Abstract
Introduction:
Statins reduce low-density lipoprotein cholesterol (LDL-C) levels, which, when elevated, represent a significant risk factor for cardiovascular (CV) disease. Hyperlipidemic patients at risk of CV events initiated on simvastatin or atorvastatin may be less likely to meet LDL-C goals (defined in National Cholesterol Education Program guidelines) and more likely to experience CV events than patients initiated on rosuvastatin. A 3-year budget impact model was developed to estimate the clinical impact and cost to a US managed care organization (MCO) with 1 million members of initiating high-risk hyperlipidemic patients on rosuvastatin rather than simvastatin or atorvastatin.
Methods:
A total of 1000 adult patients were assumed to initiate statins. The average baseline LDL-C level was 189 mg/dL. In scenario 1, all patients were initiated on simvastatin or atorvastatin and titrated to a higher dose, or switched to atorvastatin (if initiated on simvastatin) or rosuvastatin; in scenario 2, 50% of the 520 high-risk patients were initiated on rosuvastatin. Drug acquisition and administration costs were considered. Product labeling, clinical trial results, national prescription claims data, and published literature were used to populate the model.
Results:
Over 3 years, 75 additional patients reached their LDL-C goal in scenario 2, compared with scenario 1 (633 vs 558, respectively), at an increased cost of $240,628 ($1,415,516 vs $1,174,888, respectively). The additional per member per month (PMPM) cost of scenario 2 was $0.007.
Limitations:
This analysis assumed that statin efficacy is the same in real life as in trials, and used titration and switching patterns not based on patients’ goal attainment. However, sensitivity and scenario analyses showed that the model was less sensitive to these parameters than to cost-related parameters.
Conclusions:
Initiating high-risk hyperlipidemic patients on rosuvastatin may increase the number of patients reaching LDL-C goal at a relatively modest increase in PMPM cost to an MCO.
Introduction
The burden of cardiovascular disease in the US
Cardiovascular disease (CVD) is the leading cause of death and disability in the USCitation1. In 2011, more than 778,000 Americans died of CVD; coronary heart disease (CHD), in particular, accounted for more than 596,000 deaths. The disease places a significant burden on healthcare system resources, with CVD reported as the primary diagnosis for more than 6 million hospitalizations and 95 million physician visits in 2008–2009Citation2. The impact of CVD is expected to increase, as 40.5% of the American population is projected to experience some form of CVD by 2030Citation3.
CVD also generates significant costs to the healthcare system. The American Heart Association (AHA) estimated that direct costs attributable to CVD in the US amounted to $273 billion in 2010, and projected them to triple to $818 billion by 2030Citation3. The impact of CVD on indirect costs (due to productivity loss) amounted to $172 billion in 2010 and were projected to rise to $276 billion by 2030. At the patient level, the average payment per Medicare beneficiary discharged following a short hospital stay with a principal diagnosis of CVD was reported to amount to $10,201 in 2006Citation4.
Cardiovascular disease and lipid-lowering therapy
Multiple studies have shown that high cholesterol levels, and in particular low-density lipoprotein cholesterol (LDL-C), are a significant risk factor for CVDCitation5, and CHD in particularCitation5–7. Data from the National Health and Nutrition Examination Survey (NHANES) suggested that, in 2005–2006, although more than a third of Americans (71 million) had high LDL-C levels (defined as being above their LDL-C goal level or subject to cholesterol-lowering medication), less than half of these were being adequately treated, with the remainder being placed at an increased risk of CVD and associated complicationsCitation8.
Reducing LDL-C levels has been shown to decrease patients’ risk of experiencing a cardiovascular (CV) event, thereby reducing their mortality and morbidityCitation9–13. Statins have been shown to reduce LDL-C levelsCitation14–17, and of this drug class, rosuvastatin has been established as the most efficacious for this purpose: the Statin Therapies for Elevated Lipid Levels compared Across doses to Rosuvastatin (STELLAR) trial, a 6-week, parallel-group, randomized, multi-center trial involving over 2200 patientsCitation14 reported that statins decreased patients’ LDL-C levels by 20–55%, with rosuvastatin consistently achieving the highest reductions (42–55%).
