Abstract
Objective:
To assess healthcare resource utilization and costs in a cohort of US managed care patients with systemic lupus erythematosus (SLE).
Methods:
Claims data from a large managed care plan were used to identify patients of 18–64 years old with SLE-related claims from 2004–2005. Algorithms were developed to retrospectively categorize patients by disease severity and identify flare episodes by flare severity. Descriptive and multivariate analyses were performed to estimate healthcare resource utilization and costs over a 2-year period for the cohort overall and by disease and flare severity.
Results:
Among the 2990 patients in the study cohort, disease severity was mild in 789 (26.4%), moderate in 1558 (52.1%), and severe in 643 (21.5%). During the 2-year follow-up period, SLE patients utilized the following categories of care: office visit (99.7%), laboratory service (99.5%), outpatient hospital visit (76.0%), emergency room visit (45.6%), and inpatient hospital stay (26.4%). Mean total unadjusted healthcare cost per patient was $30,010 over the 2-year follow-up period, with medical and pharmacy costs comprising 76.5% and 23.5% of total expenditures, respectively. Additionally, 95.7% of patients had one or more flares, with a mean (SD) of 6.7 (3.6) flares during the 2-year follow-up period. The average unadjusted cost per mild, moderate, and severe flare, respectively, was $909, $1539, and $17,059, most of which was for medical cost rather than pharmacy cost. The frequency and cost of flares increased with disease severity.
Limitations:
The disease severity and flare severity algorithms were based upon managed care claims data; the algorithm was not verified clinically and may not be generalizable to other health plans.
Conclusions:
SLE is associated with high levels of healthcare utilization and costs in a managed care health plan. Inpatient hospital stays were the primary medical cost drivers, followed by physician office visits and outpatient hospital visits.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, inflammatory, autoimmune disorder characterized by periods of exacerbation (flares) and remission. SLE has an estimated incidence of ∼5 per 100,000 persons and a prevalence of ∼100 per 100,000 persons in the USCitation1–6. The majority of cases occur in women of childbearing age, although SLE can also occur in children and menCitation7. SLE is linked to functional impairment, poor quality-of-life, reduced productivity, unemployment, and increased mortalityCitation8–16.
SLE causes significant morbidity and mortality through effects on multiple organ systems including mucocutaneous, musculoskeletal, renal, hematologic, neurologic, and cardiovascular systemsCitation17,Citation18. Flares associated with SLE are increases in disease activity over a defined period in one or more organ systems involving new or worse clinical signs and symptoms and/or laboratory measurementsCitation19,Citation20. Over time, flares contribute to progressive, irreversible organ damage, which is present in approximately half of patients within an average of 10 years after diagnosis of SLECitation21–24.
Current treatments, including non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, anti-malarials, and immunosuppressants, are directed at symptom management, reducing disease activity, and preventing or reducing disease flaresCitation7. Most of these treatments have been associated with significant morbidity and organ damageCitation25,Citation26. Survival rates for SLE have improved in recent decades with earlier diagnosis, better disease monitoring, and treatment with immunosuppressantsCitation6,Citation18. While the long-term prognosis of SLE remains poor because of chronic tissue and organ damage from the disease and its treatment, newer treatments for SLE may offer promise for further reducing morbidity, improving quality-of-life, reducing organ damage over time, and potentially extending lifeCitation18.
The economic burden of SLE is ill-defined, particularly in comparison with other chronic rheumatologic conditions such as rheumatoid arthritis and osteoarthritisCitation18. Estimating the economic impact of SLE is complicated by its unpredictable clinical course, the heterogeneity of clinical manifestations, and the varying severity of the disease and of flares—complexities that have generally not been accounted for in the few economic assessments of SLE that have been conducted to dateCitation8,Citation18,Citation27. In this study, healthcare resource utilization and healthcare costs of patients with SLE were determined overall and by disease and flare severity over a 2-year follow-up period in a large US managed care health plan.
