Abstract
Objective:
In Finland, regional rates of schizophrenia exceed those in most countries, impacting the healthcare burden. This study determined the cost-effectiveness of long-acting antipsychotic (LAI) drugs paliperidone palmitate (PP-LAI), olanzapine pamoate (OLZ-LAI), and risperidone (RIS-LAI) for chronic schizophrenia.
Method:
This study adapted a decision tree analysis from Norway for the Finnish National Health Service. Country-specific data were sought from the literature and public documents, guided by clinical experts. Costs of health services and products were retrieved from literature sources and current price lists. This simulation study estimated average 1-year costs for treating patients with each LAI, average remission days, rates of hospitalization and emergency room visits and quality-adjusted life-years (QALY).
Results:
PP-LAI was dominant. Its estimated annual average cost was €10,380/patient and was associated with 0.817 QALY; OLZ-LAI cost €12,145 with 0.810 QALY; RIS-LAI cost €12,074 with 0.809 QALY. PP-LAI had the lowest rates of hospitalization, emergency room visits, and relapse days. This analysis was robust against most variations in input values except adherence rates. PP-LAI was dominant over OLZ-LAI and RIS-LAI in 77.8% and 85.9% of simulations, respectively. Limitations include the 1-year time horizon (as opposed to lifetime costs), omission of the costs of adverse events, and the assumption of universal accessibility.
Conclusion:
In Finland, PP-LAI dominated the other LAIs as it was associated with a lower cost and better clinical outcomes.
Introduction
Schizophrenia is a life-long disease which exerts a huge impact on the afflicted individuals, their families, and caregiversCitation1. It affects people in every country, creating a worldwide burden that ranks in the top 10 of the World Health OrganizationCitation2. The burden is extensive in terms of both costs and resource utilization, which must be managed appropriately to optimize healthcareCitation3,Citation4.
Finland is no exception; the lifetime prevalence of schizophrenia in this country is 0.87%Citation5, and the incidence of new cases is 0.56 per 10,000 or 272 new cases each yearCitation6. Studies have determined that some areas of the country have rates of schizophrenia that are very highCitation7. The highest was in the cohort born in 1940–1944, which had a rate of 3.67%Citation7. However, despite Finland’s comprehensive healthcare systemCitation8, it has recently been criticized in an OECD report which stated that ‘that those suffering mental illness get insufficient treatment at a high cost for the rest of society’Citation9. As well, the report stated that ‘Schizophrenia was … Finland’s most expensive disease, incurring costs that vary considerably from one region to another’.
Therefore, new cost-effective approaches would be welcome to mitigate such problems. The introduction of long-acting antipsychotics (LAIs) was intended to do precisely that; namely, to improve symptom control and reduce overall costs of care, thereby improving the patient’s quality-of-lifeCitation10,Citation11. These depot forms were introduced in the 1960s and have been used extensively ever since. They have been especially useful for managing patients with chronic schizophrenia who have difficulty in adhering to their medications and appointments, whether intentional or unintentional.
The addition of atypical antipsychotics such as risperidone and olanzapine was another improvement, in that these drugs have enhanced effects against the negative symptoms of schizophreniaCitation12. A decade ago, the first atypical depot antipsychotic, risperidone (RIS-LAI; Risperdal Consta®) was introducedCitation13. Injections are administered biweekly, which serves to decrease patient non-adherence, at least in part. Further research resulted in the development of depots that could be administered every 4 weeks, which decreased administration costs and further enhanced adherenceCitation14. These more recent additions have included olanzapine pamoate (Zypadhera®)Citation15,Citation16 and paliperidone palmitate (Xeplion®)Citation17,Citation18. The three available LAIs may differ somewhat with respect to their adverse event profiles which could potentially affect clinical effectiveness and associated costs. A notable example is that 1.4% of patients receiving OLZ-LAI suffer from post-injection delirium/sedation syndrome which requires close monitoringCitation19,Citation20, whereas RIS-LAI and PP-LAI have not been associated with that drug reactionCitation21.
