Abstract
Objective:
To evaluate costs and outcomes associated with initial tapentadol ER vs oxycodone CR for the treatment of chronic non-cancer pain (CNCP) in the US.
Methods:
This study developed a Monte-Carlo simulation based on the scientific foundation established by published models of long-acting opioids (LAO) in patients having moderate-to-severe CNCP. It estimates costs and outcomes associated with the use of tapentadol ER vs oxycodone CR over a 1-year period from the perspective of a US payer. LAO effectiveness and treatment-emergent adverse event (TEAE) rates are derived from clinical trials of tapentadol ER vs oxycodone CR; other inputs are based on published literature supplemented sparingly with clinical opinion. Sensitivity analyses consider the impact of real-world dosing patterns for LAO on treatment costs.
Results:
Initial tapentadol ER consistently demonstrates better outcomes than initial oxycodone CR (proportion of patients achieving adequate pain relief and no GI TEAE; acute TEAE-free days; days free of chronic constipation; quality-adjusted life days; productive working hours). While total costs with initial tapentadol ER are slightly (2.2%) higher than with initial oxycodone CR, nearly twice as many modeled patients in the initial tapentadol ER arm (29% vs 15%) achieve adequate pain relief and no GI TEAE compared to initial oxycodone CR. In sensitivity analyses, tapentadol ER becomes a dominant strategy when real-world dosing patterns are considered.
Conclusion:
The additional costs to produce better outcomes (pain relief and no GI TEAE) associated with tapentadol ER are small in the context of double the likelihood of a patient response with tapentadol ER. When daily average consumption (DACON) for oxycodone CR is factored into the analysis, initial tapentadol ER becomes a dominant strategy. Our findings are both strengthened, and limited by the use of randomized trial-centric input parameters. These results should be validated as inputs from clinical practice settings become available.
Introduction
Chronic non-cancer pain (CNCP) is pain that persists beyond the expected period of normal healing, is maladaptive, and provides no protective function. It is a major problem in modern societyCitation1. Chronic pain often causes substantial psychological distress as well as interference with daily activitiesCitation1–4.
Long-acting opioid (LAO) therapy can be an effective therapy for carefully selected and monitored patients with CNCPCitation5. Effective analgesia of LAO in such patients has been demonstrated in clinical studies of oxycodoneCitation6,Citation7. Further, published guidelines have endorsed the use of opioids with appropriate patients to treat these conditions when other clinical management strategies do not provide sufficient reliefCitation1,Citation8.
The process of titrating opioid therapy requires finding a balance between pain relief and treatment-emergent adverse effects (TEAE). Even after initiation of opioid treatment has been successful—that is, finding a treatment that achieves analgesia without unacceptable side-effects—subsequent change in the opioid regimen may be required, driven by events such as TEAE. Thus, differences in adverse event rates between LAO agents may give rise to differences in the tolerability of and, in turn, patient persistence with the prescribed treatment.
Tapentadol ER (tapentadol extended release tablet) is a novel, centrally-acting analgesic combining µ-opioid receptor agonism and norepinephrine reuptake inhibition in a single molecule. Randomized clinical trials (RCT) have shown consistently that tapentadol ER effectively reduces moderate-to-severe pain with superior tolerability compared to oxycodone CR (oxycodone controlled release) at equianalgesic dosesCitation9–11.
This study uses a decision model framework to evaluate the outcomes (pain relief, tolerability, persistence with therapy) and costs (direct and indirect) associated with the use of tapentadol ER compared with oxycodone CR for the treatment of CNCP in the US.
Methods
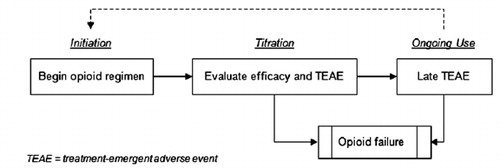
We constructed a Monte Carlo simulation using Microsoft Excel® 2007 software to assess the relative costs and outcomes associated with tapentadol ER compared to oxycodone CR in opioid-naïve and opioid-experienced patients with moderate-to-severe CNCP (specifically, patients with chronic pain due to osteoarthritis or low back maladies) who are appropriate candidates for LAO therapy. The analysis begins with LAO initiation and continues through titration and long-term use. It considers adequacy of pain relief, TEAE, opioid switching, stable LAO use over a 1-year time horizon and, if warranted, opioid failure (). The model builds on foundations established by earlier models of LAO use in CNCPCitation12–15. For instance, much like Neighbors et al.Citation14, we represent LAO-related adverse events in a stepwise, sequential manner, and we assume that patients repeat the titration phase in the event of a switch in opioid therapy. We also embrace the concept put forward by Ward et al.Citation15 that the situation of prescribing opioids to manage chronic pain is best served by a modeling structure that avoids the restrictive assumptions of Markov models.