Similarly, in the ECLIPSE trial, patients at high-risk of CVD who titrated to the full range of rosuvastatin doses (10–40 mg) were significantly more likely to reach their LDL-C goal than patients receiving atorvastatin (10–80 mg)Citation18. Moreover, these patients displayed significantly greater improvements in components of their atherogenic lipid profile compared to patients treated with atorvastatin, further supporting the use and adequate titration of rosuvastatin, particularly in patients at high risk of CVD.
National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III guidelinesCitation19 categorize patients into five main risk categories on the basis of their LDL-C levels; target LDL-C goals have been recommended, depending on patients’ category and associated LDL-C level, to facilitate the management of hypercholesterolemia. However, it has been reported that more than a third of patients receiving cholesterol-lowering therapy do not reach their target LDL-C levelsCitation8. The implications of this finding were highlighted by the NCEP Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, which suggested that 80% of patients experiencing CHD were not achieving their LDL-C goal level at the time of the eventCitation14. This highlights the inadequacies of existing treatment patterns in limiting the incidence of life-threatening events.
The cost of improved management of patients with hyperlipidemia
Hyperlipidemic patients tend to be initiated on a generic statin (usually simvastatinCitation20) regardless of their CV risk, in what is referred to across managed care organizations (MCOs) as ‘step edit’Citation21–23. Patients who do not reach their LDL-C goal while receiving these initial statins are subsequently titrated to an increased dose of the initial statin or switched to more efficacious alternatives, as necessary. However, titration and switching are associated with increased resource use and costs in the form of additional clinic visits, laboratory tests, and laboratory technician and pharmacist timeCitation24–26. A retrospective analysis of Medicaid administrative claims from a multi-state, Northeastern MCO showed that statin laboratory and office visit costs increased by 39% when patients switched from atorvastatin to other statins, compared with before the switchCitation27.
Therefore, the step-edit approach may not be appropriate for all patients, especially those at higher risk, who may initially require more aggressive treatment to reach their LDL-C goal and, therefore, are potentially at risk of treatment failure and subsequent switching. Indeed, a cost-effectiveness analysis of lipid-lowering therapies in patients with acute coronary syndrome in the UK showed that initiating patients on rosuvastatin 40 mg rather than simvastatin 40 mg or atorvastatin 80 mg was the most cost-effective approach, avoiding recurrent CV events at a cost below the cost-effectiveness threshold of £20,000 ($32,000) per quality-adjusted life-year (QALY)Citation28,Citation29. Healthcare policies intended to control primary care drug acquisition costs were associated with sub-optimal care. Moreover, as highlighted in NHANES 2005–2008Citation30 and the NCEP ATP III guidelinesCitation19, some patients who do not reach their LDL-C goal may not even have their dose increased or be switched to more efficacious treatments despite their persistently high LDL-C levels; these inadequately treated patients may remain at increased risk of CV events.
Initiating and maintaining patients with higher risk of CVD on rosuvastatin could increase their likelihood of reaching their LDL-C goal, and thereby decrease the clinical burden and cost of switching from less effective treatments, with potential downstream clinical and economic benefits through reduced CV events and associated costs. To investigate this, we developed a model to estimate the budget impact of initiating high-risk patients with dyslipidemia on rosuvastatin rather than simvastatin or atorvastatin from a US MCO’s perspective.
Methods
Model structure and assumptions
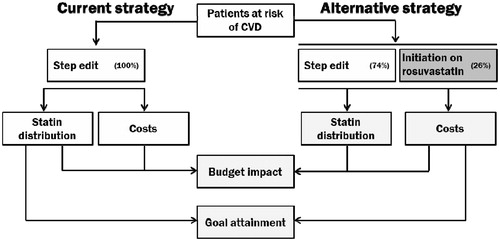
A prevalence-based, deterministic budget impact model was developed in Microsoft Excel to estimate the drug costs over 3 years of the current strategy in which all studied patients with dyslipidemia are initiated on atorvastatin or simvastatin, and of an alternative strategy in which 50% of high-risk patients were initiated on rosuvastatin (). Patients entered the model at the start of the first year and were followed for 3 years, over which drug acquisition and administration costs associated with statin treatment were calculated. Data from US sources were used where possible for model parameters; otherwise, the next most appropriate sources were used.
Over 3 years, it was assumed that patients went through four 90-day cycles. They, therefore, had three opportunities to switch, titrate, or continue receiving the same statin dose.