Aim of the study
The cost-effectiveness of these three atypical depots in Finland is currently unknown; therefore, we undertook the present economic analysis.
Methods
A similar research question was examined in NorwayCitation22. We adjusted that model for use in Finland, with the assistance of local clinical experts. In the simulation model, we inserted country-specific data whenever possible. Some of the changes made include local costs for all resources, frequency of visits to psychiatrists, primary care physicians, day care, etc., duration of stay in hospital, day hospital, long-term care, etc. Patients examined in the Finland and Norway models were the same; they all had stable chronic schizophrenia and were receiving LAIs because of frequent problems adhering to their drug regimens. In this analysis, we compared PP-LAI, RIS-LAI, and OLZ-LAI (see ).
Figure 1. Pharmacoeconomic decision tree model comparing PP-LAI (paliperidone palmitate; Xeplion®), OLZ-LAI (olanzapine pamoate; Zypadhera®), and RIS-LAI (risperidone; Risperdal Consta®) for chronic schizophrenia in Finland.
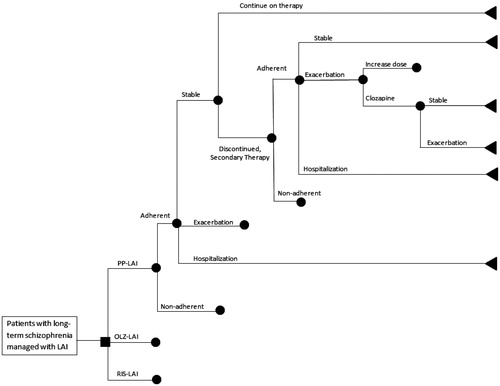
The decision tree was populated with rates derived from clinical trials and observational studies of these drugs in clinical practice. We previously observed that standard recommended doses often varied from actual doses used and we wished to reflect the costs in actual useCitation18. The literature-based doses used to treat patients with each drug under each treatment scenario are presented in Citation13,Citation16,Citation18,Citation23–39 and the probabilities used in the decision tree arms are listed in Citation14,Citation24,Citation31,Citation35,Citation36,Citation40–43. The doses and rates are weighted averages; thus, they represent how the average patient is treated, which is a hallmark of economic analyses. Patients begin with one treatment and progress through the model based on the probabilities in . In the event of failure to the primary drug or inability to tolerate it, patients would be switched to another atypical LAI. Those receiving either PP-LAI or RIS-LAI would be switched to OLZ-LAI and those on OLZ-LAI would receive PP-LAI. In the event of failure with two LAIs, patients are switched to clozapineCitation44,Citation45.
Table 1. Doses used to treat patients (source of data).
Table 2. Probability rates used in the model and sources of information.
The analysis was conducted for a 1-year period from the point of view of the Finnish National Health Service. It was carried out pursuant to the Finnish government’s submission guidelines for pharmacoeconomic analysesCitation46. Costs were extracted from available price lists or published articles and were input in 2011 euros. Prices from other years were extrapolated to 2011 currency using the consumer price index for FinlandCitation47. Costs used in the calculations appear in Citation48–50.
Table 3. Costs (in 2011 euros) used in the model.
The model produced a set of clinical outcomes (days with stable disease, quality-adjusted life-years [QALYs], rates of hospitalization and emergency room visits) and economic outcomes (i.e., average cost per patient treated) for each drug. To estimate QALYs, we used literature derived utility weights that were based on either standard gamble or time trade-off methods. A simple average weight was used for each of the three model states. Stable disease had a utility of 0.890Citation51–55, relapse was 0.659Citation51–53, and hospitalization was 0.490Citation54,Citation55.