describes patients’ movement through the system in greater detail. The model assumes that each patient begins LAO therapy (tapentadol ER or oxycodone CR) and then follows up in clinic visits. At each visit, the clinician enquires about the patient’s level of pain relief and experience of TEAE. The duration of each phase in the model depends on individual patients’ simulated experience, as described below.
Opioid initiation
All modeled patients are assumed to initiate therapy with either tapentadol ER or oxycodone CR. Dosing schedules are drawn from RCTsCitation9–11 and consist of a starting dose that adjusts upward to a minimum therapeutic dose over a fixed period of 7 days. Thus, the model assumes that total daily initial dosing (bid regimen) is tapentadol ER 100 mg/day (oxycodone CR 20 mg/day) for the first 3 days, increasing to tapentadol ER 200 mg/day (oxycodone CR 40 mg/day)—the minimum therapeutic dose—for the next 4 days. This dosing schedule is consistent with clinical guidelines which recommend beginning a trial of opioid therapy with a low dosage and increasing the dosage gradually until an optimal opioid dose is attainedCitation1,Citation5.
Titration
The duration of LAO therapy in the model depends on each patient’s simulated experience with pain relief and TEAE. During titration each patient’s opioid dose is individualized in order to achieve an acceptable balance between pain relief and opioid-related adverse effectsCitation1,Citation5.
The goal of titration in the model is to establish a stable opioid dose, achieving pain control with no unacceptable opioid-related TEAE. After a patient completes the initial 7-day regimen of tapentadol ER or oxycodone CR (opioid initiation), the model assumes that there will be 3 days (minimum) between any further dose titrations, and that any dosage adjustments will be made in increments of tapentadol ER 100 mg/day or oxycodone CR 20 mg/day. Maximum allowable doses in the model are tapentadol ER 500 mg/day or oxycodone CR 100 mg/day, again corresponding to tapentadol ER vs oxycodone CR clinical trial protocolsCitation9–11.
During titration, patients’ experience of LAO efficacy (pain relief) as well as the most common TEAE (constipation; nausea/vomiting; dizziness; somnolence; pruritus)Citation16 are considered simultaneously. If all other options have been exhausted, the model allows for patients to fail opioid therapy. Running the model produces a dataset containing 365 days of simulated experience for each patient in each of the modeled scenarios. Aggregating these data produces modeled outputs.
Efficacy
Baseline efficacy is modeled with reference to pooled Kaplan-Meier analyses of time-to-adequate-pain-relief (responders) from tapentadol ER vs oxycodone CR trials, where adequate pain relief is defined as a 30% or greater reduction in painCitation9–11. For example, by day 10 of the trial, about one-quarter of patients had achieved a 30% or greater reduction in pain; thus, the model assumes that the likelihood of adequate pain relief for any given patient on day 10 is 25%. Head-to-head clinical trial data showed no statistical difference in efficacy between the two LAO; thus, the model assumes equivalent efficacy for tapentadol ER and oxycodone CRCitation9–11.
Treatment-emergent adverse events
In the model, patients are at risk of experiencing TEAE for 15 weeks after beginning opioid therapy; i.e., a patient may experience TEAE even after a (temporarily) stable opioid dose has been achieved. TEAE (constipation; nausea/vomiting; dizziness; somnolence; pruritus) incidence rates are derived from Kaplan-Meier analyses of time-to-first-event from tapentadol ER vs oxycodone CR trialsCitation9–11. Thus, due to data limitations, the model assumes that TEAE are drug-specific but not dose-specific, and that patients will experience a maximum of one treatment-emergent episode of each event per LAO agent.