Methods
Overview
The objective of this retrospective, observational study was to quantify healthcare resource utilization and healthcare costs of patients with SLE as a function of disease severity and flare severity. Claims data from a large managed care health plan were used to identify patients with SLE and to stratify them by disease severity and flare severity according to algorithms developed based on the literature and clinical consultation. Descriptive and multivariate analyses were performed to estimate healthcare resource utilization and healthcare costs.
Data source
The data source was the Optum Research Database, a proprietary administrative claims database with linked enrollment information from a large managed care health plan with geographically diverse enrollees demographically representative of the US population. During the 5-year span of the study (1 January 2004 to 31 December 2008), data from more than 16 million individuals with both medical and pharmacy benefit coverage were available. Patient-level data were de-identified, and the study was conducted in accordance with the principles of the Health Insurance Portability and Accountability Act (HIPAA). This study protocol was exempt from institutional review board review and ethics committee approval because the claims data did not utilize personal identifiable information.
Study design
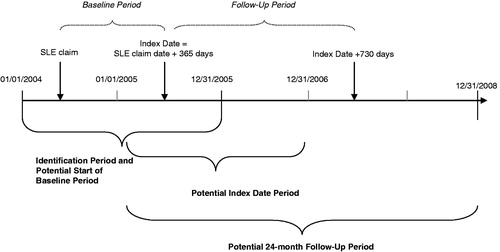
shows a study design schematic. Patients with a claim with a diagnosis code for SLE (International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code [ICD-9-CM] 710.0x) were identified during a 2-year identification period spanning 1 January 2004 through 31 December 2005. Each patient with a claim for SLE during the identification period was assigned an index date, defined as the date 1 year after the first observed SLE claim; this date was selected in order to capture healthcare utilization and costs following the initial diagnostic period. Baseline characteristics were identified during the 1-year Baseline Period prior to the Index Date. Outcomes were identified during the 2-year follow-up period after the Index Date.
Patient selection criteria
The cohort comprised commercial health plan members 18–64 years old who (1) had a claim for SLE from 1 January 2004 to 31 December 2005; (2) had, during the 36 months after the date of the first observed claim, ≥3 visits to a rheumatologist on separate dates with ICD-9-CM SLE diagnosis code 710.0x, or ≥2 visits to a rheumatologist ≥60 days apart with SLE diagnosis code, or ≥2 visits to a rheumatologist on separate dates with SLE diagnosis code and at least one filled prescription for an SLE medication (defined as an oral or IV corticosteroid, anti-malarial, or immunosuppressive); and (3) were continuously enrolled in the health plan for 1 year before and 2 years after the Index Date. Patients who died during the 2 years after the Index Date were required to be continuously enrolled only during the Baseline Period and between the Index Date and death date. No specific exclusion criteria existed as the population was included specifically by age, enrollment, and the SLE criteria outlined above.
Disease severity and flare severity algorithms
Since SLE disease severity and the severity of SLE flares had not been previously assessed using claims data, algorithms for categorizing patients by SLE severity and for identifying and categorizing the severity of SLE flares were developed specifically for this study (). The algorithm for determining disease severity combines elements of disease activity with elements of cumulative damage and/or usage of SLE medications, based on the SLEDAI, SLAM, and BILAG measures of SLE disease activity and consensus of expert clinical opinion to adapt to claims dataCitation28–30. To enable the application of the algorithm to claims data, the ICD-9-CM diagnosis codes and HCPC and CPT procedure codes associated with conditions used to categorize subjects into severity states were identified. The algorithm for identifying SLE flares and categorizing their severity is based on the Lupus Foundation 2nd International Lupus Flare Conference definition categorizing the severity of flares as mild, moderate, and severeCitation20, consensus of expert clinical opinion, and additional criteria of outpatient visits, hospitalizations, and emergency room visits supported by a qualifying SLE diagnosis or SLE-related condition.
Table 1. Algorithms for assigning patients to categories for disease severity and flare severity.