Costs and outcomes were then compared in an incremental analysis. One-way sensitivity analyses were applied to drug costs, adherence rates, hospitalization rates, and rates of stable disease. All inputs were then varied across plausible ranges in a probabilistic sensitivity analysis using 10,000 iterations. For rates, we used their 95% confidence intervals and a normal distribution, for costs we used gamma distributions and 10% ranges. In that manner, the sensitivity of input estimates could be tested to determine the robustness of the results from the model.
The final step was to estimate the approximate impact to the Finnish National Health Service over the following 3 years if they were to adopt PP-LAI to replace existing LAIs. We first obtained sales data from IMS summarizing the number of units of LAIs sold in Finland as well as total daily defined doses (DDDs)Citation56 and expenditures in euros. To determine cost impact, we considered only PP-LAI as the drug entering the market which was already composed on RIS-LAI and the traditional depots. In the calculations, we did not consider OLZ-LAI, which has also been recently introduced and would likely be competing for the same patients. We then calculated the cost/DDD for PP-LAI, RIS-LAI, and traditional LAIs as a group. Then the difference was found between PP-LAI and the other LAIs. Those differences were then multiplied by 365 to obtain the total difference per patient over 1 year. It was assumed that patients would be fully adherent (i.e., the best case scenario), receiving 365 DDDs per year. The numbers of patients receiving LAIs was then estimated using literature-based values applied to the Finnish populationCitation57. Data were summed and multiplied by plausible rates of market penetration of 2% in the first year, followed by 13% in the second and 20% in the third year.
Results
The primary results of the pharmacoeconomic analysis appear in . In the initial scenario (i.e., base case), PP-LAI dominated the other two alternatives, as it was associated with a lower overall cost to treat each patient and a higher quality-of-life. The remaining clinical outcomes are also presented in . All results favoured PP-LAI.
Table 4. Results of the economic analysis.
The major cost was hospitalization (including all forms of institutional care), which was responsible for ∼48% of the total cost for PP-LAI, 46% for OLZ-LAI, and 52% for RIS-LAI. Drug costs were next highest, with 35% for PP-LAI, 32% for OLZ-LAI and 29% for RIS-LAI. In each case, the primary drugs made up the majority of those expenses (i.e., 79% for PP-LAI, 86% for OLZ-LAI and 82% for RIS-LAI), with secondary treatments adding very little. Medical care, including outpatient care provided by physicians and other healthcare professionals, was responsible for the lowest amount.
In one-way sensitivity analyses, results were robust against changes to most input variables, including drug price, rates of stable disease and hospitalization. PP-LAI would maintain its dominance over OLZ-LAI up to a price of €6.95/mg (i.e., an increase of 61%) and over RIS-LAI up to €6.84/mg (a 59% increase). If we convert the NICE threshold of £20,000/QALYCitation58, it would be equivalent to ∼€23,000Citation59. Using that value, PP-LAI would be cost-effective compared to OLZ-LAI up to a cost of €7.16/mg (66% increase) and €7.10/mg (65% increase) compared with RIS-LAI. Similarly, OLZ-LAI and RIS-LAI prices would have to decrease to €0.27/mg (70% lower) and €2.37/mg (59% lower), respectively, to reach the threshold for dominance, and PP-LAI would remain cost-effective at prices as low as €0.22/mg (76% lower) and €2.16/mg (51% lower), respectively. However, they were sensitive to very small changes in adherence rates. PP-LAI no longer dominates OLZ-LAI when its adherence rate decreased by 11% or if that of OLZ-LAI increased by 15.5%. Similarly, when the adherence rate of PP-LAI decreases by 32% or that of RIS-LAI increases by 17.8%, it no longer dominates. Results were relatively insensitive to changes in hospitalization rates.