This model assumed that the size of the population was fixed over 3 years (i.e., the impact of mortality or incident patients initiating statins was not modeled). The time taken to switch was assumed to be negligible, and patients switched/titrated immediately at the end of a given cycle. Costs were fixed over the time horizon of the model (i.e., there was no adjustment for inflation or discounting).
Parameters
Population
This model analyzed a hypothetical MCO with 1 million members aged ≥18 years, 0.1% (1000) of whom were assumed eligible to be initiated on statins, based on the proportion of MCO plan members initiating statins, as reported in an evaluation of the effectiveness of rosuvastatin in real-world clinical practiceCitation31. By default, 24%, 24%, and 52% of patients were defined as having low, moderate, or high risk of CVD, respectively, based on a retrospective observational study of 1654 patients newly initiated on simvastatinCitation32.
All patients in the model were assumed to have an average LDL-C level of 189 mg/dL (with a standard deviation of ±20 mg/dL) based on the weighted average LDL-C level of patients in the STELLAR trialCitation14. The LDL-C goals for each risk category were based on the LDL-C goals defined in the NCEP ATP III guidelines for patients at low (0–1 risk factor), moderate (≥2 risk factors), or high risk (≥2 risk factors and a 10-year risk of CVD >20%)Citation19, as shown in .
Table 1. Patient population.
Starting dose
In the step-edit approach, it was assumed that 66% and 34% of patients were initiated on simvastatin and atorvastatin, respectively, based on market research of statin prescribing patterns in the US in 2012Citation20. This market research was also used to estimate the specific starting dose of patients initiated on rosuvastatin and atorvastatin; the breakdown of starting simvastatin doses was based on Wiley et al.Citation32 ().
Table 2. Starting doses.
Goal attainment
The statin- and dose-specific percent reductions in patients’ LDL-C level were based on data from the STELLAR trialCitation14 (). As rosuvastatin and simvastatin 5 mg doses were not studied in the STELLAR trial, the efficacy (defined as LDL-C reduction) of these doses was extrapolated: the line of best fit based on the reported efficacy of their other doses was derived and used to extrapolate the estimated efficacy of the 5 mg dose of each statin. The probability of a patient in a given risk group reaching his/her LDL-C goal was assumed to be normally distributed, as biological variables tend to be normally distributed, and was calculated using the average LDL-C level, standard deviation, and LDL-C goal in that risk group, as well as the percent LDL-C reduction afforded by the statin dose received.
Table 3. Percent reduction in LDL-C levelsCitation14.
By default, it was assumed that the efficacy of statin doses was the same as that reported in the STELLAR trial. However, evidence suggests that the effectiveness of statins is lower in real-life settings than in clinical trials due to discontinuation, imperfect adherence, and other elements affecting the uptake of, or response to, statinsCitation33. We, therefore, ran a scenario analysis in which the efficacy of all statin doses was decreased by 21%, based on a comparison between expected LDL-C reductions from projections contained in the package inserts for atorvastatin, simvastatin, and pravastatin, and those observed in 367 hyperlipidemic patients in a preventive cardiology practiceCitation33.
Titration and switching
Patients were assumed to titrate to the dose immediately above their current dose (e.g., from 5 mg to 10 mg, 10 mg to 20 mg, etc.). The model did not allow patients to titrate from the highest dose of a statin (i.e., rosuvastatin 40 mg, simvastatin 80 mg, atorvastatin 80 mg); in this case, patients were assumed to either continue receiving the same dose or switch to a more efficacious statin. Therefore, patients receiving atorvastatin were assumed to switch only to rosuvastatin, as it was deemed unlikely for patients to switch to the less efficacious simvastatinCitation14. For the same reason, patients receiving rosuvastatin were assumed not to switch to other statins, and either continued receiving the same dose or titrated to the next highest dose. Moreover, patients were not able to titrate to a lower dose of the statin they were currently receiving.
The statin- and dose-specific proportion of patients initiated and subsequently failing to reach goal who switch, titrate, or remain on the same dose was based on market research dataCitation20 describing the proportion of patients who switched to a different statin, titrated to a higher dose, or continued receiving the same dose (). (The data did not indicate the reason for switching, titrating, or staying on the same dose.)
Table 4. Treatment decision in patients failing to reach goalCitation20.