Measurements and outcomes
Demographics and baseline clinical characteristics were summarized using data from the 1-year baseline period. In addition, patient co-morbidities were collected based on a pre-specified list of conditions commonly associated with SLE (e.g., mononeuropathy/polyneuropathy, kidney disease, seizure, psychosis, aortitis), as well as conditions that may be considered in the differential diagnosis of SLE (e.g., discoid lupus, rheumatoid arthritis, fibromyalgia with positive antinuclear antibody, Sjogren’s syndrome, primary anti-phospholipid syndrome, undifferentiated connective tissue disease, idiopathic thrombocytopenic purpura, drug-induced lupus).
Outcomes assessed during the 2-year follow-up period included healthcare resource utilization (office visits, outpatient hospital visits, emergency department visits, laboratory services, inpatient admissions), and healthcare costs (total costs, pharmacy costs, medical costs [office visit, outpatient, emergency department, inpatient, laboratory, and other medical costs]). Healthcare costs were computed as the combined costs paid by the health plan and the patient during the follow-up period. Costs included all medical and pharmacy costs incurred by the SLE patients identified for inclusion in the study. Differentiating between SLE-related costs and non-SLE related costs was not attempted because of the heterogeneous nature of the disease. Costs were adjusted using the annual medical care component of the Consumer Price Index (CPI) to reflect inflation between 2005–2008 (adjusted up to 2008)Citation31. Medication use was assessed as the proportion of patients using SLE medications and the mean number of prescriptions for the following categories of SLE medication: prescription NSAIDs, oral or intravenous corticosteroids, immunosuppressive medications (azathioprine, cyclosporine, cyclophosphamide, lefluonomide, methotrexate, mycofenolate mofetil, intravenous immune globulin), anti-malarial drugs, rituximab, and other biologics (abatacept, adalimumab, certolizumab, golimumab, etanercept, infliximab, anakinra).
Flare severity and the number of flares overall were determined for the 2-year follow-up period. The cost of each flare was computed as the combined costs paid by the health plan and the patient during each flare period. Costs were calculated as total costs, medical costs, and pharmacy costs from all claims (regardless of diagnosis code, procedure, or medication) occurring between the start and end date of each flare. Because expert clinical opinion indicated the majority of flares should resolve within a period of 30 days, this length of time was established as length of a flare. For flares with hospitalizations that started during the flare but did not end before the end of the flare period, the cost of the entire hospitalization was counted toward the cost of the flare.
Data analysis
All data were summarized with descriptive statistics, and comparisons among disease-severity sub-groups (mild, moderate, severe) were conducted with appropriate tests (e.g., t-test, Mann Whitney-U test, chi square) based on the distribution of the measure. In addition, multivariate analyses were conducted on cost outcomes. Because healthcare costs are extremely skewed, estimated cost measures were modeled using a generalized linear model (GLM) with a gamma distribution and log linkCitation32, which avoids potential difficulties introduced by transformation and retransformation of the dependent variableCitation33. A multivariate model comparing the total healthcare costs over the 2-year follow-up period among severity sub-groups was estimated. Independent variables in the model included disease severity sub-group (mild, moderate, severe), gender, age (continuous variable), region (Midwest, South, West, Northeast), baseline count of pre-specified comorbidities, baseline SLE treatments (yes/no for each category of treatment), and whether or not the patient had a principal treating provider. Similarly, a multivariate model was estimated on the costs of a flare at the flare level. The dependent variable was the total cost of the flare during the flare period (continuous variable). Independent variables in the final flare model were gender, age (dichotomized as <45 or ≥45), geographic region, flare severity as determined by the algorithm, whether the flare was identified from medical or pharmacy claims or both, the specialty of the provider treating the flare, length of the flare, and the order of the flare (i.e., whether it was the first, second, third, etc. flare). SAS was used for data management and generation and STATA 10 was used for cost analysis.
