studies found lower rates of pneumonia or LRTI in those treated with antiviral drugs.
Table 3. Probability of pneumonia or lower respiratory tract infection or chest infiltrates, given influenza.
presents estimates of the probability of hospitalization by age and high-risk sub-group. The probability of hospitalization given influenza ranged from 0% for those aged 65 years and older who were otherwise healthy and living in the community in the US in 1999–2003Citation25 to 20.8% among those with cancer or taking chronic corticosteroids with an influenza diagnosis in 1996–1997 in the USCitation30 or 20% in those with congestive heart failure or chronic lung disease with LCI in the USCitation25.
Table 4. Probability of hospitalization, given influenza.
Estimates of the probability of ICU admission for those hospitalized with influenza are presented in . The probability of ICU admission was 4.2% for those aged 65 years or older with influenza as either a primary or secondary diagnosis code for influenza, but not necessarily with LCI, in France from a hospital database study in 2006–2007Citation31, and ranged between 11.4–28.6% for seven US and Canadian studies reviewed in people hospitalized with LCI.
Table 5. Probability of intensive care unit admission given hospitalized for influenza.
Estimates of the probability of mechanical ventilation given hospital or ICU admission by age and high-risk sub-group are presented in . The probability of mechanical ventilation for those with LCI ranged from 0% among immunocompromised patients of all ages admitted to the hospital without a pneumonia diagnosis in France in 1998–2008Citation32 to 21.0% for those with a pneumonia diagnosisCitation32. The probability of mechanical ventilation for patients admitted to the ICU ranged from 69–78% in the USCitation33,Citation34. In other studies of all hospital admissions, the probability of mechanical ventilation ranged between 3.8% for those aged 65 years and over with cancer and an influenza diagnosisCitation35 to 26% of those with COPD and LCICitation33.
Table 6. Probability of mechanical ventilation given intensive care unit or hospital admission for influenza.
Estimates of the probability of death at the time of an influenza episode from all causes by age and high-risk sub-group are shown in . The estimates of death rates in hospitalized patients from the French hospital database study that included those with a primary or secondary diagnosis code for influenza, but not necessarily with LCICitation31, were lower (0.3% for those aged 5–64 years and 3.1% for those aged 65 years and over) than estimates in studies that estimated the death rates for those hospitalized with LCI (2.9–14.3% for all ages and 13.5% for those aged 65 years and over) in US and Canadian studiesCitation25,Citation33,Citation36–39 and in those with an influenza diagnosis and cancer (8.3% for those aged 18–64 years and 9.5% for those with cancer aged 65 years and over)Citation35. The estimated probability of death in those with stem cell and other transplants ranged from 4–9% in those treated with antiviral therapy and from 17–27% for those not treated with antiviral therapyCitation28,Citation29. However, these estimates were for all-cause death and included deaths associated with the underlying disease. The estimated death rates for those admitted to the ICU depended on the high-risk group analysed and ranged from 13.6% for those with hypertension to 52.4% for immunocompromised patients in a multi-site US studyCitation34.
Table 7. Probability of death with influenza episode.
Finally, estimates of the length of stay in the hospital and ICU and the duration on mechanical ventilation for those using these hospital resources, by age and high-risk sub-group, are presented in . Some of the studies reviewed presented mean total length of stay, and some presented the median length of stay. Four studies presented both valuesCitation30,Citation31,Citation40,Citation41, and all four showed that the mean length of stay was longer than the median length of stay by between 1.2–5 days. Two studies estimated a longer length of stay for those with pneumonia or other poor outcomes than for those with less serious symptomsCitation32,Citation42. The two studies that estimated length of stay for those admitted to the ICU did not find a longer total length of stay in the hospital than the estimates for those admitted to the hospitalCitation34,Citation36. There did not seem to be a clear pattern for different hospital resource use by country. Length of stay in the ICU and duration on mechanical ventilation varied considerably between studies.
Table 8. Length of stay in hospital, in intensive care unit, or on mechanical ventilation.
Discussion
The articles identified in the literature review found only limited data available to differentiate between high-risk groups in rates of influenza infection and associated complication rates and resource use. In addition, estimates of complication rates or resource use from different studies in the same high-risk group varied substantially. Estimates of complications or resource use also varied according to whether the study included all those with an influenza diagnosis or influenza-like illness or included only those with LCI.