Acute TEAE (nausea/vomiting, somnolence, dizziness, pruritus) are modeled as time-limited occurrences for which only a proportion of affected patients will seek medical contact (). In contrast, constipation is modeled as an acute occurrence (initial event) that has chronic management implications; thus, patients experiencing a constipation adverse event who are treated acutely are assumed to remain on treatment (stool softener; laxative) for the duration of therapy with the LAO agent.
Table 1. Days duration, management, and utilization associated with TEAE.
The model does not make an explicit distinction between mild, moderate, and severe TEAE. However, the model implicitly acknowledges TEAE severity (and the degree to which patients are bothered by the TEAE) by assuming that patients may, or may not seek medical attention due to TEAE according to rates documented in published literatureCitation17. The model assumes further that medical contact is necessary for a patient’s experience of TEAE to impact therapy (e.g., prescription treatment for TEAE; change in LAO therapy). Any prescribed interventions for TEAE are assumed by the model to be effective. Only the direct costs of healthcare services due to TEAE are considered. The model does not address adverse impacts of TEAE treatment. These assumptions apply equally in both modeled arms.
Clinical assessment schedule
The model assumes patients will receive routine clinical re-assessment to evaluate adequacy of pain relief and TEAE at 14-day intervals until an optimal (stable) opioid dose is found. Once an optimal dose is found, patients are assumed to be reassessed clinically at scheduled, 30-day intervals, in keeping with clinical guidelines for the use of opioid therapy in CNCPCitation5. The occurrence of TEAE may result in an unscheduled clinical re-evaluation at any point in the modeled year.
Clinical management
All scheduled clinical encounters are assumed to occur in a primary care office. Unscheduled clinical encounters for TEAE, however, may prompt unscheduled clinical contact, such as a telephone call or an office, walk-in clinic, or emergency department (ED) visit. Utilization may vary by healthcare setting; however, the model assumes that the longer-term clinical management decision regarding LAO therapy (e.g., increase dose, opioid switch) will not vary by healthcare setting.
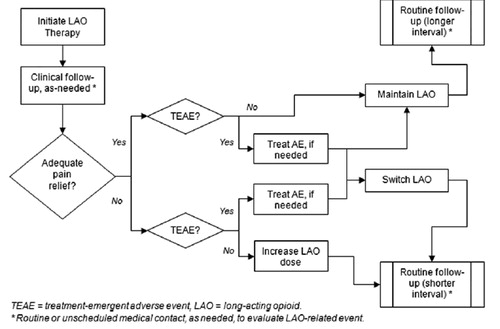
Clinical management during titration is modeled simply depending on each patient’s simulated experience with regard to LAO efficacy and TEAE. We developed decision logic with reference to published clinical practice guidelinesCitation1,Citation8 and informed by expert clinical opinion. The same decision logic governs patient flow in the model, regardless of LAO:
Adequate pain relief
If pain relief is adequate and there are no TEAE then maintain current LAO regimen.
If pain relief is adequate and patient has one or more TEAE then either (a) treat TEAE (if applicable) and maintain current LAO regimen; or (b) treat TEAE (if applicable) and switch LAO agent.
Inadequate pain relief
If pain relief is inadequate and there are no TEAE then increase dose of current LAO.
If pain relief is inadequate and patient has one or more TEAE then switch LAO agent.
Opioid switching
Patients begin the model on initial LAO therapy with tapentadol ER or oxycodone CR. The model assumes there is one alternate LAO, represented as an amalgam (e.g., weighted average efficacy, cost) of available alternative LAO therapies (branded and generic fentanyl, hydromorphone, morphine sulfate).
Patients switching LAO therapy move to a morphine-equivalent dose of the next agentCitation18 and repeat titration on the new LAO regimen (i.e., they are again vulnerable to TEAE). Patients who switch to the alternate LAO may stay on that LAO for the duration of the modeled year or they may fail opioid therapy, but they may not subsequently switch to another LAO therapy.
Ongoing use
Once an optimal opioid dose of LAO is found, patients are assumed to be reassessed clinically at 30 day intervals. However, this stable pattern of LAO use may be interrupted by subsequent TEAE.