Results
Patient sample
presents the study cohort. Among more than 16 million enrollees in the plan, 25,128 had at least one claim for SLE during the Identification Period. Of those patients, 2990 met eligibility criteria and comprised the study cohort. As assessed by the severity algorithm, disease severity was classified as mild in 789 patients (26.4%), moderate in 1558 patients (52.1%), and severe in 643 patients (21.5%).
Patients had a mean age of 44 years and 92% were female. Demographics did not differ significantly by disease severity (). The frequency of co-morbid conditions at baseline was higher in the moderate and severe sub-groups than in the mild sub-group (). The top three common SLE-related comorbidities at baseline were nephritis, mononeuropathy/polyneuropathy, and other renal impairments (). Of the conditions that may be considered as differential diagnoses during the assessment of patients with SLE, rheumatoid arthritis, fibromyalgia, and discoid lupus were the most common, with each found to present in ∼20% of patients at baseline. Fifty-two patients died during the follow-up period (seven in the mild sub-group, 19 in the moderate sub-group, and 26 in the severe sub-group (p < 0.001)).
Table 2. Demographics and comorbidities of patient sample.
Healthcare utilization
Medical services
During the 2-year follow-up period, almost all patients had at least one office visit (99.7%) and at least one laboratory service (99.5%), followed by utilization of at least one outpatient hospital visit (76.0%), emergency room visit (45.6%), and inpatient hospital stay (26.4%) (). The mean number of visits followed a similar pattern: 31.4 office visits, 24.6 laboratory services, 6.6 outpatient hospital visits, 1.9 ER visits, and 0.5 inpatient hospital stays (average length of stay 3.5 days) over the 2-year period. For each of the medical services categories, healthcare utilization was highest in the severe sub-group and lowest in the mild sub-group ().
Table 3. Healthcare utilization and costsa during the 2-year follow-up period.
Medications
The most commonly used medications in this cohort of SLE patients were oral or intravenous corticosteroids, anti-malarial drugs, prescription NSAIDs, and immunosuppressive medications (). Corticosteroid usage was highest in the severe sub-group and lowest in the mild sub-group. Use of anti-malarials, NSAIDs, and immunosuppressants (moderate and severe groups only, by definition) was more consistent across severity sub-groups. Mycofenolate mofetil, methotrexate, and azathioprine were the most commonly used immunosuppressive medications.
Healthcare costs
Mean total unadjusted healthcare costs per patient were $30,010 (2008 USD) over the 2-year follow-up period, and significantly differed (p < 0.001) by disease severity, with highest costs in the severe sub-group ($72,429) followed by the moderate sub-group ($22,250) and the mild sub-group ($10,762) ().
Figure 3. Mean unadjusted per-patient medical, pharmacy, and total costs over the 2-year follow-up period (2008 USD). Costs were significantly different (p < 0.001) between disease severity groups.
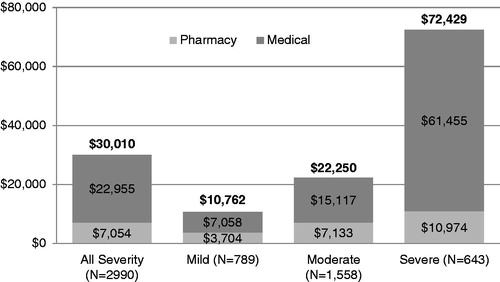
In the cohort as a whole, medical costs accounted for 76.5% and pharmacy costs accounted for 23.5% of total healthcare expenditures. Inpatient hospital stays were the primary medical cost drivers, followed by outpatient hospital visits, physician office visits, and laboratory services (). The proportion of healthcare expenditures accounted for by medical costs increased with increasing disease severity (65.6% mild sub-group, 67.9% moderate sub-group, 84.9% severe sub-group).