The framework displayed in for the presentation of the data postulated a clinical case of influenza that might (1) prompt a visit to the doctor or hospital emergency room, (2) include symptoms of LRTI such as pneumonia, (3) require inpatient hospital treatment both in regular and intensive care for influenza complications or exacerbations of an underlying chronic condition, (4) require respiratory support with mechanical ventilation, and (5) end in death. The probability of clinical influenza that prompts a visit to a physician or hospital is impacted by many factors other than the type of high-risk factor, including healthcare-seeking behaviour, the presence of co-morbidity, the dominant virus strain in the influenza season, the magnitude of the influenza epidemic, vaccination status, and the match of the vaccine with the circulating viruses. The outcome of clinical influenza infection is also dependent on the type of care received, including antiviral treatment. All of these factors are likely to vary by influenza season and country.
presents an overview of the data abstracted from the published studies where outcomes and resource use were presented for specific high-risk groups. It shows wide ranges for each estimate, with only limited data available for some high-risk groups, including those with chronic respiratory disease. When the data are viewed for individual categories of high-risk patients, there are some differences between the different groups. Many studies reviewed did not differentiate between high-risk groups, presenting, for example, estimates of outcomes for all people hospitalized with either an influenza diagnosis or with LCI.
Figure 2. Overview of clinical outcomes and resource-use data associated with influenza complications by high-risk group. CV, cardiovascular; HIV, human immunodeficiency virus; ICU, intensive care unit; LCI, laboratory-confirmed influenza; LOS, length of stay; LRTI, lower respiratory tract infection. a Including those with HIV infection, post-transplant, and with cancer. b Rate for those hospitalized with a confirmed influenza diagnosis.
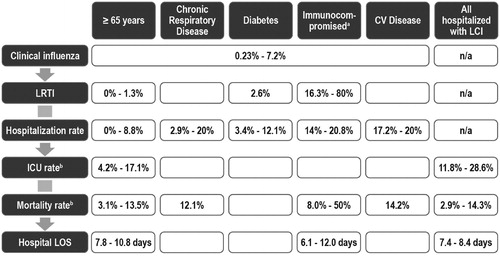
We identified several studies that estimated the probability of a clinical case of influenza and its complications and resource use for those aged 65 years and older. However, the estimates, shown in , show a wide range in the estimates for all elements of the burden of influenza, including hospitalization and death rates. Part of this range may be attributable to the grouping in many of these studies of those with or without a condition that puts them at higher risk of influenza complications. Thus, the Falsey et al.Citation25 prospective cohort study, which sub-divided those aged 65 years and older into those with and without chronic conditions, found a large difference in rates of pneumonia and hospitalization between the two groups, although clinical influenza attack rates were similar in the two groups. Thus, in viewing the burden of complications in those aged 65 years and older, it is important to differentiate between those with and without underlying chronic medical conditions.
Although chronic respiratory disease is generally known to place a person at increased risk of influenza complication, there are only limited data that estimate the burden of influenza for this important high-risk group. Similarly for those with diabetes, although there are estimates of their risk of LRTI or hospitalization, no data were identified estimating the outcomes in this high-risk group once hospitalized. Mortality rates for those hospitalized with cardiovascular disease were similar to those with chronic respiratory disease and those aged 65 years and older, but hospitalization rates were higher.
In contrast, several studies were identified among immunocompromised patients, defined as including those who are post-transplant, those with HIV infection, and those with cancer. All of these studies indicated that this high-risk group had higher rates of LRTI or hospitalization and higher mortality rates for those hospitalized. The mortality rates presented in are for all causes, including the underlying condition, but in those studies of the immunocompromised where the influenza mortality rate was presented separately, influenza mortality rates were also higher than in the other high-risk groups. In the immunocompromised groups, there were also some small observational studies that indicated that the risk of complications was lower when the influenza was treated with antiviral drugsCitation28,Citation29.
Although the very young are recognized as one of the high-risk groups, data are lacking on what, besides age, puts them at risk for hospitalizations due to influenza complications. Furthermore, with the recent 2009 influenza A H1N1 pandemic and the replacement of the previous influenza A seasonal H1N1 strain with the pandemic strain as the new seasonal strain, it is becoming evident that younger people are experiencing influenza-related complications requiring hospitalization at a higher rate than in the past, when infected with 2009 influenza A H1N1. In only 50% of the children hospitalized with influenza, but in 87% of the adults, an underlying chronic medical condition putting them at high risk for complications was identifiedCitation54.