Opioid failure
Opioid failure is a treatment alternative of last resort in the model; it may occur at any time (titration; ongoing use) if all other modeling options have been exhausted. Opioid failure is an absorbing state. The model assumes that patients who fail LAO therapy continue to have underlying chronic pain; thus, they are assigned a fixed, one-time macro-cost to manage and/or re-establish pain control ($3440) as well as an assumed daily cost of subsequent pain control therapy ($17) () The subsequent approach to pain management is not detailed in the model nor is it intended to be exhaustive; rather, it is intended to represent costs associated with key clinical management practices—such as additional diagnostics, office visits, and interventional treatments —that may occur in this patient population. For patients who fail opioid therapy, the model assumes 14 days of inadequate pain relief while pain control is being re-established, leading to a temporary decrement in health-related quality-of-life (). Clinical management for those who fail opioids is assumed to impact workplace productivity (). The fixed, one-time cost does not vary by LAO therapy or by the elapsed time between the start of therapy and the time of opioid failure.
Table 2. Opioid failure: component costs.
Table 3. Utilities.
Table 4. Workplace productivity.
Health-related quality-of-life
Traditionally, patient preferences for certain health statuses are arranged on an interval scale, with 1.0 assigned to an optimal level of health and well-being (“perfect health”) and 0.0 assigned to the worst health status possible, or death. Each health status in between is assigned a preference weight ranging from 0.0–1.0. Quality-adjustments allow differences in morbidity effects between alternate interventions to be assessed.
In the present application, disutilities are calculated additively relative to the reference case of patients who achieve an adequate level of pain control and experience no LAO-related adverse outcomes (). From this baseline, utility decrements associated with events such as TEAE or periods of uncontrolled pain are subtracted for the period of time that the adverse health status is experienced.
In order to avoid the accumulation of disutilities that create a quality-adjusted life day less than zero, the summary calculations in the model assume that a patient may accrue disutilities for inadequate pain relief and one other TEAE in any given day. Thus, if a patient has more than one TEAE on the same day, only the event with the greatest disutility is accrued for that day. Utility decrements for each event in the model are the same regardless of LAO. However, the rate at which patients experience each event may differ depending on the opioid agent.
Workplace productivity
Workplace productivity is operationalized in terms of productive work hours lost due to inadequate pain relief and TEAE, as well as productivity losses related to clinical encounters (). These lost productive working hours may include absenteeism (e.g., sick leave, personal time off) and presenteeism (e.g., decreased quality/quantity of work while in the workplace) due to chronic pain and/or its treatmentCitation22. In order to avoid the accumulation of productivity decrements that create less than zero productivity hours in any 1 day, the summary calculations in the model assume that a patient will lose productive work hours for inadequate pain relief and one other LAO-related event in any given day. Thus, if a patient has more than one TEAE on the same day, only the event with the greatest loss in productive work hours is accrued for that day.
Costs
Costs, including medications, clinical assessments, and treatment of TEAE and opioid failure, are reported in 2012 US dollars (). Five strengths of tapentadol ER (50 mg, 100 mg, 150 mg, 200 mg, 250 mg) and five strengths of oxycodone CR (10 mg, 20 mg, 30 mg, 40 mg, 50 mg) are considered. Pharmacy prices represent drug wholesale acquisition cost (WAC), blended among branded and generic versions of the same agent, as applicable.
Table 5. Unit costs.
The base case analysis assumes a daily average consumption (DACON) of 2.0 for both tapentadol ER and oxycodone CR. Sensitivity analysis examine a daily cost for oxycodone CR that is based on real-world DACONCitation23.
Model outputs
The model keeps ‘score’ for each patient depending on his/her path through the simulated treatment year (clinical management, events, direct costs, utilities, productivity), creating a simulated database of patient experience. At the end of the 365 modeled days each patient will either (a) be on the initially prescribed LAO; (b) be on the alternate LAO; or (c) have failed opioid therapy. In each case, a full year of direct and indirect cost estimates is created for all patients who enter the model.
Primary model end-points are patients who achieve adequate pain relief and no GI TEAE, and costs. Additional outputs include days free of any (acute) TEAE; days free of chronic constipation; number of routine office visits; number of unscheduled office visits; number of TEAE; number of opioid failures; percentage of patients who remain on initial LAO; quality-adjusted life days; and productive work hours.
