Abstract
Purpose:
The Czech Republic is faced with making choices between pharmaceutical products, including depot injectable antipsychotics. A pharmacoeconomic analysis was conducted to determine the cost-effectiveness of atypical depots.
Methods:
An existing 1-year decision-analytic framework was adapted to model drug use in this healthcare system. The average direct costs to the General Insurance Company of the Czech Republic of using paliperidone palmitate (Xeplion®), risperidone (Risperdal Consta®), and olanzapine pamoate (Zypadhera®) were determined. Literature-derived clinical rates populated the model, with costs adjusted to 2012 Euros using the consumer price index. Outcomes included quality-adjusted life-years (QALYs), days in remission, and proportions hospitalized or visiting emergency rooms. One-way sensitivity analyses were calculated for all important inputs. A multivariate probability analysis was used to examine the stability of results using 10,000 iterations of simulated input over reasonable ranges of all included variables.
Results:
Expected average costs/per patient treated were €5377 for PP-LAI, €6118 for RIS-LAI, and €6537 for OLZ-LAI. Respective QALYs were 0.817, 0.809, and 0.811; ER visits were 0.127, 0.134, and 0.141; hospitalizations were 0.252, 0.298, and 0.289. Results were generally robust in sensitivity analyses. PP-LAI dominated RIS-LAI and OLZ-LAI in 90.2% and 92.1% of simulations, respectively. Results were insensitive to drug prices but sensitive to adherence and hospitalization rates.
Conclusions:
PP-LAI dominated the other two drugs, as it had a lower overall cost and superior clinical outcomes, making it the preferred choice. Using PP-LAI in place of RIS-LAI for chronic relapsing schizophrenia would reduce the overall costs of care for the healthcare system.
Introduction
The Czech Republic has an estimated population of just over 10 millionCitation1. The country has adopted a compulsory national health insurance model with essentially universal coverageCitation2,Citation3. The total healthcare budget in 2007 was €8.5 billion, of which 51.2% was for hospital care, 24.0% for ambulatory care, 18.6% for drugs, and 6.2% otherCitation2. The majority of these costs (∼86%) are managed by the General Insurance Company of the Czech RepublicCitation2.
Pharmaceuticals are included as benefits and are managed by the State Institute for Drug Control (SÚKL). When setting allowable prices for new drugs, they use international price comparisons; however, they do not officially require the use of pharmacoeconomic guidelinesCitation2. They do require a wide range of evidence be presented, including expected costs to the healthcare system.
A search of Medline and Embase found a single full peer reviewed pharmacoeconomic analysis addressing schizophrenia in this country, which was published more than a decade agoCitation4. Hosák and BahbouhCitation4 compared costs and outcomes between 67 patients treated with oral risperidone and 67 with traditional antipsychotics with data derived from chart reviews. Clinical outcomes were similar between groups, but hospitalization costs were 40% lower in the risperidone group. The increased acquisition cost of risperidone was not offset by this reduction, which was attributed to the very low labor costs in the healthcare field at that time. Two other pharmacoeconomic analyses from the Czech Republic have appeared in abstract formCitation5,Citation6. Both studies examined risperidone long-acting injectable (RIS-LAI) in a mirror image model and found that it was cost-effective compared to the previous treatment for schizophrenia and schizoaffective disorder.
In all three of these studiesCitation4–6, the main cost driver was hospitalization, which comprised 60–70% of the total costs of care. A major causative factor in rehospitalization among persons with chronic schizophrenia is poor adherence to prescribed drug regimensCitation7,Citation8. This problem has been identified in the Czech Republic, and is more problematic than in persons with somatic disordersCitation9,Citation10. Even partial adherence is associated with large increases in hospitalizations and costsCitation10. Therefore, if adherence can be improved, patients can have better management of their disease and thereby avoid relapses. As well, their quality-of-life (QoL) would be improvedCitation11.
To that end, depot medications have been developed. Because their clinical effect is prolonged, they have the potential to preclude both intentional and unintentional non-adherence by the patientCitation12. In a meta-analysis of data from 1700 patients, Leucht et al.Citation13 found that depot antipsychotics were superior to oral drugs in reducing hospitalizations due to relapse. Some of the older depot drugs required weekly or biweekly injectionsCitation14; however, improved formulations have extended their effect to the point that some of them can be administered monthly. Such is the case with paliperidone palmitateCitation15 (PP-LAI) and olanzapine pamoateCitation16 (OLZ-LAI).