The same pattern of results was observed for the 2-year follow-up mean total adjusted healthcare costs, which were $16,624 for the mild sub-group, $23,977 for moderate and $59,610 for the severe sub-group over the 2-year follow-up period. Significant (p < 0.05) predictors of higher healthcare costs in the multivariate models were moderate or severe disease severity, male gender, number of baseline comorbidities, baseline anemia, baseline immunosuppressive drug use, higher office visit count, and interaction of having a principal treating provider and office visit count. In the multivariate models baseline anti-malarial drug use and presence of principal treating provider were significant predictors of lower healthcare costs.
Frequency and cost of flares
The percentage of patients with at least one flare as defined by the algorithm was 95.7%, and the mean (SD) number of flares per patient was 6.7 (3.6) during the 2-year follow-up period in the overall cohort (). Both the percentage of patients with at least one flare and the mean number of flares increased with increasing disease severity (p < 0.001 for both comparisons). Moderate flares were most common (86.5% of the cohort) followed by mild flares (71.5% of the cohort) and severe flares (19.9% of the cohort). The severe disease sub-group was substantially more likely to have severe flares than the other two sub-groups although, the majority of patients in the severe sub-group also experienced mild and moderate flares ().
Table 4. Frequency of flares by disease severity and costs per flare (2008 USD) during the 2-year follow-up period.
The average unadjusted cost of flares increased with flare severity, as did both medical and pharmacy component costs (). The average unadjusted cost per flare was $909 for mild flares, $1539 for moderate flares, and $17,059 for severe flares. The same pattern was observed in the multivariate analysis, in which the average adjusted cost per flare was $1896 for mild flares, $1936 for moderate flares, and $2556 for severe flares. Significant (p < 0.05) predictors of higher healthcare costs of a flare in the multivariate models were moderate or severe flare severity, age ≥45 years, identification of flare through medical claims, alone or in combination with pharmaceutical claims (vs by pharmaceutical claims alone), having a provider other than a rheumatologist treating the flare, a higher-order flare, and longer length of flare; lower costs were associated with the Midwest or South region (vs Northeast).
Discussion
To our knowledge, this study is the first to use administrative claims data to examine utilization and costs in SLE patients and generate algorithms classifying subjects by disease severity and flare severity. The results show that SLE in a large US managed care population was associated with high levels of healthcare utilization and costs, particularly for patients with moderate or severe disease, and that flares are frequent and costly, particularly when they are severe. Medical costs rather than pharmacy costs were the primary contributor to total healthcare expenditures. Because flares, particularly moderate and severe flares, are important cost determinants in patients with SLE, earlier identification and treatment of patients at risk for experiencing any flare, especially moderate or severe flares, could help reduce flare frequency and/or severity and potentially result in reduction of healthcare utilization and cost.
The number of patients meeting criteria for SLE in this managed care population of more than 16 million enrollees was 7428 patients for an estimated prevalence rate of 44 patients with SLE per 100,000 people. This value falls within the range of 20–70 per 100,000 persons found in a recent review paper (which includes non-US studies)Citation6, but is slightly lower than the ∼100 per 100,000 rate found in other recent US studiesCitation1–4. The lower prevalence rate in this study may be due to the stringent entry criteria employed, the purpose of which was an attempt to exclude patients who may have been assessed for SLE but not diagnosed with SLE. The current study included only patients enrolled in commercial health plans; prevalence rates may differ in other populations such as patients enrolled in Medicare or Medicaid or uninsured patients. However, the plans analyzed in this study do have wide geographic distribution across the US; therefore, the results can be generalized to managed care populations on a national level.
This population of patients with SLE frequently used all types of healthcare services during the 2-year follow-up period. The high use of healthcare services translated into high healthcare costs, which averaged just over $30,000 per patient during the 2-year follow-up period. This value is slightly below the total medical costs in a recent claims study in which healthcare costs totaled $19,502 over 12 months for patients with SLE compared with $7264 for matched controls without SLECitation8.