Model validation
Model inputs were derived from RCTs and published sources wherever possible. The model structure and assumptions were confirmed with two practicing pain specialists. Technical validity was assessed by a thorough quality check of programming and by setting inputs to extreme values. Formal stability tests of the model were conducted; these tests demonstrated that the variability of model results from one run to the next leveled off when the number of replications (simulated patients) in each modeled arm exceeded 900. In addition, we confirmed that the distribution of model-generated efficacy outcomes at 1, 30, 60, and 90 days were, as intended, comparable to RCT results. At each time point the model-generated efficacy outcomes were within 1–3% of trial results; differences between model- and trial-generated results were evenly distributed (positively and negatively) around zero.
In addition, one-way sensitivity analyses were conducted on user-modifiable variables to test the robustness of the modeled observations.
Results
Summary results for 50 runs of the model using 1000 patients in each model arm (total of 100,000 simulated patients) and baseline input values for all variables are presented in (clinical, humanistic variables) and 7 (costs). All end-points refer to per-patient average annual outcomes. All results are calculated on an intent-to-treat basis. For example, shows that, after 50 runs of the model, an average of 29% (SD = 1.41%) of patients in the initial tapentadol ER arm achieved adequate pain relief and no GI TEAE vs 15% (SD = 1.03%) of patients in the initial oxycodone CR model arm. This (average 14 percentage point) difference was observed in 50 out of 50 runs of the model; that is, the initial tapentadol ER arm (vs initial oxycodone CR) consistently yielded a greater proportion of patients with adequate pain relief and no GI TEAE at the end of the modeled year.
Table 6. Summary 1-year results (clinical, humanistic variables).
Table 7. Summary results (costs).
Compared to the oxycodone CR group, a greater number of modeled patients in the initial tapentadol ER arm achieve adequate pain relief and no GI TEAE (29% vs 15%). Patients in the initial tapentadol ER arm consistently have more days free of any (acute) TEAE, more days free of chronic constipation, and fewer TEAE. A greater number of patients who initiate opioid therapy with tapentadol ER remain on the original LAO for the entirety of the modeled year (42% vs 38%). Total direct costs for patients in the initial tapentadol ER arm are slightly (2.2%) higher than for patients in the initial oxycodone CR model arm. In addition, modeled patients in the initial tapentadol ER arm achieve greater non-monetary benefits, such as more quality-adjusted life days and more productive working hours, compared to patients initiating treatment with oxycodone CR.
Sensitivity analysis
One-way sensitivity analyses on all user-modifiable variables demonstrated the robustness of the model findings. Results of these analyses show that the advantage of initial therapy with tapentadol ER vs oxycodone CR increases as (a) the cost of a routine office visit decreases relative to an unscheduled office visit (because a strategy of initial tapentadol ER consistently results in more routine, but fewer unscheduled office visits); (b) the direct treatment and patient management costs associated with TEAE increase (because the incidence of TEAE is consistently lower with initial tapentadol ER); and (c) the costs associated with switching LAO therapy increase (because a higher proportion of patients remain on initial tapentadol ER without switching).
Estimates of LAO effectiveness and TEAE rates used in the model are derived from a comparative RCT of tapentadol ER vs oxycodone CR. Changes in these variables will influence model outcomes. In general, an agent’s advantage relative to the comparator LAO will increase as (a) the proportion of patients for whom the LAO is effective increases; (b) efficacy (pain relief) is achieved earlier following the start of the LAO therapy; and (c) TEAE rates decrease.
Our baseline model conservatively assumes twice-daily dosing for both LAO, following clinical trial protocols. However, while a recent audit of 100,000+ prescriptions confirms real-world DACON for tapentadol ER (all strengths) of ∼2.0 (50 mg, 2.11; 100 mg, 1.99; 150 mg 1.98; 200 mg, 1.96; 250 mg, 1.95), it reveals that patients tend to use more than twice-daily dosing for oxycodone CR (10 mg, 2.36; 20 mg, 2.46; 30 mg, 2.43; 40 mg 2.69; 50 mg 2.45)Citation23. All other things being equal, the advantages of any therapy will tend to decrease as the price of that therapy increases relative to its comparator (because the increased cost will offset any cost savings). In the context of the current model, we executed 50 model runs after adjusting the daily cost of LAO in keeping with real-world, strength-specific DACON for each agent (tapentadol ER 50 mg, $5.38; 100 mg, $9.37; 150 mg $12.02; 200 mg, $15.15; 250 mg, $15.07 and oxycodone CR 10 mg, $4.77; 20 mg, $9.52; 30 mg, $13.29; 40 mg, $18.45; 50 mg, $22.84). In this scenario, the clinical advantages of initial tapentadol ER vs oxycodone CR remain unchanged while the mean, per patient difference in total cost is $268 (SD = $108; advantage tapentadol ER). Thus, initial treatment with tapentadol ER becomes a dominant strategy (reflecting better outcomes at a lower cost) in 50/50 model runs when the analysis uses a DACON-adjusted price for oxycodone CR.