The economics of these newer drugs in the Czech Republic healthcare system is currently unknown. We therefore undertook this research to determine the cost-effectiveness of the newer atypical depot antipsychotics from the viewpoint of the General Insurance Company of the Czech Republic.
Methods
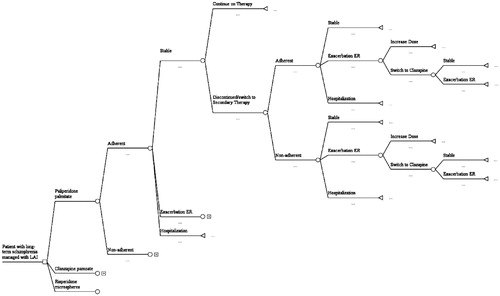
A decision tree model with a 1-year time horizon that was previously developed for used in another country was adapted for the Czech healthcare system. Drugs of interest included paliperidone palmitate (Xeplion®), risperidone (Risperdal Consta®), and olanzapine pamoate (Zypadhera®). depicts the model and clinical events. Patients began with stable disease and were managed as described in Citation15,Citation17–35. Data for that table were extracted from the published literature and represent standard care. Local variations were implemented based on the input from experts. Patients would move along the branches of the decision tree until they arrived at a decision node, which occurred every 3 months. They would either remain stable or deteriorate according to rates found in the literature ()Citation17,Citation23,Citation32,Citation36–39. Their symptoms could relapse, prompting a visit to the emergency room (ER) where they would be treated. Those who could be managed as outpatients would be treated and discharged with follow-up in the community. The others would be admitted to hospital and managed as inpatients. Their course of illness would continue until they reached the 1 year time horizon.
Table 1. Clinical management of schizophrenia and sources of information.
Table 2. Clinical rates used as inputs for calculations in the model.
Patients who had relapsed because they had discontinued their primary drug for reasons other than intolerance or ineffectiveness would be restarted on the same drug using recommended dosing regimens (). Those who stopped due to intolerance or lack of efficacy would be switched to an alternative drug. Those failing PP-LAI or RIS-LAI would be switched to OLZ-LAI, and those who failed OLZ-LAI would be switched to RIS-LAI. After two failures, patients would receive clozapine.
The outcomes incorporated into the analyses were those related to patient care such as number of days with stable disease, number of relapses requiring treatment at the ER, number of hospitalizations, and quality-adjusted life years. The utility weights were those previously calculated for these patients, based on data from the literature (0.890 for stable disease)Citation40–44, 0.659 for a relapse treated in the ERCitation40–42, and 0.490 for hospitalizationCitation43,Citation44.
Since the perspective was that of the General Insurance Company, the analysis incorporated all direct costs of care such as drug costs, medical care, and hospital care. Unit prices for cost inputs are listed in Citation45–48. Each year, the company issues a list of drugs and services that will be reimbursed for the following year. If generic drugs are available and are considered acceptable, they are put on the list and only that price is allowed. Otherwise, the brand name drug is used. Such is the case in this analysis; olanzapine and risperidone tablets are both available as generics and those prices have been used. We used a simple average cost per mg across all available strengths of each drug. Indirect costs were not considered, nor were costs accrued by other agencies such as the justice system, police, or welfare.
Table 3. Units costs for resources.
The primary pharmacoeconomic outcome was the incremental cost-effectiveness ratio based on the additional cost that would be required to pay for one additional quality-adjusted life year (QALY). Model robustness was tested using a series of one-way sensitivity analyses on all major cost and clinical inputs. As well, a multivariate probability-based analysis was conducted for all model inputs. Clinical values and costs were varied over plausible ranges using 10,000 iterations in a Monte Carlo synthesis.
Results
presents the results from the pharmacoeconomic analyses. PP-LAI had the lowest overall expected cost per patient treated. At the same time, it was associated with the greatest number of QALYs and days with stable disease as well as the lowest numbers of ER visits and hospitalizations for relapses. Thus, PP-LAI dominated the other alternatives in the base case analysis.
Table 4. Pharmacoeconomic results.
In the one-way sensitivity analyses, PP-LAI was insensitive to alterations in acquisition costs, since it would require a 37% increase in the price of PP-LAI to lose its dominance over OLZ-LAI and 24% for RIS-LAI. On the other hand, dominance would be lost if the price of OLZ-LAI were to decrease by 41% or RIS-LAI by 21%. In addition, modest changes in adherence rates or hospitalization rates could result in a loss of dominance.
