Abstract
Objective:
The objective of this analysis was to compare costs of paricalcitol or cinacalcet plus low dose vitamin D, and of phosphate binders, in patients in the IMPACT SHPT study; and to extrapolate those to estimate expected annual maintenance costs.
Methods:
IMPACT SHPT was a 28-week, randomized, open-label trial. Subjects from 12 countries received intravenous (IV) or oral paricalcitol, or oral cinacalcet plus fixed IV doxercalciferol or oral alfacalcidol. The primary end-point was the proportion of subjects who achieved a mean intact parathyroid hormone (iPTH) value of 150–300 pg/mL during weeks 21–28 (evaluation period). This study compares the costs of study drugs and phosphate binders among participants during the study and annualized. This analysis includes only those subjects that reached the evaluation period (134 in each group).
Results:
The mean total drug costs over the study period were €2606 (SD = €2000) in the paricalcitol group and €3034 (SD = €3006) in the cinacalcet group (difference €428, p = 0.1712). The estimated annualized costs were €5387 (SD = €4139) in the paricalcitol group and €6870 (SD = €6256) in the cinacalcet group (difference €1492, p = 0.0395). In addition, a significantly greater proportion (p = 0.010) of subjects in the paricalcitol arm (56.0%) achieved an iPTH of 150–300 pg/mL during the evaluation period compared to the cinacalcet arm (38.2%).
Limitations:
This was a secondary analysis of the IMPACT SHPT study which was not designed or powered for costs as an outcome. The dosing of study drugs and phosphate binders in the IMPACT study may not reflect actual practice, and patients were followed for 28 weeks, while the treatment of SHPT is long-term.
Conclusion:
Patients with SHPT requiring hemodialysis who were treated with a paricalcitol-based regimen for iPTH control had lower estimated annual drug costs compared to those treated with cinacalcet plus low-dose vitamin D.
Introduction
Chronic kidney disease (CKD) is a disorder that is increasing in prevalence worldwideCitation1–4. Age, hypertension, diabetes, and obesity are all important risk factors for CKDCitation1,Citation5. A common complication of CKD is secondary hyperparathyroidism (SHPT), which is defined by elevated serum levels of intact parathyroid hormone (iPTH). SHPT causes changes in calcium, phosphorous, and vitamin D metabolism, and these changes can eventually result in complications such as renal osteodystropy, fractures, cardiovascular disease, and even deathCitation6–11. Many individuals with CKD will eventually progress to end-stage renal disease (ESRD) and require renal replacement therapy, such as dialysis or kidney transplantation, making CKD extremely expensive to both patients and societyCitation4.
The National Kidney Foundation (NKF) has published guidelines for the management of SHPT with the goal of preserving calcium, phosphate, and parathyroid hormone (PTH) homeostasis, and correcting inadequate vitamin D receptor activationCitation12,Citation13. The 2003 guidelines of the NKF Kidney Disease Outcomes Quality Initiative (KDOQI) recommended a target of 150–300 pg/ml serum iPTH, while the 2009 guidelines of the NKF Kidney Disease–Improving Global Outcomes (KDIGO) suggest an iPTH goal of 2–9-times the upper limit of normal (which corresponds to a range of 130–600 pg/ml). Both guidelines recommend vitamin D receptor (VDR) activators to control PTH levels. Phosphate binders are also recommended in the treatment of hyperphosphatemia. There are two categories of phosphate binders—calcium-containing and non-calcium-containing. Calcium-containing phosphate binders are often used with cinacalcet to reduce phosphate levels and the likelihood of cinacalcet-induced hypocalcemia; however, calcium load must be monitored to avoid hypercalcemia. The more expensive non-calcium-containing phosphate binders do not affect calcium levels.
Compared to other VDR activators, more evidence exists supporting the association between paricalcitol and improved patient outcomes. For example, paricalcitol use in long-term hemodialysis has been associated with reduced hospitalizations, improved survival in observational data, and reduced incidence of hypercalcemia compared to calcitriol in a randomized clinical trialCitation14–16. Compared to doxercalciferol and calcitriol, paricalcitol has been associated with less elevation of serum phosphorusCitation17,Citation18. Compared to alfacalcidol, paricalcitol has been shown to both decrease PTH levels faster and be more cost-effectiveCitation19,Citation20.