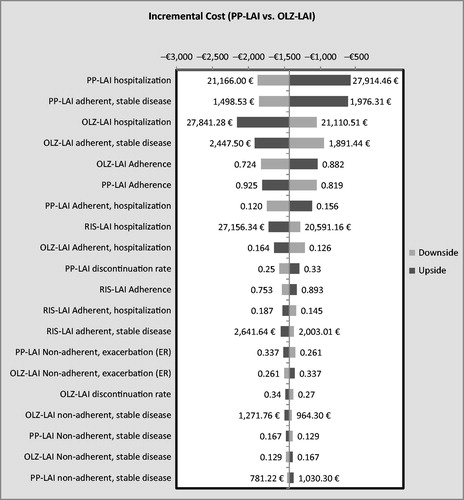
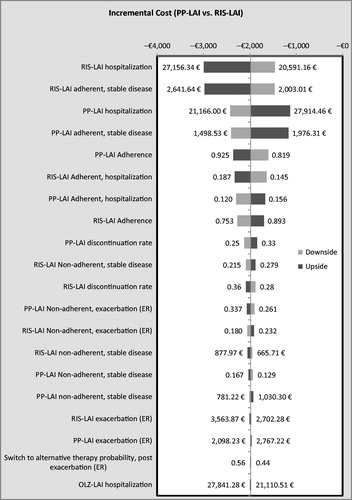
In the multivariate sensitivity analysis, PP-LAI dominated OLZ-LAI in 77.8% of the 10,000 simulations. In 88.7%, it had a lower cost and was associated with more QALYs in 85.5%. Compared with RIS-LAI, PP-LAI dominated in 85.9% of the simulations; in 94.6% it had a lower cost and in 89.8% it provided more QALYs. Overall, PP-LAI was dominated in 2.6% of the 20,000 simulations (i.e., 10,000 against each of the two comparison drugs). Tornado diagrams appear in and showing the impact on costs of variations in the 20 most influential variables.
Budget impact
This analysis found that the overall cost of treatment would be lower if patients were started on PP-LAI rather than OLZ-LAI or RIS-LAI. During 2010, RIS-LAI had the largest proportion of the market for LAIs in Finland, comprising ∼59% of the units consumed, with traditional LAIs making up the remainder. We assumed that PP-LAI would be adopted under the scenario presented above in the Methods section. The expected impact of PP-LAI would be €164,698 in the first year, €1.1 million in the second, and €1.7 million in the third year. The maximum expected impact was €12.1 million for a 100% share, which would be a highly unlikely scenario.
Discussion
A Medline search for pharmacoeconomic analyses or related analyses identified only two studies that quantified costs associated with schizophrenia in Finland. Koskinen et al.Citation60 examined the utilization of antipsychotic drugs in Finland over the 5-year period 1999–2005. They reported a dramatic increase in cost, even though the number of patients had remained relatively constant. The major reason appeared to be a large increase in the use of the newer atypical drugs as opposed to the traditional agents. In the other study from 1971, Niskanen and PihkanenCitation61 compared hospital care with home care for 102 persons with chronic schizophrenia in a randomized trial. They found that, after 1 year, hospital care costs were triple those for home care, while clinical outcomes were equal. In effect, they conducted a cost minimization study long before the era of pharmacoeconomics.
Therefore, we believe that this is the first pharmacoeconomic study of schizophrenia in Finland. The results are comparable to those we previously found in Norway, that is, PP-LAI dominated other LAIsCitation22. We also found similar results when this model was applied in GreeceCitation62, despite having a much different healthcare system and costing patterns. The most probable reason is that these models are driven by rates of adherence and hospitalization, while local variations make a comparatively minor contribution to the overall cost.
PP-LAI has advantages over RIS-LAI due to its monthly dosing as opposed to biweekly administration. Fewer administration times mean not only lower administration costs but also less chance for non-adherence. Such clinical advantages appear to translate into savings for the healthcare system.
We were conservative in dosing OLZ-LAI every 4 weeks. In the clinical trials from which the data were derived, 43% of the patients were dosed every 2 weeks and only 57% were dosed every 4 weeksCitation13. Each dose is associated with additional costs for administration and monitoring.