Discussion
Our analysis demonstrates that CNCP patients started on initial tapentadol ER show consistently better outcomes compared to initial oxycodone CR with respect to the proportion of patients achieving adequate pain relief and no GI TEAE; days free of acute TEAE; days free of chronic constipation; QALD; and productive working hours. In the base case, total costs for patients with initial tapentadol ER are slightly (2.2%) higher than for patients with initial oxycodone CR; however, nearly twice as many patients in the initial tapentadol ER arm (29% vs 15%) achieve adequate pain relief and no GI TEAE compared to initial oxycodone CR. Sensitivity analyses show that a strategy of initial tapentadol ER becomes dominant (reflecting better outcomes at lower cost) when the cost of oxycodone CR considers real-world DACON. Our findings are broadly similar to those of two other independently conducted cost-effectiveness evaluations of tapentadol ER vs oxycodone CR, one from the perspective of the UKCitation24 and the other from the perspective of SpainCitation25.
The differences in tolerability between LAO therapies have important economic implications. Opioid-induced side-effects can be costly to the healthcare system; they have a substantial impact on the costs of pain management by preventing titration to the optimal dose or leading to treatment discontinuationCitation17,Citation26–28. Further, ambulatory patients experiencing opioid-related TEAE may seek additional care (e.g., primary care office visits, prescription medication, sometimes hospitalization) in order to manage the undesired outcomesCitation28–31.
Notice that in our analysis a strategy of initial tapentadol ER consistently produces more routine, and fewer unscheduled clinical encounters. This is because the lower TEAE rate for tapentadol ER (vs oxycodone CR) demonstrated in clinical trials produces fewer adverse events and, as a result, fewer patients seeking unscheduled treatment due to TEAE. Because our model assumes that a care provider will evaluate LAO efficacy and TEAE at every clinical encounter (scheduled or unscheduled), patients who present for an unscheduled visit will, in essence, ‘reset the clock’ on their routine follow-up schedules. Thus, an unscheduled visit on Monday will obviate the need for a previously scheduled visit, say, on Friday the same week.
Of course the story does not stop with clinical care; the impact of reductions in nausea, vomiting, and constipation events is also felt by patients on LAO therapy for CNCP as well as by their employers. In our analysis, the strategy of initial tapentadol ER consistently resulted in more patients achieving optimal LAO therapy on the initially prescribed agent, more QALD and more productive working hours compared to a strategy of initial oxycodone CR. However, these indirect benefits go beyond the immediate payer’s perspective and were not explicitly valued as part of the present analysis.
Our model builds on the scientific foundation established by earlier published models of LAO use in CNCP and incrementally advances the existing body of literature in four ways. First, we created a patient-centric model capable of representing the management of patients on LAO probabilistically, explicitly influenced by both adequacy of pain relief (opioid efficacy) and TEAE. Second, our model describes TEAE using a stepwise, sequential approach. Five possible adverse events are modeled independently, accompanied by underlying decision logic intended to acknowledge that each patient’s experience of opioid-related efficacy and adverse events occurs simultaneously in clinical practice. Further, our model assumes that management decisions are made, over time, on the basis of both parameters. Third, our model represents rates of LAO efficacy and adverse events, by day, for 15 weeks of therapy based on data from RCTs because, in clinical practice, the duration of opioid titration and stable opioid use does not occur over an arbitrary number of days; rather, it may vary substantially by patient depending on his/her experience of pain relief and adverse events. Fourth, our model considers workplace productivity and quality-of-life impacts associated with LAO efficacy and tolerance, using baseline estimates from published sources.