Dose- and drug-specific proportions of patients were assumed to have switched to individual doses of atorvastatin or rosuvastatin, also based on market research dataCitation20 ().
Table 5. Statin dose received by switching patientsCitation20.
Drug costs
Wholesale acquisition costs (WAC) were based on market research conducted in 2012Citation20 and 2013Citation3Citation4 (). Different companies produce generic simvastatin, but the WAC reported for Mylan were preferred over others, as they were the lowest, making the analysis more conservative.
Table 6. WAC for statins.
Market research identified the dispensing fee for simvastatin, which was also applied to atorvastatin (as both are generic); for the same reason, it was assumed that both commanded no rebate and both required tier 1 copayment amounts. Branded rosuvastatin was assumed to require tier 3 copayment. Copayment amounts were based on those paid by Yale University’s managerial and professional staff and faculty as of June 2012—publicly-available data from Yale University were selected as these fell within the range of copayment amounts identified on websites for other MCOsCitation35. All dispensing fees and copayments were applied on a monthly basis (i.e., every 30 days). All data relating to copayments are shown in .
Table 7. Rebates, dispensing fees, and copaymentsCitation20,Citation34.
Monitoring costs
The model assumed that each dose change was associated with one physician visit and one laboratory test, the costs of which were applied to all patients during each cycle, regardless of statin, dose, or treatment decision (i.e., titration, switching). The cost associated with a physician visit ($139.89) was based on the cost of outpatient visits as per the Centers for Medicare and Medicare Services (CMS) Physician Fee ScheduleCitation36, and the cost of a lipid panel ($18.97) was based on the CMS Clinical Laboratory Fee SchedulesCitation37.
Event costs
By default, event costs were excluded from the model, although a scenario analysis was run in which we projected cost savings from avoided CV events, as the benefits of targeted statin therapy can be measured clinically as well as economically. The average event rate in patients not reaching their LDL-C goal (0.9%, 1.8%, and 2.7% for low-, moderate-, and high-risk patients, respectively) was estimated using CHD rates from the Framingham Heart Study (a prospective, single-center study of 5345 patients in MassachusettsCitation6), adjusted for the incidence of CHD as a proportion of overall CVD based on a systematic review and economic evaluation of statins for the prevention of coronary eventsCitation38. Patients reaching their LDL-C goal were assumed to have a 25% reduction in the incidence of CV events, in line with a retrospective MCO database analysis of claims from 21,856 patients with high CV riskCitation39.
An average cost ($17,488) was applied to each CV event, based on the proportion of individual CV events as reported in Ward et al.Citation38 and the cost associated with each, based on data from studies used in a cost-effectiveness analysis of rosuvastatin in patients at risk of CV eventsCitation40 ().
Table 8. Proportional distribution of CV events and associated costsCitation38,Citation39.
Sensitivity analysis
One-way sensitivity analyses were performed by varying the following model parameters by ±10% relative to the base-case parameter value:
Population parameters:
Average population LDL-C level (189 mg/dL),
Proportion of patients with high CV risk (52%), and
LDL-C goal of patients with high CV risk (100 mg/dL);
Dose-specific titration and switching parameters:
Proportion of atorvastatin and simvastatin patients continuing to receive the same dose,
Proportion of atorvastatin and simvastatin patients titrating, and
Proportion of atorvastatin and simvastatin patients switching;
Cost parameters:
Dispensing fee of rosuvastatin ($2.00),
Rebate of rosuvastatin (41%),
Tier 1 copayment ($5.00), and
Tier 3 copayment ($50.00).
The WAC of the different statins was not investigated in the sensitivity analyses as it is a parameter with a known value—the variability in price was explored via sensitivity analyses on dispensing fees, rebate, and copayment amounts.
In addition, the following scenarios were modeled:
Event costs were included based on the average cost per CV eventCitation38,Citation40 and the number of patients experiencing CV events among those reaching and failing to reach their LDL-C goalCitation6,Citation38,Citation39, and
Statin efficacy was decreased by 21%Citation33.