Cinacalcet, a calcimimetic agent, is also effective in reducing PTH and, when used in combination with low dose vitamin D, has the added advantage of minimizing risk of hypercalcemiaCitation21–23. The ACHIEVE study compared flexible-dose vitamin D alone (paricalcitol or doxercalciferol) to a cinacalcet-based therapy in patients with SHPT on dialysis and found no significant difference in the proportion of subjects who simultaneously achieved a mean iPTH between 150–300 pg/ml and a mean Ca X P product value of <55 mg2/dl2 Citation23. However, the study did not directly compare cinacalcet to paricalcitol.
More recently, the Improved Management of iPTH with Paricalcitol-centered Therapy vs Cinacalcet Therapy with Low-dose Vitamin D in Hemodialysis Patients with Secondary Hyperparathyroidism (IMPACT SHPT) study compared optimal dose titration of paricalcitol, with supplemental cinacalcet for possible hypercalcemia only, to the combination of cinacalcet and low-dose vitamin D in controlling iPTH in patients with SHPT requiring dialysis, and found paricalcitol to be superior to cinacalcet in achieving iPTH 150–300 pg/mL during weeks 21–28Citation24. Not reported in the main results of IMPACT SHPT were any differences in costs between patients who received paricalcitol vs cinacalcet.
Understanding the cost differences between paricalcitol and cinacalcet treated patients could provide an additional perspective from which to interpret the clinical results of the IMPACT SHPT study. Therefore, the objective of the current analysis was to compare the costs of paricalcitol or cinacalcet plus low dose vitamin D treatment for SHPT, including phosphate binder costs, in patients enrolled in the IMPACT SHPT study, and to extrapolate those costs to estimate the expected annual maintenance costs of treatment. This study was conducted from a global payer perspective, with all costs expressed in Euros.
Methods
This was an analysis of drug utilization data collected during the IMPACT SHPT study but not reported in the publication of the main resultsCitation24. The methods of the IMPACT SHPT study have been described in detail elsewhereCitation25. In brief, the IMPACT SHPT study was a 28-week, multi-center, randomized, open-label, phase 4 trial (ClinicalTrials.gov identifier: NCT00977080). Subjects were randomly assigned to receive intravenous (IV) or oral paricalcitol, or oral cinacalcet plus fixed IV doxercalciferol or oral alfacalcidol, for 28 weeks using a stratified randomization. The stratification was based on the mode of paricalcitol administration (IV at US and Russian sites, oral at other sites which included Czech Republic, Germany, Denmark, Spain, Great Britain, Greece, Italy, Netherlands, Portugal, and Sweden). The paricalcitol and cinacalcet doses used in the study were adjusted per protocol based on chemistry results as recommended in the drug package insertsCitation25.
The primary end-point of the IMPACT SHPT study was the proportion of subjects in each treatment group per stratum who achieved a mean iPTH value of 150–300 pg/mL during weeks 21–28 (evaluation period). A pre-specified secondary end-point was the proportion of subjects in each treatment group who achieved a mean iPTH value of 150–300 pg/mL during weeks 21–28 (evaluation period) using a Cochran–Mantel–Haenszel (CMH) test to control for stratum.
For this cost analysis we examined the utilization of study drug (IV or oral paricalcitol, or oral cinacalcet plus fixed IV doxercalciferol or oral alfacalcidol) and of all phosphate binders used by participants during the trial. In the IMPACT SHPT study patients randomized to paricalcitol could receive supplemental cinacalcet administered if serum calcium was ≥10.5 mg/dL (2.61 mmol/L) in two consecutive blood tests in the presence of high iPTH. Cinacalcet use in the paricalcitol treatment group was also accounted for in this secondary cost analysis.