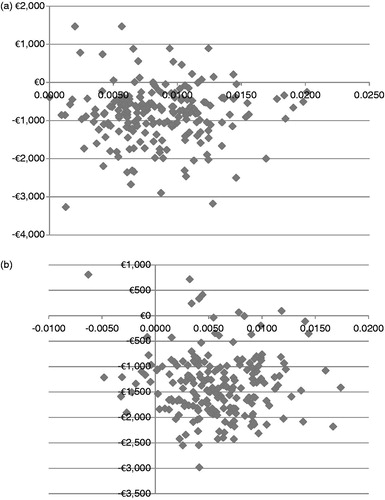
In the 10,000 iteration probability sensitivity analyses, PP-LAI dominated OLZ-LAI in 92.1% and RIS-LAI in 90.2%, and was itself dominated 1.3% of the time. illustrate those findings. Using the cost-effectiveness threshold of ∼€30,000 suggested by NICECitation49 (∼750,000 Kč), PP-LAI was cost-effective in 98.8% of simulations when compared with RIS-LAI and in 93.8% when compared with OLZ-LAI. Thus, we conclude that the results were quite stable over usual variations that would be encountered in the majority of situations.
Discussion
Since PP-LAI is the drug with the lowest cost per patient treated and the highest number of QALYs, it dominates the other two atypical antipsychotic depots. Sensitivity analyses generally confirmed the robustness of those results. Unless dramatic changes were made, the likelihood that PP-LAI would not be the cost-effective alternative is low. Therefore, it should be considered the drug of choice in many situations. The lower costs appear to be associated with the higher efficacy of PP-LAI, which decreases hospitalizations and ER visits. Similar findings in the Czech Republic have been reported previously. In a review of the use of clozapine, Zvolský and HulínskýCitation50 concluded that the drug’s efficacy reduced the number of hospital re-admissions and length of stay, which offset the added costs of monitoring.
QoL is the standard outcome measured in pharmacoeconomic evaluations as it captures many aspects of the patient’s being. Sidlova et al.Citation51 found that patients with chronic schizophrenia have much lower quality-of-life than do persons not suffering from the disease. Therefore, it is an especially important outcome to measure in these people and drugs that can improve QoL are welcome additions as therapeutic choices.
Among the limitations in this study, not all costs were considered in the calculations. They did not include the costs of adverse events, despite the fact that they can be quite common with antipsychotic drugs. A previous analysis in the Czech Republic reported that adverse events accounted for only ∼1.2% of the total cost of careCitation52. Other government agencies have made similar statementsCitation53,Citation54. Therefore, it was assumed that results would not be affected to any great extent due to their omission.
In addition, indirect costs, such as loss of work days, were not included in the analysis. One reason is that few of these people regularly participate in the work force. A multi-country study in Europe indicated that <10% from a sample of almost 4000 persons with chronic schizophrenia worked full-time and ∼12% worked part-timeCitation55. Therefore, the overall cost burden may have been a little under-estimated; however, the difference between drugs would have been minimal. Similarly, violence does occur with some patientsCitation56, which would also add to the under-estimation, but not bias.
It must be kept in mind that a model such as this represents the average patient from the defined population treated with average doses for an average length of time. There will be much variation in real life situations, as has been described in the literature. There has especially been so with Risperdal Consta®, which has few blinded trialsCitation57. However, the literature must be reviewed with caution, as there are issues of different forms of bias such as selection bias, channeling bias, and sponsorship bias, among others.
Conclusions
Among the atypical depot antipsychtoics examined in this analysis, paliperidone palmitate (Xeplion®) emerged as the drug having the best pharmacoeconomic profile. It had the lowest cost per patient treated, which would result in cost savings for the system. At the same time, it had the best clinical outcomes, which would result in better patient care and improved quality-of-life. If feasible, paliperidone palmitate should possibly be considered the first choice when selecting from among these alternatives for persons with chronic relapsing schizophrenia in the Czech Republic.
Transparency
Declaration of funding
This study was funded by Janssen Cilag.
Declaration of financial/other interest
TTE and RZ have disclosed they are consultants to Janssen Cilag and received funding from Janssen to help conduct this study and prepare it for publication. JS has no relevent financial relationships to disclose. SV, MG and MEHH have disclosed that they are employees of Janssen Cilag. JME Peer reviewers on this manuscript have no relevant financial relationships to disclose.