Results
Base case scenario
Clinical outcomes
In an MCO consisting of 1 million members, 1000 of whom were assumed to initiate statins at the beginning of the model, titration and switching patterns across different risk groups eventually led to 260 patients receiving rosuvastatin after 3 years of the current strategy. In the alternative treatment strategy in which 50% of high-risk (259) patients were initiated on rosuvastatin, 452 patients were receiving rosuvastatin after 3 years. The majority of low- and moderate-risk patients who switched had been initiated on simvastatin (rather than atorvastatin), although some may also have received atorvastatin during intermediary cycles.
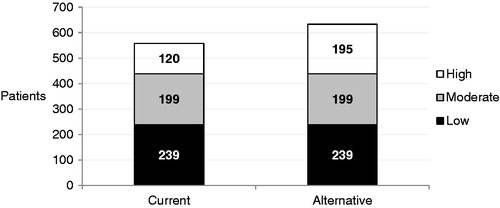
Initiating half of high-risk patients on rosuvastatin in the alternative strategy led to 633 patients reaching their LDL-C goal, compared with 558 in the current strategy (), a 13% increase for the overall population, but a 63% increase among high-risk patients alone. This improved goal attainment was reflected in the average LDL-C goal of the population, which was 111 mg/dL in the alternative strategy compared with 114 mg/dL in the current one.
Economic outcomes
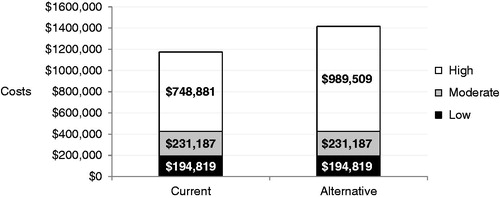
Over 3 years, net spending in the alternative strategy was estimated at $1,415,516, compared with $1,174,888 in the current strategy, leading to a budget impact of $240,628, or a 20% increase in spending compared with the current strategy (). On a per member per month (PMPM) basis, this net spending translates into an increase of $0.007 PMPM (from $0.033 PMPM in the current strategy to $0.039 PMPM in the alternative strategy).
The cost per additional patient reaching his/her LDL-C level in the alternative compared with the current strategy, calculated by dividing the budget impact by the number of additional patients reaching their LDL-C goal, was $3208.
Sensitivity analysis results
Parameters directly affecting the cost of rosuvastatin (i.e., rebate, tier 3 copayment) have the greatest impact on the budget impact, as do the average LDL-C level of the population and the LDL-C goal of patients with high CV risk (). Titration and switching decisions for patients failing to reach goal have less of an impact on the model results. Varying certain parameters—e.g. titration and switching rates, and cost parameters—only affected the results for patients with high risk, as the same proportion of patients with low and moderate risk were assumed to be initiated on simvastatin or atorvastatin in the current and revised treatment patterns.
Figure 4. Sensitivity analyses. One-way sensitivity analyses were performed by varying the following model parameters by ±10% relative to the base-case value: [1] Rebate of rosuvastatin; [2] Average population LDL-C level; [3] Tier 3 copayment; [4] LDL-C goal of patients with high CV risk; [5] Proportion of patients with high CV risk; [6] Proportion of atorvastatin and simvastatin patients continuing to receive the same dose; [7] Proportion of atorvastatin and simvastatin patients switching to a different statin; [8] Tier 1 copayment; [9] Dispensing fee of rosuvastatin; [10] Proportion of atorvastatin and simvastatin patients titrating to a higher dose. Parameters directly affecting the cost of rosuvastatin ([1], [3]) have the greatest impact on the budget impact, as do the average LDL-C level of the population ([2]) and the LDL-C goal of patients with high CV risk ([4]); titration and switching decisions ([6], [7], [10]) for patients failing to reach goal have less of an impact on the model results.
![Figure 4. Sensitivity analyses. One-way sensitivity analyses were performed by varying the following model parameters by ±10% relative to the base-case value: [1] Rebate of rosuvastatin; [2] Average population LDL-C level; [3] Tier 3 copayment; [4] LDL-C goal of patients with high CV risk; [5] Proportion of patients with high CV risk; [6] Proportion of atorvastatin and simvastatin patients continuing to receive the same dose; [7] Proportion of atorvastatin and simvastatin patients switching to a different statin; [8] Tier 1 copayment; [9] Dispensing fee of rosuvastatin; [10] Proportion of atorvastatin and simvastatin patients titrating to a higher dose. Parameters directly affecting the cost of rosuvastatin ([1], [3]) have the greatest impact on the budget impact, as do the average LDL-C level of the population ([2]) and the LDL-C goal of patients with high CV risk ([4]); titration and switching decisions ([6], [7], [10]) for patients failing to reach goal have less of an impact on the model results.](/cms/asset/1708e2e5-ca4c-45e6-adb1-84d89de867e0/ijme_a_801350_f0004_b.jpg)
Figure 5. Scenario analyses. Event costs were included based on the average cost per CV eventCitation38,Citation40 and the number of patients experiencing CV events among those reaching and failing to reach their LDL-C goalCitation6,Citation38,Citation39. Real-life efficacy was estimated by decreasing the efficacy of all statin doses by 21%Citation33.