The budget impact analysis revealed a modest effect on the drug budget. If OLZ-LAI were to have an equal proportion of that market penetration, these estimates for PP-LAI would be halved, but the total impact of the two drugs (PP-LAI and OLZ-LAI) would be similar or slightly higher due to the use of OLZ-LAI. These calculations were based on DDDs. However, the expert panel indicated that they customarily dosed most antipsychotics, including risperidone, at ∼1 DDD, but they used 1.5 DDD of olanzapine. The same pattern of dosing was reported in the long-term clinical trials for these two drugs. In the two trialsCitation24,Citation25 using PP-LAI in 667 patients, the average dose was 69.3 mg, or 0.99 DDD, whereas the OLZ-LAI trialCitation36 in 599 patients used a weighted average dose of 432 mg, which is 1.54 DDDs. Therefore, calculations would have to be adjusted accordingly.
Adverse effects were not included in the cost analysis because PP-LAI is a metabolite of RIS-LAI and would, therefore, be expected to have a similar profile with respect to adverse events. In addition, official reports from authorities have concluded that adverse events have little influence on pharmacoeconomic analysesCitation63,Citation64. Problems that do arise from the use of these drugs have been captured in this analysis through the rates of discontinuation and switching to other products. Therefore, omission of adverse event costs can be considered reasonable.
There are limitations to this simulation study. We attempted to populate the decision tree with data specific for Finland, which may have a different profile than other countries. It was not always possible to locate such country-specific information (e.g., duration of hospitalization, complete accounting for all resources consumed in treatment, etc.); therefore, we used the best available estimates. We examined only out-patients with stable chronic schizophrenia who required LAIs. Results would probably differ with other clinical situations, such as relapsed inpatients requiring acute care or patients suffering their first psychotic episode. Other patient groups not assessed included persons with schizoaffective disorder or other forms of psychoses or mixed syndromes. Finally, head-to-head trials that reported all of the required information were not available, so we were forced to rely on indirect data. However, the selected studies were all performed in a similar manner on the same population of patients, so the data should be comparable. This approach has been taken in previous research of this natureCitation65. Nonetheless, further research would be required to address those issues.
This study excluded indirect costs of the disease and its consequences, such as time lost from productive work or reduced ability to work and caregiver burden. Many individuals in this population are unable to work or to persist at a meaningful occupation. They often require sheltered accommodation and supervision. We also did not address the risk of suicide, which is not infrequent in this patient populationCitation66,Citation67. Another omitted aspect was violence, which exerts a costly toll on victims (e.g., hospitalization or death) and on perpetratorsCitation68,Citation69.
Conclusions
In this analysis, due to its lower overall cost of treatment and better clinical results, PP-LAI dominated both OLZ-LAI and RIS-LAI. Because of these favourable attributes, PP-LAI appears to be a favourable addition to the formulary of antipsychotics in Finland as a cost-effective option for treating patients with chronic schizophrenia.
Acknowledgements
Declaration of funding
This article was supported by Janssen Pharmaceutica NV, Beerse, Belgium.
Declaration of financial/other relationships
RJ is an employee of Janssen. TE, HP, RZ, and CV have received consultant/advisory board fees from Janssen. TE has received funding from the sponsor for this research. He has also received travel funding to present a portion of these results at the Latin American ISPOR conference in Mexico City, 2011. In the past, he has received funding for unrelated work from Novo Nordisk, Lundbeck, BMS, Generex, and Janssen-Ortho. This was not a clinical trial; it was a pharmacoeconomic analysis. Therefore, the protocol is presented in the Methods section of this paper.
Acknowledgements
The authors would like to thank the following persons for medical advice and clinical input into this project. MD, PhD Jari Tiihonen, Hospital of Niuvaniem; MD, Antti Liuska, Hospital district of Pohjois-Karjala; MD, Ilkka Larmo, HUS; MD, Sirpa Lindroos, Health care district of Forssa.