We multiplied utilization by unit prices for each drug to get the total cost per patient for the 28 week study. The drug unit prices were country-specific and were obtained from the Midas Quantum database (supplied by IMS Health, Inc., Danbury, CT, USA). In the Midas database, country-specific unit costs are calculated by deriving the weighted average of either local manufacture or trade level (wholesalers, retail pharmacies, or hospitals) per mg prices derived based on average country utilization of all the different dosage forms, strengths, and packaging size for each drug. Note that for phosphate binders, while brand names may differ, the drugs and formulations are generally the same. Taxes, if applied, are not included in the unit price determination. In the Midas data, all country-specific unit prices were provided in Euros (2011) and were converted, if necessary, using the mid-point historical exchange rate from the second quarter of calendar year 2011 (obtained from http://www.oanda.com/currency/historical-rates/). Because the number of subjects in some countries was small, we did not stratify any of the results by country.
Descriptive statistics, including means with standard deviations and percentages, were used to characterize the cost data in each group for both the study drugs and phosphate binders. We examined univariate associations between baseline characteristics and both the treatment group and the outcome variable to determine if a multivariable approach was necessary. By definition a confounder must be associated with both the treatment group and the outcome. While some variables were different between treatment groups (see results), these were not associated with the outcome, and consequently did not require adjustment. Therefore, we used a one-way ANOVA to compare costs per patient in the two groups.
To examine the long-term costs expected among the two groups, we extrapolated maintenance costs per patient over 12 months. The maintenance phase was defined as the evaluation period which was weeks 21–28 of the study. We took the average daily drug utilization for each patient during the maintenance phase and multiplied that by the drug unit costs described above, and then annualized the result using the same linear regression. We made the assumption that dosages at 6–7 months will remain the same for the following 12 months.
In addition to cost per patient, we report the mean costs during the study period in the sub-group of patients who achieved a mean iPTH value of 150–300 pg/mL during weeks 21–28 (evaluation period).
All statistical tests were two-tailed, with p-values <0.05 considered statistically significant. Data were summarized and analyzed using SAS version 11.0 (SAS Institute, Cary, NC).
Results
Of 746 patients screened for the IMPACT SHPT study, 474 (63.5%) did not meet eligibility criteria for reasons that have been described previouslyCitation25. Among the 272 patients randomized, 268 received ≥1 dose of study drug and were included in the study analyses. describes the demographic and baseline characteristics of the patients in each treatment group. The majority of patients in the paricalcitol and cinacalcet treatment groups were males (64.9% and 60.5%, respectively) and the mean age was 63.6 and 62.6 years. Most patients had been on dialysis for 4 years. There were some minor differences in baseline laboratory values and most concomitant medication use was the same across groups. As shown in the table, a few comorbidities also differed between groups. Specifically, diabetes was significantly more prevalent in the paricalcitol group (53.7%) than in the cinacalcet group (34.3%), and this was mainly because of differences in the proportions of those with type II diabetes, 48.5% vs 32.1% in the paricalcitol and cinacalcet groups, respectively. The paricalcitol group also had a higher proportion of patients with angina, 14.2% vs 6.7% in the paricalcitol and cinacalcet groups, respectively; but the mean diastolic blood pressure was higher in the cinacalcet group.
Table 1. Demographic and baseline characteristics and concomitant medications by treatment group.
Control of iPTH
The main results of the IMPACT SHPT study are presented elsewhere but are briefly summarized hereCitation24. As stated previously, the primary efficacy end-point was the percentage of patients achieving the treatment goal of a mean iPTH value between 150–300 pg/mL during weeks 21–28 of therapy per stratum. The Cochran–Mantel–Haenszel analysis of the primary end-point showed that the proportion of subjects treated with paricalcitol who achieved iPTH 150–300 pg/mL during weeks 21–28 was significantly greater (56.0%) than the corresponding proportion in the cinacalcet arm (38.2%; p = 0.010). Ten subjects treated with paricalcitol received cinacalcet, and eight of those reached the evaluation period. The results remained consistent even when those eight subjects were excluded.