References
- Index Mundi. Czech Republic demographics profile 2012. http://www.indexmundi.com/czech_republic/demographics_profile.html. Accessed February 13, 2013
- Bryndová L, Pavloková K, Roubal T, et al. Czech Republic: health system review. Health Systems in Transition 2009;11:1-122. www.euro.who.int_data/assets/pdf_file/0003/75144/E86823.pdf. Accessed November 21, 2012
- Kinkorová J, Topolčan O. Overview of healthcare system in the Czech Republic. EPMA J 2012;3:1-8
- Hosák L, Bahbouh R. Costs and outcomes of risperidone treatment in schizophrenia in the Czech Republic. Eur Psychiatry 2002;17:213-21
- Skoupá J, Cerna V, Dolezal T. Cost-effectiveness-analysis of risperidone long-acting injection in schizophrenia: 12 month data from Czech Republic. Presented at the 11th Annual ISPOR European Congress, Athens, Greece, November 9-11, 2008
- Skoupá J. Cost-effectiveness analysis of risperidone ling-acting injection in schizophrenia: 24-month data from Czech Republic. Value Health 2010;13:A451-2
- Thieda P, Beard S, Richter A, et al. An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatr Serv 2003;54:508-16
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004;55:886-91
- Cesková E. [Compliance in psychiatry]. Cas Lek Cesk 2009;148:489-92
- Svestka J, Bitter I. Nonadherence to antipsychotic treatment in patients with schizophrenic disorders. Neuro Endocrinol Lett 2007;28(1 Suppl):95-116
- Mohr P. Quality of life in the long-term treatment and the role of second-generation antipsychotics. Neuro Endocrinol Lett 2007;28(1 Suppl):117-33
- Nasrallah H. The case for long-acting antipsychotic agents in the post-CATIE era. Acta Psychiatr Scand 2007;115:260-7
- Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia–a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res 2011;127:83-92
- Adams CE, Fenton MK, Quraishi S, et al. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br J Psychiatry 2001;179:290-9
- European Medicines Agency. Xeplion® summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002105/WC500103317.pdf. Accessed November 21, 2012
- European Medicines Agency. Zypadhera® summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000890/WC500054429.pdf. Accessed November 21, 2012
- Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schiz Res 2010;116:107-17
- Gopal S, Hough D, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol 2010;25:247-56
- Pandina GJ, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:218-26
- Hough D, Lindenmayer J-P, Gopal S, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:1022-31
- Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 2010;35:2072-82
- Pandina GJ, Lindenmayer J-P, Lull JM, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychiatry 2010;30:235-44
- Gopal S, Vijapurkar U, Lim P, et al. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol 2010;25:685-97
- Fleischhacker WW, Gopal S, Lane L, et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol 2011:1-12 . [Epub ahead of print]
- European Medicines Agency. Risperdal Consta® summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Risperdal_Consta_30/WC500008170.pdf. Accessed November 21, 2012
- Kissling W, Heres S, Lloyd K, et al. Direct transition to long-acting risperidone - analysis of long-term efficacy. J Psychopharmacol 2005;19:15-21
- Lee M, Ko Y, Lee S, et al. Long-term treatment with long-acting risperidone in Korean patients with schizophrenia. Hum Psychopharmacol 2006;21:399-407
- Lindenmayer J-P, Khan A, Eerdekens M, et al. Long-term safety and tolerability of long-acting injectable risperidone in patients with schizophrenia or schizoaffective disorder. Eur Neuropsychopharmacol 2007;17:138-44
- Olivares JM, Rodrigues-Morales A, Diels J, et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry 2009;24:287-96
- Möller H-J, Llorca P-M, Sacchettii E, et al. Efficacy and safety of direct transition to risperidone long-acting injectable in patients treated with various antipsychotic therapies. Int Clin Psychopharmacol 2005;20:121-30
- Lauriello J, Lambert T, Andersen S, et al. An 8-week, double-blind, randomized, placebo controlled study of olanzapine long-acting injection in acutely ill patients with schizophrenia. J Clin Psychiatry 2008;69:790-9
- Kane J, Detke H, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry 2010;167:181-9