This analysis is not without limitations. Our model evaluates treatment efficacy with reference to clinical trial protocols in which adequate pain relief is standardized as a 30% or greater reduction in pain. This definition is somewhat arbitrary in the context of real-world management where patients may have different starting pain levels, thresholds, and treatment goals. Further, because efficacy data beyond 15 weeks were unavailable, our model assumes that patients’ pain relief status at 15 weeks will carry forward for the duration of the modeled time horizon. Published reports of other modeled evaluations show similar assumptions by some authorsCitation13,Citation14, while other authors have addressed the issue by assuming a constant, non-zero rate of withdrawal due to lack of efficacy for both LAO agentsCitation24,Citation25. Different approaches to this issue will naturally lead to different absolute results; however, any assumption of no difference in long-term efficacy for the two agents should reach similar relative conclusions.
Our model further did not consider longer-term dose adjustments that might be necessitated by analgesic tolerance. Results of a pre-clinical study suggest that the rate of analgesic tolerance with tapentadol is half that of morphineCitation32. If these study results are upheld in the context of oxycodone CR it could yield a more favorable cost-effectiveness profile for tapentadol ER.
Another limitation of our analysis is that TEAE incidence rates are derived from Kaplan-Meier analyses of time-to-first-event from clinical trials Thus, due to data limitations, the model assumes that patients will experience a maximum of one treatment-emergent episode of each event per LAO agent. This is a potentially conservative assumption to the extent that tapentadol has a more advantageous adverse event profile compared with oxycodone, as has been shown in clinical trials and reported in several non-randomized studiesCitation17,Citation33–35.
Finally, baseline model estimates are derived from a variety of sources since there is no one study that has collected data on all of the modeled parameters. Where published estimates were lacking, it was necessary to rely on expert clinical opinion. That notwithstanding, one distinct advantage of a decision model is that it applies a consistent theoretical framework to clarify immediate and downstream cost and outcome tradeoffs between alternative therapeutic approaches. By design, the baseline assumptions of the model are easily changed in light of new information and/or to meet the needs and local clinical practices of decision-makers.
Conclusions
The results from this cost-effectiveness analysis demonstrate that, while total costs for patients with initial tapentadol ER are slightly (2.2%) higher than for patients with initial oxycodone CR, nearly twice as many patients in the initial tapentadol ER arm (29% vs 15%) achieve adequate pain relief and no GI TEAE compared to initial oxycodone CR. This marginal increase in costs to produce better outcomes associated with tapentadol ER may be acceptable to payers. If real-world DACON for oxycodone CR is considered in the analysis, initial tapentadol ER becomes a dominant strategy (i.e., better outcomes at a lower cost). Even beyond the direct costs to managed care, the higher tolerability of tapentadol ER (resulting in fewer TEAEs, fewer unscheduled office visits, more patients achieving optimal LAO therapy on the initial agent, more QALD, and more productive working hours) may produce favorable outcomes of considerable interest to patients as well as to their employers.
Transparency
Declaration of funding
This research was supported by Janssen Global Services, LLC.
Declaration of financial/other relationships
NN, DP, and KO received consulting fees from Janssen Global Services, LLC for the development of this paper. SM is an employee of Janssen Global Services, LLC. SHM is an employee of Janssen Scientific Affairs, LLC. Janssen Pharmaceuticals, Inc. manufactures tapentadol ER.
Acknowledgments
The authors gratefully acknowledge the contributions made to this research by Stacy Rattana.