In the current study, medical costs accounted for a much larger proportion of total costs than did pharmacy costs (76.5% vs 23.5%), a finding consistent with previous claims research showing that pharmacy costs accounted for 20% of total costs in patients with SLE and nephritis and 27% of total costs in patients with SLE without nephritisCitation34. The disproportionately large contribution by the severe sub-group to overall cost is attributable in part to the larger proportion of patients in this sub-group who used inpatient hospital services. Although emergency room use was also frequent in the study population as a whole, it did not appear to be a particularly important cost driver or contributor to severity-related differences in costs. The direct relationship between disease severity and healthcare costs in the current study is consistent with the finding in a large Medicaid population, that mean annual medical costs for patients with SLE increased steadily as they were followed over a 5-year periodCitation35. Presumably, increasing disease severity with time accounted for the steady increase in cost in this Medicaid population.
Previous research has established flares as important determinants of the humanistic and economic burdens of SLECitation27,Citation36. As identified by the flare severity algorithm, flares were common in the studied population, with more than 95% of patients having at least one flare over the 2-year follow-up period. Medical costs of a flare were substantially greater than pharmacy costs, and, as expected, costs increased with flare severity. The average cost of a severe flare at $17,059 was substantially higher than the cost of a moderate flare ($1539) or a mild flare ($909). These results are consistent with results of a study that used chart review and patient-reported questionnaires to study the impact of flares on costs in patients with SLE in Hong KongCitation27. Patients with flares used more healthcare resources and incurred significantly higher annual costs with mean total (direct and indirect) costs per patient-year of $22,580 for patients with flares and $10,870 for patients without flares (2006 USD)Citation27. The incremental cost of flares reveals how reduction of flare activity could facilitate important net economic benefits and reduce the cost burden of SLE.
Several study limitations should be noted in interpreting the results of this study. First, the algorithms developed in this study to categorize patients by disease and flare severity relied primarily on the utilization of healthcare services and prescriptions for SLE treatments. Accordingly, the study findings that severe flares and patients categorized as having severe disease are more costly than less severe flares and less severe disease, respectively, may be confounded by the fact that inpatient hospitalization was one of the factors used for categorization. Since it is not generally possible to determine the duration of a flare episode using only claims data, an average duration of 30 days was assigned for all flares. The actual duration of the flare episodes could have been shorter or longer and therefore would affect the utilization and costs calculated during the flare period. Secondly, due to the heterogeneous nature of SLE, we did not attempt to separate SLE-related healthcare utilization and costs from non-SLE related utilization, but rather evaluated the average total cost for all healthcare services for an SLE patient during the 2-year observation period. Therefore, the cost per SLE patient may include services rendered for treating conditions not related to SLE. Third, retrospective claims data, which are collected for payment purposes rather than research purposes, can be inaccurate and subject to coding errors. In addition, some information that could impact study outcomes, including clinical or disease-specific parameters and patient characteristics such as ethnicity, is not readily available in claims data and so not included in the analyses. Although the algorithms used in this study to determine disease and flare severity levels were designed based upon clinical elements of existing disease activity and flare measures and definitions, misclassification of patients into incorrect disease severity or flare severity categories could occur. Fourth, the presence of a prescription claim does not indicate that a drug was taken or taken as prescribed. Therefore, the data on pharmacotherapy cannot be regarded as a definitive indicator of drug use. Moreover, patients in this study could have received drugs in the absence of a prescription claim by receiving drug samples or by filling a prescription outside of the healthcare pharmacy system. Finally, the degree to which the results of this study can be generalized to patients covered under other types of healthcare plans and other populations is unknown. This study did not include patients younger than 18 years and older than 65 years although SLE can occur in these populations. Future research should address healthcare utilization and the cost of treatment in children and the elderly, particularly since currently available studies suggest that the costs of treatment of childhood-onset SLE might exceed those of adult SLECitation37. In addition, prolonged life expectancy plus the cumulative effect and complications of SLE disease and its treatments on key organ systems may increase healthcare utilization in an elderly populationCitation14,Citation38.