Dosing of paricalcitol, cinacalcet, and phosphate binders
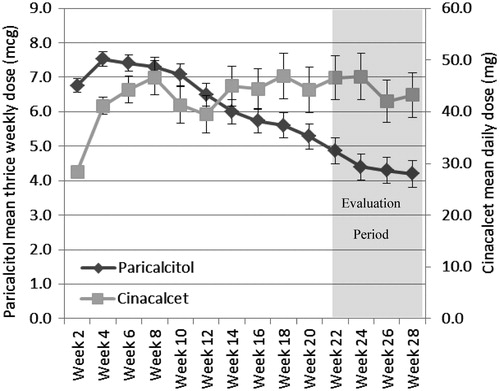
shows the mean doses of paricalcitol and cinacalcet used during the study period in their respective treatment groups. A paricalcitol dose (IV or oral) was administered 3-times a week at each dialysis session and a cinacalcet dose was administered every day. For paricalcitol-treated patients, the mean dosage decreased over the study period. The overall mean dose of paricalcitol for the entire study period was 6.0 (SD = 2.8) mcg per dose (three doses per week), whereas the mean dose during the evaluation period (weeks 21–28) was 4.5 (SD = 3.7) mcg. In contrast, cinacalcet doses increased in the first 2 months of the study period and then stabilized. The mean cinacalcet dose was 44.7 (SD = 27.4) mg per day for the entire study period and 45.9 (SD = 39.9) mg during the evaluation period.
Figure 1. The mean thrice weekly dose of paricalcitol (diamonds and left vertical axis) and mean daily dose of cinacalcet (squares and right vertical axis) for each week of the study period. The mean dose of paricalcitol declined over the study, whereas for cinacalcet it increased. Weeks 21–28 was considered the evaluation period.
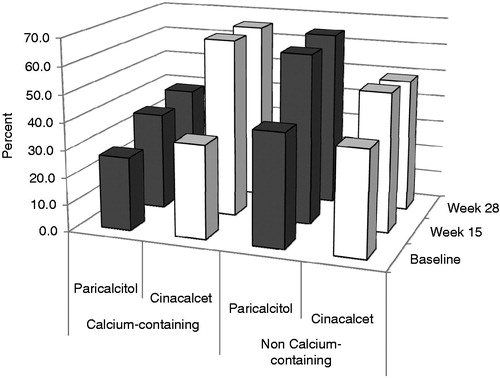
The percentage of study subjects requiring calcium and non-calcium-containing phosphate binders at baseline, week 15, and week 28 is shown in . Among the paricalcitol group a lower percentage received calcium-containing phosphate binders compared to those in the cinacalcet group (35.6% and 38.5% at weeks 15 and 28, respectively, in the paricalcitol group, vs 65.5% at both weeks 15 and 28 in the cinacalcet group, p < 0.01 at both time periods). For non-calcium-containing phosphate binders the opposite was true but there were smaller differences between groups. Specifically, 62.5% of patients at week 15 and 64.4% at week 28 in the paricalcitol group received non-calcium-containing phosphate binders compared to 51.0% and 49.0% in the cinacalcet group (p = 0.12 at week 15 and p = 0.03 at week 28).
Figure 2. The percentage of patients in the paricalcitol and cinacalcet groups who were receiving concomitant calcium and non-calcium phosphate binders at baseline, week 15, and week 28. Only those subjects completing the study are included. Patients were counted if they had used a phosphate binder the day of or 7 days prior to the visit. A greater percentage of patients in the cinacalcet group required calcium-containing phosphate binders, whereas the opposite is true for non calcium-containing phosphate binders.
Drug costs
As shown in , the mean total drug costs per subject over the entire study period was €2606 in the paricalcitol group and €3034 in the cinacalcet group (difference €428 or 16.4%, p = 0.1712). The cost of study drug only was €1242 and €1689 for the paricalcitol and cinacalcet group, respectively, the difference was statistically significant (p = 0.0031), while the cost of phosphate binders was €1364 and €1346 for the paricalcitol and cinacalcet group, respectively, and was not statistically significant (p = 0.9372).
Table 2. Costs by treatment group.