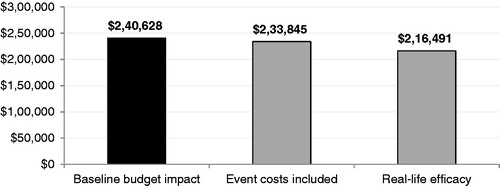
Factoring in real-life efficacy (i.e., a decrease of 21% in the efficacy of all statinsCitation33) leads to the greatest decrease (−10%) in the budget impact, whereas considering event costs results in a more modest decrease (−3%) in the cost of initiating 50% of high-risk patients on rosuvastatin (Figure 5).
Discussion
Our model, which is the first to account for the updated cost of generic atorvastatin in the US, estimated that initiating 50% of high-risk patients (26% of all patients initiating statins) on rosuvastatin rather than generic simvastatin or atorvastatin, led to 75 additional patients—out of 259 high-risk patients—reaching their LDL-C goal in the alternative strategy compared with the current strategy. In line with the higher WAC of rosuvastatin, this resulted in a budget impact of $240,628 over 3 years, although at $0.007 PMPM, this increase in spending is minimal. Budget impact was most sensitive to parameters decreasing the overall cost of rosuvastatin (i.e., rebate, tier 3 copayment); increasing the LDL-C level of the population or decreasing the LDL-C goal of high-risk patients also decreased the budget impact, which further suggests targeting high-risk patients may offer the greatest value for money.
It has been shown that costs associated with prescribed medicines, although substantial, account for less than a fifth of direct CVD costs, and a tenth of direct and indirect CVD costsCitation41. This suggests that, although the cost per additional patient reaching his/her LDL-C goal was estimated at $3208, it should be considered in the context of other factors, such as decreased morbidity and mortality in patients receiving cholesterol-lowering therapyCitation9–13. Moreover, the step-edit approach, although it may eventually allow identification of the ideal statin and dose for patients, places high-risk patients at unnecessary risk by delaying the time to adequate control of their LDL-C level.
In the base case scenario, event costs were excluded to focus on the costs that are most relevant to MCOs. However, this conservative assumption did not consider the costly implications of high LDL-C levels and associated CVD hospitalizations and care, which is why a scenario analysis including CV event costs was run. This analysis showed that initiating half of high-risk patients on rosuvastatin may result in a budget impact of $233,845, or 16% of net spending, 4% lower than a budget impact that does not consider event costs. At the PMPM level, the difference in net spending between both strategies was negligible (∼$0.007).
A limitation of this approach includes the simplified calculation of costs (using CHD rates adjusted to overall CVD levels) and the use of data from a cost-effectiveness analysis of statins in the British populationCitation38. It should be noted, however, that event rates in that publication originally stemmed from the Framingham Heart StudyCitation6, which was conducted in the US. Moreover, the model only considers event costs over 3 years; the fact that the majority of CV events and associated costs are usually incurred over a longer time period would suggest that the model under-estimates the overall burden of CV events in relation to treatment and administrative costs.
This analysis has several additional limitations. First, it assumed that statin efficacy is the same in real-life situations as it is in clinical trial settings; however, a study by Frolkis et al.Citation33 demonstrated that such is not the case. We chose to use data from the STELLAR trial because data for a rosuvastatin-specific decrease in efficacy was not available in Frolkis et al., but ran a scenario analysis using a pooled reduction in statin efficacy based on the combined reductions for patients receiving simvastatin, atorvastatin, or pravastatin in a non-clinical settingCitation33. The budget impact in this analysis was of $216,491, or 17% of net spending, suggesting that drug costs may be lower in a real-life, rather than in clinical trial, setting—perhaps due to increased switching and titration rates associated with lower atorvastatin and simvastatin efficacy.