Additional research could include indirect cost assessments and cost assessments of specific organ involvement. Determination of the indirect costs, in addition to the direct costs, of SLE in US patients would be of interest in the context of previous findings suggesting that the direct costs of SLE are dwarfed by its indirect costsCitation8,Citation14,Citation39. SLE imposes a significant burden on patients’ work-related disability and loss of productivity due to occupational changesCitation40.
Cost assessments of patients with different organ involvement would be noteworthy since previous studies have shown higher cost burdens in different organ systems. Two studies of administrative claims databases have shown SLE patients with nephritis consume more healthcare resources and incur significantly higher direct medical costsCitation8,Citation14. In one study, the mean annual total medical expenditures for SLE patients with nephritis was almost 4-times higher than those for SLE patients with no nephritisCitation8. Similar to this study, the majority of added costs were attributable to inpatient, medical costs and less were attributable to pharmacy costs. Neuropsychiatric SLE has also been found to be associated with increased higher direct and indirect costsCitation41. Overall, multi-organ flares are more costly than single-organ flaresCitation27.
Conclusions
This study is the first to categorize SLE disease severity and flare severity using a claims-based algorithm. These results show that adult patients with SLE who have increased disease severity and flare severity used more healthcare resources and incurred higher direct costs. Further clinical and economic evaluation of new SLE therapies is worth pursuing, as controlled disease activity and/or decreased flare activity has the potential to manage and reduce the costs of SLE.
Transparency
Declaration of funding
This study was funded by GlaxoSmithKline and Human Genome Sciences.
Declaration of financial/other relationships
CG, PJ, and DB have disclosed that they are employees of GlaxoSmithKline. GD has disclosed that he was an employee of Human Genome Sciences, Inc. NE-N and AR have disclosed that they are employees of OptumInsight, a consulting firm paid by GlaxoSmithKline to assist with conducting this research.
Acknowledgments
The authors acknowledge the following contributors: Mike Ward, MD and Mary Ann Dooley, MD for their clinical consultation on study design; Jessica Wegner, George Goldberg, James Burke, Jim Hartje, Margaret Burgess, Suzi Adams, Laura Becker, and Yvette Dennis of OptumInsight for assistance with study design and analysis; Jane Saiers, PhD of The WriteMedicine, Inc. for writing assistance, which was paid for by GlaxoSmithKline; and Anna Oh of GlaxoSmithKline for editorial assistance.
References
- Naleway AL, Davis ME, Greenlee RT, et al. Epidemiology of systemic lupus erythematosus in rural Wisconsin. Lupus 2005;14:862-6
- Uramoto KM, Michet CJ, Thumboo J, et al. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum 1999;42:46-50
- Balluz L, Philen R, Ortega L, et al. Investigation of systemic lupus erythematosus in Nogales, Arizona. Am J Epidemiol 2001;154:1029-36
- Chakravarty EF, Bush TM, Manzi S, et al. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum 2007;56:2092-4
- Bertsias GK, Salmon JE, Boumpas DT. Therapeutic opportunities in systemic lupus erythematosus: state of the art and prospects for the new decade. Ann Rheum Dis 2010;69:1603-11
- Pons-Estel GJ, Alarcon GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 2010;39:257-68
- D’Cruz DP, Khamashta MA, Hughes GRV. Systemic lupus erythematosus. Lancet 2007;369:587-96
- Carls G, Li T, Panopalis P, et al. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J Occup Environ Med 2009;51:66-79
- Campbell R, Jr Cooper GS, Gilkeson GS. Two aspects of the clinical and humanistic burden of systemic lupus erythematosus: mortality risk and quality of life early in the course of disease. Arthritis Rheum 2008;59:458-64
- Scofield L, Reinlib L, Alarcon GS, et al. Employment and disability issues in systemic lupus erythematosus: a review. Arthritis Rheum 2008;59:1475-9