The annualized total maintenance drug costs per subject was €5387 in the paricalcitol group and €6879 in the cinacalcet group (difference €1492, or 27.7%), as shown in . The difference was statistically significant (p = 0.0395). The annualized cost of the study drug was €2110 and €3855 for the paricalcitol and cinacalcet group, respectively (p < 0.0001), while the annualized cost of phosphate binders was €3276 and €3024 for the paricalcitol and cinacalcet group, respectively (p = 0.6444).
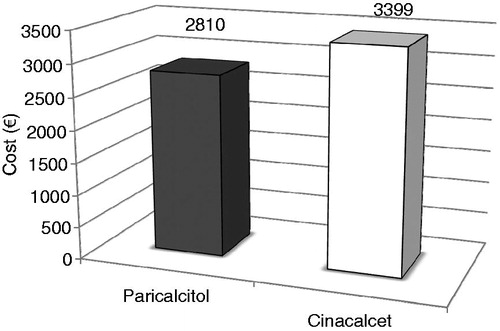
We also examined costs in only those patients who were considered responders (i.e., achieved a mean iPTH value of 150–300 pg/mL during weeks 21–28). There were 61 responders in the paricalcitol group and 39 responders in the cinacalcet group. The mean cost for the study drugs was €1317 in the paricalcitol group and €1797 in the cinacalcet group (p = 0.0285), while the mean cost of phosphate binders was €1494 and €1602, respectively (p = 0.8209). The total drug costs were €2810 in the paricalcitol group and €3399 in the cinacalcet group (p = 0.3123), and are shown graphically in .
Discussion
The main findings of the IMPACT SHPT study were that paricalcitol-based therapy used in patients with SHPT requiring hemodialysis resulted in a greater portion of patients reaching the iPTH goal (based on KDOQI guidelines) compared with the combination of cinacalcet and low-dose vitamin DCitation24. The clinical improvement observed in the IMPACT SHPT study occurred despite a mean dosage of paricalcitol that declined over the study period (), a pattern that has been observed in other studiesCitation24,Citation26,Citation27. However, our annualized cost estimates are based on the assumption that dosing of SHPT drugs during the evaluation period were stabilized. If this is not the case, and paricalcitol doses continue to decrease, then the annualized study drug costs in the paricalcitol group may be even lower than we estimated in our results. Still, our conclusion, that the paricalcitol-based regimen is less expensive than the cinacalcet-based regimen, would not change.
The mean dosages of paricalcitol and cinacalcet observed in their respective treatment groups in the IMPACT SHPT study were important to the cost differences between the two therapies. The mean dose of paricalcitol over the entire study period was 6.0 (SD = 2.8) mcg trice weekly (18.0 mcg/week), while that of cinacalcet was 44.7 (SD = 27.4) mg per day. While it is not possible to make a direct dose comparison, the mean dose in the cinacalcet group of the 27-week ACHIEVE study was cinacalcet 49.3 mg/day plus 10.5 mcg/week of the selective or non-selective vitamin D receptors activators (doxercalciferol or paricalcitol). In that study a 1 mcg dose of doxercalciferol was considered to be equal to 2 mcg of paricalcitolCitation28. In two large clinical trials cinacalcet doses ranged from 30–180 mg dailyCitation21,Citation29.
Our analysis found that mean study drug cost was significantly higher in those who received cinacalcet compared to paricalcitol (in both the study period and annualized), while the difference in phosphate binder costs was not significant. This clearly had an impact on total cost which was numerically higher both in the study period and when annualized (but the difference was only statistically significant in the later). Only one previous study has examined costs in paricalcitol and cinacalcet-treated patients directly, and that focused on costs related to parathyroidectomy—which occurred less frequently in those treated with paricalcitolCitation30. However, our results are consistent with a post-hoc economic analysis of the ACHIEVE study, which found 48% higher average medication costs per patient for those treated with cinacalcet plus low dose vitamin D compared to those receiving either paricalcitol or doxercalciferol ($5501 vs $3709 in each group, respectively)Citation28. Statistical analyses of the difference between groups were not reported.
It is notable the phosphate binder costs were essentially equal in the two groups in our analysis. In subjects completing week 28, use of calcium-containing phosphate binders was more common in subjects receiving cinacalcet than patients receiving paricalcitol, whereas the opposite was true for non-calcium containing phosphate binders. However, the overall cost of phosphate binders was not different between the two groups.