References
- Trescot AM, Boswell MV, Atluri SL, et al. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician 2006;9:1-39
- Cao H, Zhang Y. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev 2008;32:972-83
- Gureje O, Von Korff M, Simon G, et al. Persistent pain and well-being: a World Health Organization Study in primary care. JAMA 1998;280:147-51
- National Pharmaceutical Council Inc (NPC). Joint Commission on Accreditation of Healthcare Organizations. Pain: current understanding of assessment, management, and treatments. Reston, VA: National Pharmaceutical Council, 2001. Available at http://d.scribd.com/docs/1qeor4k1bd6nmb8g71hj.pdf. [Last accessed April 20, 2010]
- Chou R, Fanciullo G, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J P 2009;10:113-30
- Caldwell J, Hale M, Boyd R, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal anti-inflammatory drugs: a double blind, randomized, multicenter, placebo controlled trial. J Rheumatol 1999;26:862-9
- Roth S, Fleischmann R, Burch F, et al. Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: placebo-controlled trial and long-term evaluation. Arch Intern Med 2000;160:853-60
- American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology 2010;112:810–33
- Janssen-Ortho Inc and Johnson & Johnson. Tapentadol clinical trial protocol PAI-3008. TItusville, NJ. 2009
- Janssen-Ortho Inc and Johnson & Johnson. Tapentadol clinical trial protocol PAI-3009. TItusville, NJ. 2009
- Janssen-Ortho Inc and Johnson & Johnson. Tapentadol clinical trial protocol PAI-3011. TItusville, NJ. 2009
- Frei A, Andersen S, Hole P, et al. A one year economic model comparing transdermal fentanyl with sustained-release morphine in the treatment of chronic noncancer pain. J Pain Palliat Care Pharmacother 2003;17:5-26
- Greiner W, Lehmann K, Earnshaw S, et al. Economic evaluation of Durogesic in moderate to severe, nonmalignant chronic pain in Germany. Eur J Health Econ 2006;7:290-6
- Neighbors D, Bell T, Wilson J, et al. Economic evaluation of the fentanyl transdermal system for the treatment of chronic moderate to severe pain. J Pain Symptom Manage 2001;21:129-43
- Ward A, Bozkaya D, Fleischmann J, et al. Modeling the economic and health consequences of managing chronic osteoarthritis pain with opioids in Germany: comparison of extended-release oxycodone and OROS hydromorphone. Curr Med Res Opin 2007;23:2333-45
- Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372-80
- Gregorian RJ, Gasik A, Kwong W, et al. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J Pain Palliat Care Pharmacother 2010;11:1095-108
- Labby D, Koder M, Amann T. Opioids and chronic non-malignant pain: a clinician’s handbook. Portland, OR: CareOregon, Inc. 2003. Available at http://www.careoregon.org/Providers/PharmacyHelpDesk.aspx. [Last accessed August 2010]
- Brandt LJ, Prather CM, Quigley EM, et al. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005;100(1 Suppl):S5-21
- Bruyere O, Richy F, Reginster JY. Three year joint space narrowing predicts long term incidence of knee surgery in patients with osteoarthritis: an eight year prospective follow up study. Ann Rheum Dis 2005;64:1727-30
- Schmier J, Palmer C, Flood E, et al. Utility assessments of opioid treatment for chronic pain. Pain Med 2002;3:218-30
- Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003;290:2443-54
- IMS Health. IMS national prescription audit. IMS Health: Falls Church, VA, 2012 [Last accessed June 15, 2012]
- Ikenberg R, Hertel N, Moore RA, et al. Cost-effectiveness of tapentadol prolonged release compared with oxycodone controlled release in the UK in patients with severe non-malignant chronic pain who failed 1st line treatment with morphine. J Med Econ 2012;15:724-36
- Obradovic M, Ikenberg R, Hertel N, et al. Cost-effectiveness of tapentadol in severe chronic pain in Spain: a cost analysis of data from RCTs. Clin Ther 2012;34:926-43
- Oderda G, Evans S, Lloyd J, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage 2003;25:276-83
- Oderda G, Said Q, Evans R, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother 2007;41:400-7
- Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag 2009;5:137-44
- Hjalte F, Berggren A, Bergendahl H, et al. The direct and indirect costs of opioid-induced constipation. J Pain Symptom Manage 2010;40:696-703
- Iyer S, Davis K, Candrilli S. Opioid use patterns and health care resource utilization in patients prescribed opioid therapy with and without constipation. Manag Care 2010;19:44-51
- Anastassopoulos K, Chow W, Ackerman S, et al. Frequency and bothersomeness of side effects in pain patients taking oxycodone immediate release: impact on prescripti on and over-the-counter medication use [poster]. Paper presented at: International Society for Pharmacoeconomics and Outcomes Research 14th Annual International Meeting; May 16-20, 2009; Orlando, FL
- Tzschentke TM, Christoph T, Kogel B, et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 2007;323:265-76
- Chancellor J, Martin M, Liedgens H, et al. Stated preferences of physicians and chronic pain sufferers in the use of classic strong opioids. Value Health 2012;15:106-17
- Lange B, Kuperwasser B, Okamoto A, et al. Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv Ther 2010;27:381-99
- Wild J, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract 2010;10:416-27