- Campbell R, Jr., Cooper GS, Gilkeson GS. The impact of systemic lupus erythematosus on employment. J Rheumatol 2009;36:2470-5
- McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus 2006;15:633-43
- Mok CC, Ho LY, Cheung MY, et al. Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: a 2-year prospective study. Scand J Rheumatol 2009;38:121-7
- Panopalis P, Tazdany J, Gillis JZ, et al. Health care costs and costs associated with change in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum 2008;59:1788-95
- Yelin E, Tonner C, Trupin L, et al. Work loss and work entry among persons with systemic lupus erythematosus: comparisons with a national matched sample. Arthritis Rheum 2009;61:247-58
- Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550-7
- Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol 2003;56:481-90
- Lau CS, Mak A. The socioeconomic burden of SLE. Nat Rev Rheumatol 2009;5:400-4
- Isenberg DA, Allen E, Farewell V, et al. An assessment of disease flare in patients with systemic lupus erythematosus: a comparison of BILAG 2004 and the flare version of SELENA. Ann Rheum Dis 2011;70:54-9
- Ruperto N, Hanrahan LM, Alarcon GS. International consensus for a definition of disease flare in lupus. Lupus 2011;20:453-62
- Gladman DD, Urowitz MB, Rahman P, et al. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955-9
- Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine 2006;85:147-56
- Rivest C, Lew RA, Welsing PM, et al. Association between clinical factors, socioeconomic status, and organ damage in recent onset systemic lupus erythematosus. J Rheumatol 2000;27:680-4
- Gladman DD, Urowitz MB, Kagal A, et al. Accurately describing changes in disease activity in systemic lupus erythematosus. J Rheumatol 2000;27:377-9
- Kalunian K, Merrill JT. New directions in the treatment of systemic lupus erythematosus. Curr Med Res Opin 2009;25:1501-14
- Zonana-Nacach A, Barr SG, Magder LS, et al. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum 2000;43:1801-8
- Zhu TY, Tam LS, Lee VW, et al. The impact of flare on disease costs of patients with systemic lupus erythematosus. Arthritis Rheum 2009;61:1159-67
- Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum 1992;35:630-40
- Liang MH, Socher SA, Larson MG, et al. Reliability and validity of 6 systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum 1989;32:1107-18
- Isenberg DS, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus arythematosus. Rheumatology 2005;44:902-6
- Bureau of Labor Statistics. US Department of Labor, Consumer Price Index, Chained Consumer Price Index. Washington, DC. 2009 http://www.bls.gov/data/. Accessed 10 September 2009
- Blough DK, Madden CW, Hornbrook MC. Modeling risk using generalized linear models. J Health Econ 1999;1:153-71
- Manning WG. The logged dependent variable, heteroscedasticity, and the retransformation problem. J Health Econ 1998;17:283-9
- Pelletier EM, Ogale S, Yu E, et al. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a US claims database. Clin Ther 2009;31:2653-64
- Li T, Carls GC, Panopalis P, et al. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large Medicaid population. Arthritis Rheum 2009;61:755-63
- Zhu TY, Tam LS, Lee VW, et al. Relationship between flare and health-related quality of life in patients with systemic lupus erythematosus. J Rheumatol 2010;37:568-73
- Brunner HI, Sherrard TM, Klein-Gitelman MS. Cost of treatment of childhood-onset systemic lupus erythematosus. Arthritis Rheum 2006;55:184-8
- Urowitz MB, Gladman DD, Ibañez D, et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2012;64:132-7
- Sutcliffe N, Clarke AE, Taylor R, et al. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:37-47
- Zhu TY, Tam LS, Li EK. Cost-of-illness studies in systemic lupus erythematosus: a systematic review. Arthritis Care Res (Hoboken) 2011;63:751-60
- Zhu TY, Tam LS, Lee VW, et al. Systemic lupus erythematosus with neuropsychiatric manifestation incurs high disease costs: a cost-of-illness study in Hong Kong. Rheumatology (Oxford) 2009;48:564-8