As a limitation, the targeted literature review only included title keyword searches of the MEDLINE database and only reviewed articles published in English. The Embase databases were not searched. The keyword searches were supplemented by hand searches of the bibliographies from the full-text papers reviewed. This review was intended to provide a general overview of existing data on the burden on influenza disease among widely accepted high-risk conditions and, as such, has identified what appear to be gaps in the literature presenting information on complication rates and resource use for different high-risk groups. In particular, studies specifically designed to differentiate between influenza complication rates in different high-risk categories would provide valuable information for assessing the value of new management strategies in the different high-risk groups. In addition, it should be recognized that conclusions drawn from studies in different risk groups will always be complicated by the seasonal variability and unpredictability of the influenza epidemic each year and by differences across countries and regions in the access to healthcare, the vaccination rate, and the structure of the healthcare system.
Conclusions
The articles included in this review provide information indicating that different high-risk groups might have different levels of risk from a clinical case of influenza. Thus, persons 65 years of age living in the community with no other high-risk conditions may have the lowest burden of influenza complications; among those with other high-risk conditions, a middle-aged person with diabetes who is immunocompetent may be at a lower risk for influenza complications than someone who is immunocompromised, regardless of age; and a person with a high-risk condition who has LCI and is admitted to the hospital with pneumonia/LRTI has a high likelihood of ICU admission, mechanical ventilation, and death. However, there was a wide range of estimates from studies of patients with the same high-risk condition. These findings can be used to evaluate new therapies, including better influenza vaccines, prophylaxis, and/or treatments strategies for different high-risk groups.
Transparency
Declaration of funding
This review was sponsored by Janssen-Cilag, Horsham, PA, USA.
Declaration of financial/other relationships
JM has disclosed that she has received funding from Janssen to perform the literature review and to prepare this paper. She performs similar projects for many other pharmaceutical companies. MK, SL, and GH-T have disclosed that they are employed by Janssen, which is developing a compound for the treatment of influenza in high-risk groups.
Authors’ contributions
MK, SL, and JM designed the literature review study, and JM performed the literature review. MK, GH-T, and JM jointly developed the outline for the paper, interpreted the results of the literature review, and wrote sections of the paper. All authors reviewed drafts of the paper and reviewed and approved the final paper.
References
- Enserink M. Virology. Mapmaker for the world of influenza. Science 2008;320:310-11
- World Health Organization (WHO). Acute Respiratory Infections (Update September 2009). Available at http://www.who.int/vaccine_research/diseases/ari/en/index1.html. [Last accessed 28 November 2012]
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 2008;121:258-64
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010;38(Suppl 4):e91-7
- Turner D, Wailoo A, Nicholson K, et al. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess 2003;7:iii-iv, xi–xiii, 1–170
- Begum F, Pebody R. Seasonal influenza vaccine uptake among those 65 years and over and under 65 years at risk in England: Winter Season 2009-2010. London: Health Protection Agency, Department of Health; 2010. Available at http://www.dh.gov.uk/en/Publichealth/Immunisation/Keyvaccineinformation/DH_104070. [Last accessed 28 November 2012]
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;59:1-62
- World Health Organization (WHO), Regional Office for Europe: WHO/Europe recommendations on influenza vaccination during the 2010/2011 Winter Season. 2011. Available at http://www.euro.who.int/__data/assets/pdf_file/0004/128839/Euro_flu_2010-2011.pdf. [Last accessed 28 November 2012]
- van den Berg JP, Westerbeek EA, van der Klis FR, et al. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev 2011;87:67-72
- Salgado CD, Giannetta ET, Hayden FG, et al. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Infect Control Hosp Epidemiol 2004;25:923-8
- Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med 1998;158:1769-76
- Spaude KA, Abrutyn E, Kirchner C, et al. Influenza vaccination and risk of mortality among adults hospitalized with community-acquired pneumonia. Arch Intern Med 2007;167:53-9
- Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect 2009;58:446-58
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24:1159-69
- Goossen GM, Kremer LCM, van de Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev 2009;2:CD006484
- Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis 2009;9:493-504
- Jefferson T, Di Pietrantoni C, Al-Ansary LA, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010;2:CD004876
- Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:36-44
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1-25
- Hernan MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011;53:277-9
- Jefferson T, Jones M, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009;339:b5106
- Critical Appraisal Skills Programme (CASP). Making sense of evidence about clinical effectiveness: 12 questions to help you make sense of cohort study, 2010. Available at http://www.casp-uk.net/wp-content/uploads/2011/11/CASP_Cohort_Appraisal_Checklist_14oct10.pdf. [Last accessed 28 November 2012]
- Center for Reviews and Dissemination. Systematic Reviews: CRD’s guidance for undertaking reviews in health care. York, UK: York Publishing Services, Ltd, 2008
- Vila-Córcoles A, Rodriguez T, de Diego C, et al; EPIVAC Study Group. Effect of influenza vaccine status on winter mortality in Spanish community-dwelling elderly people during 2002-2005 influenza periods. Vaccine 2007;25:6699-707
- Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005;352:1749-59
- Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant 2005;11:781-96
- Yousuf HM, Englund J, Couch R, et al. Influenza among hospitalized adults with leukemia. Clin Infect Dis 1997;24:1095-9
- Boudreault AA, Xie H, Leisenring W, et al. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol Blood Marrow Transplant 2011;17:979-86
- Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85:278-87
- Irwin DE, Weatherby LB, Huang WY, et al. Impact of patient characteristics on the risk of influenza/ILI-related complications. BMC Health Serv Res 2001;1:8
- Tomas J, Lelièvre F, Bercelli P, et al. Hospital admissions related to influenza in France during the 2006/2007 epidemic. Rev Epidemiol Sante Publique 2011;59:159-67
- Schnell D, Mayaux J, de Bazelaire C, et al. Risk factors for pneumonia in immunocompromised patients with influenza. Respir Med 2010;104:1050-6
- Babcock HM, Merz LR, Fraser VJ. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol 2006;27:266-70
- Li G, Yilmaz M, Kojicic M, et al. Outcome of critically ill patients with influenza virus infection. J Clin Virol 2009;46:275-8
- Cooksley CD, Avritscher EB, Bekele BN, et al. Epidemiology and outcomes of serious influenza infections in the cancer population. Cancer 2005;104:618-28
- McGeer A, Green KA, Plevneshi A, et al; Toronto Invasive Bacterial Diseases Network. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007;45:1568-75
- Oliveira EC, Marik PE, Colice G. Influenza pneumonia: a descriptive study. Chest 2001;119:1717-23
- Dao CN, Kamimoto L, Nowell M, et al. Emerging Infections Program Network: adult hospitalizations for laboratory-positive influenza during the 2005-2006 through 2007-2008 seasons in the United States. J Infect Dis 2010;202:881-8
- Muller MP, McGeer AJ, Hassan K, et al; Toronto Invasive Bacterial Disease Network. Evaluation of pneumonia severity and acute physiology scores to predict ICU admission and mortality in patients hospitalized for influenza. PLoS One 2010;5:e9563
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-40
- Skiest DJ, Kaplan P, Machala T, et al. Clinical manifestations of influenza in HIV-infected individuals. Int J STD AIDS 2001;12:646-50
- Angelo SJ, Marshall PS, Chrissoheris MP, et al. Clinical characteristics associated with poor outcome in patients acutely infected with influenza A. Conn Med 2004;68:199-205
- Meier CR, Napalkov PN, Wegmuller Y, et al. Population-based study of incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis 2000;19:834-42
- Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2001;28:479-84
- Orzeck EA, Shi N, Blumentals WA. Oseltamivir and the risk of influenza-related complications and hospitalizations in patients with diabetes. Clin Ther 2007;29:2246-55
- Murata Y, Walsh EE, Falsey AR. Pulmonary complications of interpandemic influenza A in hospitalized adults. J Infect Dis 2007;195:1029-37
- Nichols WG, Guthrie KA, Corey L, et al. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 2004;39:1300-6
- Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis 1996;22:778-82
- Whimbey E, Elting LS, Couch RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant 1994;13:437-40
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002;2:287-91
- Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003;163:1667-72
- Sessa A, Costa B, Bamfi F, et al. The incidence, natural history and associated outcomes of influenza-like illness and clinical influenza in Italy. Fam Pract 2001;18:629-34
- Martino R, Rámila E, Rabella N, et al. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis 2003;36:1-8
- Centers for Disease Control and Prevention. 2010–2011 Influenza Season Summary. Available at http://www.cdc.gov/flu/weekly/weeklyarchives2010-2011/10-11summary.htm. [Last accessed 28 November 2012]