Another limitation of this model is that it assumes patients only titrate to the next highest statin dose or switch to a more efficacious statin. This may not fully reflect real-life titration and switching patterns, where some patients may be titrated up by several doses at once, or switch to less efficacious statins due to tolerability issues or for cost containment purposesCitation42. Similarly, discontinuation has not been factored into the model. However, as these limitations apply to all statins despite evidence of the contrary (e.g., high doses of atorvastatin have been associated with liver injuryCitation43 and subsequent discontinuation), these assumptions do not unduly favor any particular statin. Sensitivity analyses showed that the model was less sensitive to titration and switching decisions than other elements (e.g., rosuvastatin costs, average LDL-C level, and LDL-C goal of high-risk patients) in determining net spending in the revised, compared with the current, treatment pattern.
Lastly, titration and switching rates from market research were reported for the overall population, rather than specifically for patients failing to reach their LDL-C goal. However, in our model, these rates were applied exclusively to patients not reaching goal, as patients who did reach their LDL-C goal were assumed to continue receiving the same statin dose until the end of the model. To our knowledge, such data are not readily available, suggesting that more research into goal attainment–dependent titration and switching patterns may be necessary to obtain a more accurate depiction of current statin therapies.
In 2006, Huse et al.Citation44 published an analysis of the economic and humanistic impact of initiating 4900 and 34,700 high-risk patients (as defined in NCEP ATP III guidelines) on rosuvastatin in a commercial MCO and Medicare plan, respectively. They found that 36 and 727 CV events could be avoided in these health plans, resulting in cost savings of $4.03 million and $34.32 million, respectively. This suggests that our analysis may have under-estimated the decreased CV event costs due to increased rosuvastatin initiation, as we did not find these to offset increased treatment costs, whereas Huse et al. did.
Conclusion
Despite its limitations, our model highlights the potential value of appropriately tailoring statin therapy to the risks and needs of different patients. Indeed, initiating a proportion of high-risk hyperlipidemic patients on rosuvastatin may increase the number of patients reaching their LDL-C goal (as defined in the NCEP guidelines), thereby preventing the incidence of CV events, at a modest increase to an MCO’s PMPM costs.
Transparency
Declaration of funding
Funding for this research was provided by AstraZeneca LP.
Declaration of financial/other relationship
CM and LR have disclosed that they are consultants with Medaxial Ltd., which has received research funding from AstraZeneca LP to conduct this research. SB has disclosed that he is an employee of AstraZeneca LP. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
References
- Hoyert D, Hu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep 2011;61:1-65
- Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. US Department of Health and Human Services: National Heart Lung and Blood Institute, 2012. Bethesda, MD. http://www.nhlbi.nih.gov/resources/docs/2012_ChartBook_508.pdf. Accessed November 19, 2012
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933–44
- Centers for Medicare and Medicaid Services. Health Care Financing Review: Medicare & Medicaid Statistical Supplement. Table 5.5: Discharges, total days of care, and program payments for medicare beneficiaries discharged from short-stay hospitals, by principal diagnoses within Major Diagnostic Classifications (MDCs): Calendar Year 2006. Baltimore, MD: Centers for Medicare and Medicaid Services, 2005. http://www.cms.hhs.gov/MedicareMedic- aidStatSupp/downloads/2007Table5.5b.pdf. Accessed November 12, 2012
- Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986;256:2823-8
- Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:365-74
- Kuklina EV, Shaw KM, Hong Y, et al. Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol — United States, 1999–2002 and 2005—200. MMWR 2011;60:109-14
- Grundy SM. Cholesterol-lowering therapy: evaluation of clinical trial evidence. New York: Marcel Dekker Inc., 2000
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-207
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-57
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995;333:1301-7