- Ascher-Svanum H, Peng X, Montgomery W, et al. Assessing the infrequent oral supplementation of olanzapine long-acting injection in the treatment of schizophrenia. Eur Psychiatry 2011;26:313-9
- Chakos M, Lieberman J, Hoffman E, et al. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry 2001;158:518-26
- European Medicines Agency Committee for Proprietary Medicinal Products. Summary information on referral opinion following arbitration pursuant to Article 30 of Council Directive 2001/83/EC for Leponex and associated names. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Leponex_30/WC500010966.pdf. Accessed November 21, 2012
- Ascher-Svanum H, Faries D, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry 2006;67:453-60
- Nicholl D, Akhras K, Diels J, et al. Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Curr Med Res Opin 2010;26:943-55
- Olivares J, Peuskens J, Pecanek J, et al. Clinical and resource-use outcomes of risperidone long-acting injection in recent and long-term diagnosed schizophrenia patients: results from a multinational electronic registry. Curr Med Res Opin 2009;25:2197-206
- Mehnert A, Diels J. Impact of administration interval on treatment retention with long-acting antipsychotics in schizophrenia. Presented at the Tenth Workshop on Costs and Assessment in Psychiatry -Mental Health Policy and Economics, Venice, Italy, 25-27 March 2011
- Briggs A, Wild D, Lees M, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes 2008;6:105
- Cummins C, Stevens A, Kisely S. The use of olanzapine as a first and second choice treatment in schizophrenia. A West Midlands Development and Evaluation Committee Report. Birmingham, UK: Department of Public Health & Epidemiology, University of Birmingham, 1998
- Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schiz Res 2004;71:155-75
- Oh PI, Lanctôt KL, Mittmann N, et al. Cost-utility of risperidone compared with standard conventional antipsychotics in chronic schizophrenia. J Med Econ 2001;4:137-56
- Revicki DA, Shakespeare A, Kind P. Preferences for schizophrenia-related health states: a comparison of patients, caregivers and psychiatrists. Int Clin Psychopharmacol 1996;11:101-8
- State Institute for Drug Control (SÚKL). Reimbursement list (11/2012). www.sukl.cz. Accessed November 21, 2012
- Psychiatric care. Series: Health Statistics 2012. Prague: Institute of Health Information and Statistics of the Czech Republic, www.uzis.cz. Accessed February 13, 2013
- Hospitalization by classification DRG. Series: Health Statistics 2012. Prague: Institute of Health Information and Statistics of the Czech Republic, www.uzis.cz. Accessed February 13, 2013
- Act 411/2011: The list of reimbursement for medical procedures 2012. Prague: Institute of Health Information and Statistics of the Czech Republic, www.uzis.cz. Accessed February 13, 2013
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733-44
- Zvolský P, Hulínský J. [Clozapine–an atypical antipsychotic agents, its advantages and risks]. Cesk Psychiatr 1994;90:328-40
- Sidlova M, Prasko J, Jelenova D, et al. The quality of life of patients suffering from schizophrenia–a comparison with healthy controls. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2011;155:173-80
- Hosák L, Bahbouh R. Costs and outcomes of risperidone treatment in schizophrenia in the Czech Republic. Eur Psychiatry 2002;17:213-21
- All Wales Medicines Strategy Group. Final appraisal report: Olanzapine depot (ZypAdhera®). Penarth, Wales: Lilly UK, 2010. Advice No: 1510. http://www.wales.nhs.uk/sites3/Documents/371/olanzapine%20depot%20(ZypAdhera)%20schizophrenia.pdf. Accessed April 4, 2013
- Farahati F, Boucher M, Moulton K, et al. Atypical antipsychotic monotherapy for schizophrenia: clinical review and economic evaluation of first year of treatment [Technology report number 91]. Ottawa: Canadian Agency for Drugs and Technologies in Heath, 2007
- Papageorgiou G, Cañas F, Zink M, et al. Country differences in patient characteristics and treatment in schizophrenia: data from a physician-based survey in Europe. Eur Psychiatry 2011;26(1 Suppl):17-28
- Vevera J, Hubbard A, Veselý A, et al. Violent behaviour in schizophrenia. Retrospective study of four independent samples from Prague, 1949 to 2000. Br J Psychiatry 2005;187:426-30
- Chue P, Chue J. The cost-effectiveness of risperidone long-acting injection in the treatment of schizophrenia. Expert Rev Pharmacoecon Outcomes Res 2012;12:259-69