References
- Mental health: schizophrenia. http://www.who.int/mental_health/management/schizophrenia/en/. Accessed October 10, 2011
- The global burden of disease: 2004 update. Geneva: World Health Organisation, 2008
- Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schiz Bull 2004;30:279-93
- Karagianis J, Novick D, Pecanek J, et al. Worldwide-Schizophrenia Outpatient Health Outcomes (W-SOHO): baseline characteristics of pan-regional observational data from more than 17,000 patients. Int J Clin Pract 2009;63:1578-88
- Perälä J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar disorders in a general population. Arch Gen Psychiatry 2007;64:19-28
- Juvonen H, Reunanen A, Haukka J, et al. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Arch Gen Psychiatry 2007;64:894-9
- Arajärvi R, Suvisaari J, Suokas J, et al. Prevalence and diagnosis of schizophrenia based on register, case record and interview data in an isolated Finnish birth cohort born 1940–1969. Soc Psychiatry Psychiatr Epidemiol 2005;40:808-16
- Teperi J, Porter ME, Vuorenkoski L, et al The Finnish health care system: a value-based perspective. Sitra Reports 82. Helsinki: Edita Prima Ltd, 2009
- OECD report criticises Finland for inefficient treatment of mental illness. Helsingin Sanomat. http://www.hs.fi/english/article/OECD+report+criticises+Finland+for+inefficient+treatment+of+mental+illness/1135251638800. Accessed October 10, 2011
- Nasrallah H. The case for long-acting antipsychotic agents in the post-CATIE era. Acta Psychiatr Scand 2007;115:260-7
- Adams CE, Fenton MKP, Quraishi S, et al. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br J Psychiatry 2001;179:290-9
- Möller HJ. Novel antipsychotics and negative symptoms. Int Clin Psychopharmacol 1998;13(3 Suppl):S43-7
- Kane J, Eerdekens M, Lindenmayer J-P, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003;160:1125-32
- Mehnert A, Diels J. Impact of administration interval on treatment retention with long-acting antipsychotics in schizophrenia. Presented at the Tenth Workshop on Costs and Assessment in Psychiatry -Mental Health Policy and Economics, Venice, Italy, March 25-27, 2011
- European Medicines Agency. Assessment report for Zypadhera. Doc. Ref EMEA/608654/2008. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000890/WC500054428.pd. Procedure No. EMEA/H/C/000890, 1-63. 2008. Accessed August 8, 2011.
- European Medicines Agency. Zypadhera® product monograph. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000890/WC500054429.pdf. 20-4-2010. Accessed August 8, 2011.
- European Medicines Agency. Xeplion® opinion. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002105/smops/Positive/human_smop_000162.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d127. Accessed October 10, 2011.
- European Medicines Agency. Xeplion® product monograph. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002105/WC500103317.pdf. Accessed October 10, 2011
- Detke H, Mcdonnell D, Brunner E, et al. Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, I: analysis of cases. BMC Psychiatry 2010;10:32
- Devadason P. Practical limitations of prescribing olanzapine depot (Relprevv). Australas Psychiatry 2010;18:269.