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287:3215-22
- Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR Trial). Am J Cardiol 2003;92:152-60
- D’Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J 2000;139:272-81
- Anderson KM, Odell PM, Wilson PW, et al. Cardiovascular disease risk profiles. Am Heart J 1991;121:293-8
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22
- Faergeman O, Hill L, Windler E, et al. Efficacy and tolerability of rosuvastatin and atorvastatin when force-titrated in patients with primary hypercholesterolemia: results from the ECLIPSE study. Cardiology 2008;111:219-28
- National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421
- New patient treatment patterns, dispensing fees and rebate amounts. Data on file. AstraZeneca LP, 2012
- PHC Step Therapy Criteria: Statins. Physicians Health Choice. http://www.phccares.com/pdf/PHC%20Step%20Therapy%20Criteria%20Statins.pdf. Accessed November 19, 2012
- Step therapy program. Medical Health Associates Health Plans, 2012. http://www.mahealthcare.com/step_therapy/StepTherapy_MedAssoc_2011.pdf. Accessed November 28, 2012
- Prior Therapy Timeline Standardization: Oct – Dec 2012. Optima Health, 2012. Available at: http://public.optimahealth.com/Lists/OptimaFormsLibrary/form-doc-timeline-for-step-edit-drugs.pdf. Accessed November 19, 2012
- Ito MK, Lin JC, Morreale AP, et al. Effect of pravastatin-to-simvastatin conversion on low-density-lipoprotein cholesterol. Am J Health Syst Pharm 2001;58:1734-9
- Moisan J, Vaillancourt R, Grégoire JP, et al. Preferred hydroxymethylglutaryl-coenzyme A reductase inhibitors: treatment-modification program and outcomes. Am J Health Syst Pharm 1999;56:1437-41
- Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy 2001;21:1130-9
- Meissner B, Dickson M, Shinogle J, et al. Drug and medical cost effects of a drug formulary change with therapeutic interchange for statin drugs in a ministate managed Medicaid organization. J Manag Care Pharm 2006;12:331-40
- Ara R, Pandor A, Stevens J, et al. Prescribing high-dose lipid-lowering therapy early to avoid subsequent cardiovascular events: is this a cost-effective strategy? Eur J Prev Cardiol 2012;19:473-83
- XE Universal Currency Converter. Mid-market exchange rates. 2013. www.xe.com. Accessed January 8, 2013
- Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA 2009;302:2104-10
- Gandhi SK, Järbrink K, Fox KM, et al. Effectiveness of rosuvastatin in reducing LDL-C and target LDL-C goal attainment in real-world clinical practice. Curr Med Res Opin 2009;25:2817-28
- Willey VJ, Bullano MF, Shoetan NN, et al. Therapy modifications and low-density lipoprotein cholesterol goal attainment rates associated with the initiation of generic simvastatin. Curr Med Res Opin 2010;26:121-8
- Frolkis JP, Pearce GL, Nambi V, et al. Statins do not meet expectations for lowering low-density lipoprotein cholesterol levels when used in clinical practice. Am J Med 2002;113:625-9
- Data on file. AstraZeneca LP, 2013
- Yale Health Pharmacy Benefit for Managerial & Professional and Faculty. New Haven, CT: Yale Health, 2013; http://yalehealth.yale.edu/yale-health-pharmacy-benefit-managerial-professional-and-faculty. Accessed January 3, 2013
- Centers for Medicare and Medicaid Services. Physician Fee Schedule. Baltimore, MD: Centers for Medicare and Medicaid Services, 2012. www.cms.gov. Accessed June 12, 2012
- Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule. Baltimore, MD: Centers for Medicare and Medicaid Services, 2012. www.cms.gov. Accessed June 12, 2012
- Ward S, Lloyd Jones M, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11:1-160, iii-iv
- Charland SL, Cziraky MJ, Quimbo R, et al. Achieving optimal lipid values in patients with dyslipidemia is associated with reduced risk of cardiovascular events. J Clin Lipidol 2008;2:343-53
- Ohsfeldt RL, Gandhi SK, Smolen LJ, et al. Cost effectiveness of rosuvastatin in patients at risk of cardiovascular disease based on findings from the JUPITER trial. J Med Econ 2010;13:428-37
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012;125:e2-e220
- Rublee DA, Burke JP. LDL-C goal attainment in patients who remain on atorvastatin or switch to equivalent or non-equivalent doses of simvastatin: a retrospective matched cohort study in clinical practice. Postgrad Med 2010;122:16-24
- Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol 2012;56:374-80
- Huse DM, Song X, Ozminkowski RJ, et al. Impact of rosuvastatin use on costs and outcomes in patients at high risk for cardiovascular disease in US managed care and Medicare populations: a data analysis. Clin Ther 2006;28:1425-42