This study should be interpreted with an understanding of its limitations. The limitations of the IMPACT SHPT study have been described elsewhereCitation24,Citation25. Here we focus primarily on limitations that may have influenced the cost analysis. IMPACT SHPT was a multinational study with a stratified design to allow for differences in the approved dosage form (IV or oral) of paricalcitol in different countries. Again, both because of small samples sizes in some countries and because of unequal distribution of patients across IV and oral stratum by country, we did not report costs at the county-level in this analysis. Nevertheless, such information might be useful to policy-makers within selected countries. Further, the cost analysis conducted here was a secondary analysis of the IMPACT SHPT study which was not designed or powered for this outcome. Nevertheless, we did find statistically significant differences between groups.
Our results may have been impacted by the IMPACT SHPT study protocol. First, despite its randomized design, there were some differences in a few baseline comorbidities between treatment groups in the impact study. We carefully examined these differences and found that none of them were confounders in the association with costs and, therefore, did not require adjustment. Second, the dosing of study drugs and phosphate binders in the IMPACT SHPT study was based on a protocol and, thus, may not reflect actual practice. Last, the IMPACT SHPT study followed patients for 28 weeks, while in actual practice the treatment of SHPT in hemodialysis is a long-term endeavor. In addition to providing cost estimates for the study period we extrapolate these data to 1 year and in doing so make the assumption that treatments do not change once stabilized. Yet, as noted above, data from both the IMPACT SHPT study and other previous studies suggests that, while the cinacalcet dose may stabilize by week 28, paricalcitol dosing requirements may continue to decreaseCitation24,Citation26,Citation27. Again, the implications of this are that we may have over-estimated the annualized study drug costs in the paricalcitol group relative to cinacalcet. However, this potential bias would not change our conclusion that the paricalcitol-based regimen is less expensive.
Our cost analysis was restricted to study drugs and phosphate binders reported in the IMPACT SHPT study. Other costs, such as those associated with laboratory tests, hospitalization, physician visits, epoetin, or treatment of adverse events, would be important to compare but were not collected in the IMPACT SHPT study and, therefore, not available for inclusion in this secondary analysis. Some of these costs have been assessed in other studies but not for comparisons of paricalcitol and cinacalcetCitation15. In this analysis we wanted to avoid assumptions about the impact of changes in iPTH, serum calcium, or serum phosphate on resource use. Nevertheless, because paricalcitol was more effective than cinacalcet in the IMPACT SHPT study, it is likely that these costs were also lower. Furthermore, it is worthwhile noting that, while serious and severe adverse events were not significantly different between treatment groups in the IMPACT SHPT study, in the IV stratum, adverse events leading to treatment discontinuation occurred significantly less commonly in the paricalcitol compared to the cinacalcet group.
Last, actual costs were not collected in the study. Instead, we estimated costs of the study drugs and phosphate binders by applying country-level unit costs derived from an external database to the average utilization for each patient over the study period. This approach may have resulted in a slightly different estimate than would have been obtained with actual costs. However, it is not likely that such a difference would change either the direction of or magnitude of the estimates we derived.
Conclusion
Patients with SHPT requiring hemodialysis who were treated with a paricalcitol-based regimen for iPTH control had lower estimated annual drug costs compared to those treated with cinacalcet plus low-dose vitamin D.
Transparency
Declaration of funding
This and the IMPACT SHPT study were funded by AbbVie Inc.
Declaration of financial/other relationships
TM and SK are employees of Abbvie Inc. and owners of AbbVie, Inc. stock. AS has received support in the form of grant/research, consultant/advisor, or speakers bureau from AbbVie, Amgen, AMAG Pharmaceuticals, Affymax and Keryx Biopharmaceuticals. MK has received support in the form of grant/research, consultant/advisor, or speakers bureau from AbbVie, Amgen, Fresenius Medical Care, Medice, Misubishi, Sanofi, Shire, and Vifor. GS has received sponsorship from AbbVie and consulted for Merck. AbbVie, Inc. is the manufacturer of Zemplar® (paricalcitol) and Amgen is the manufacturer of Sensipar® (cinacalet). AbbVie contributed to the study design, research, and interpretation of data, writing, reviewing, and approving the publication.
CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Acknowledgments
The authors wish to acknowledge Beverly Johns and Naijun Chen, employees of AbbVie, Inc. for assistance with statistical analyses. Glen Schumock, who is an author, is a partner in Second City Outcomes Research LLC which received payment from AbbVie Inc. to assist with manuscript preparation.
References
- CDC. National Chronic Kidney Disease Fact Sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2010
- Zhang Q-L, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 2008;8:117
- Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med 2006;354:997-9
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038-47
- Haroun MK, Jaar BG, Hoffman SC, et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 2003;14:2934-41
- Rix M, Andreassen H, Eskildsen P, et al. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int 1999;56:1084-93
- Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 2000;36:1115-21
- Tsuchihashi K, Takizawa H, Torii T, et al. Hypoparathyroidism potentiates cardiovascular complications through disturbed calcium metabolism: possible risk of vitamin D(3) analog administration in dialysis patients with end-stage renal disease. Nephron 2000;84:13-20
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305
- Weiner DE, Tighiouart H, Stark PC, et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis 2004;44:198-206
- Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 2005;16:1788-93
- National Kidney Foundation. KDOQI Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S1-S202
- National Kidney Foundation. KDIGO Clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD). Chapter 4.1: treatment of CKD-MBD targeted at lowering high serum phosphorus and maintaining serum calcium. Kidney Int 2009;76:S50-S99
- Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 2003;349:446-56
- Dobrez DG, Mathes A, Amdahl M, et al. Paricalcitol-treated patients experience improved hospitalization outcomes compared with calcitriol-treated patients in real-world clinical settings. Nephrol Dial Transplant 2004;19:1174-81
- Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 2003;63:1483-90
- Coyne DW, Grieff M, Ahya SN, et al. Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. Am J Kidney Dis 2002;40:1283-8
- Joist HE, Ahya SN, Giles K, et al. Differential effects of very high doses of doxercalciferol and paricalcitol on serum phosphorus in hemodialysis patients. Clin Nephrol 2006;65:335-41
- Rosery H, Bergemann R, Marx SE, et al. Health-economic comparison of paricalcitol, calcitriol and alfacalcidol for the treatment of secondary hyperparathyroidism during haemodialysis. Clin Drug Investig 2006;26:629-38
- Hansen D, Rasmussen K, Danielsen H, et al. No difference between alfacalcidol and paricalcitol in the treatment of secondary hyperparathyroidism in hemodialysis patients: a randomized crossover trial. Kidney Int 2011;80:841-50
- Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004;350:1516-25
- Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 2010;78:578-89
- Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008;3:1718-25
- Ketteler M, Martin KJ, Wolf M, et al. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study. Nephrol Dial Transplant 2012;27:3270-8
- Ketteler M, Martin KJ, Cozzolino M, et al. Paricalcitol versus cinacalcet plus low-dose vitamin D for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: study design and baseline characteristics of the IMPACT SHPT study. Nephrol Dial Transplant 2012;27:1942-9
- Ross EA, Tian J, Abboud H, et al. Oral paricalitol for treatment of secondary hyperparathyroidism in patients on hemodialysis or peritoneal dialysis. Am J Nephrol 2008;28:97-106
- Llach F, Yudd M. Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis 2001;38(5 Suppl):S45-50
- Shireman TI, Almehmi A, Wetmore JB, et al. Economic analysis of cinacalcet in combination with low-dose vitamin D versus flexible-dose vitamin D in treating secondary hyperparathyroidism in hemodialysis patients. Am J Kidney Dis 2010;56:1108-16
- Sprague SM, Evenepoel P, Curzi M, et al. Simultaneous control of PTH and CaxP is sustained over three years of treatment with cinacalcet HCl. Clin J Am Soc Nephro 2009;4:1465-76
- Schumock GT, Walton SM, Lee TA, et al. Comparative effectiveness of paricalcitol versus cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. Nephron Clin Pract 2011;117:c151-9