- Alphs L, Gopal S, Karcher K, et al. Are long-acting intramuscular formulations of risperidone or paliperidone palmitate associated with post-injection delirium sedation syndrome? An assessment of safety databases. Curr Drug Saf 2011;6:43-5
- Einarson TR, Vicente C, Zilbershtein R, et al. Pharmacoeconomic analysis of paliperidone palmitate versus olanzapine pamoate for chronic schizophrenia in Norway. Acta Neuropsychiatrica 2012 (In press). Article first published online: 11 JUL 2012 | DOI: 10.1111/j.1601-5215.2012.00670.x
- European Medicines Agency. Risperdal Consta® product monograph. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Risperdal_Consta_30/WC500008170.pdf. Accessed October 10, 2011
- Gopal S, Vijapurkar U, Lim P, et al. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol 2010;25:685-97
- Fleischhacker W, Gopal S, Lane R, et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol 2011;22:1-12
- Gopal S, Hough D, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol 2010;25:247-56
- Pandina GJ, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:218-26
- Hough D, Lindenmayer J-P, Gopal S, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:1022-31
- Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 2010;35:2072-82
- Pandina GJ, Lindenmayer J-P, Lull JM, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol 2010;30:235-44
- Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schiz Res 2010;116:107-17
- Kissling W, Heres S, Lloyd K, et al. Direct transition to long-acting risperidone - analysis of long-term efficacy. J Psychopharmacol 2005;19:15-21
- Lee M-S, Ko Y-H, Lee S-H, et al. Long-term treatment with long-acting risperidone in Korean patients with schizophrenia. Hum Psychopharmacol 2006;21:399-407
- Lindenmayer J-P, Khan A, Eerdekens M, et al. Long-term safety and tolerability of long-acting injectable risperidone in patients with schizophrenia or schizoaffective disorder. Eur Neuropsychopharmacol 2007;17:138-44
- Olivares J, Rodrigues-Morales A, Diels J, et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry 2009;24:287-96
- Kane J, Detke H, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry 2010;167:181-9
- Lauriello J, Lambert T, Andersen S, et al. An 8-week, double-blind, randomized, placebo controlled study of olanzapine long-acting injection in acutely ill patients with schizophrenia. J Clin Psychiatry 2008;69:790-9
- Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005;15:111-17
- Eerdekens M, Van Hove I, Remmerie B, et al. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004;70:91-100
- Olivares J, Peuskens J, Pecanek J, et al. Clinical and resource-use outcomes of risperidone long-acting injection in recent and long-term diagnosed schizophrenia patients: results from a multinational electronic registry. Curr Med Res Opin 2009;25:2197-206
- Ascher-Svanum H, Faries D, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry 2006;67:453-60
- Weiden P, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004;55:886-91
- Morken G, Widen J, Gråwe R. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry 2008;8:32.
- Simonsen E, Friis S, Opjordsmoen S, et al. Early identification of non-remission in first-episode psychosis in a two-year outcome study. Acta Psychiatr Scand 2010;122:375-83
- Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry 1999;156:990-9
- Ministry of Social Affairs and Health, Finland. Guidelines for preparing a health economic evaluation. Helsinki, 2009. http://www.ispor.org/PEguidelines/source/GuidelinesinFinland_EnglishVersion.pdf. Accessed October 10, 2011
- Finland inflation rate (Consumer prices). http://www.indexmundi.com/finland/inflation_rate_(consumer_prices).html. Accessed October 10, 2011
- SLD price. Suomen LääkeData Oy (Finland). Helsinki: IMS Health Oy. http://www.sld.fi/finland.html. Accessed October 10, 2011
- Hujanen T, Kapienen S, Tuominen U, et al. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006. Helsinki: Stakes, 2008. http://www.stakes.fi/verkkojulkaisut/tyopaperit/T3-2008-VERKKO.pdf. Accessed December 1, 2011
- Hus Palveluhinnasto 2011. Osa 1, Tuotteistetut sairaanhoidolliset palvelut; Osa 2 Suoriteperusteiset sairaanhoidolliset palvelut. Helsinki: Helsingin Ja Uudenmaan Sairaanhoitopiiri (Hospital District of Helskinki and UUsimaa), http://www.hus.fi/default.asp?path=1,28,820,823,3018. Accessed December 1, 2011
- Briggs A, Wild D, Lees M, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes 2008;6:105.
- Cummins C, Stevens A, Kisely S. The use of olanzapine as a first and second choice treatment in schizophrenia. A West Midlands Development and Evaluation Committee Report. Birmingham, UK: Department of Public Health & Epidemiology, University of Birmingham, 1998
- Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schiz Res 2004;71:155-75
- Oh PI, Lanctôt KL, Mittmann N, et al. Cost-utility of risperidone compared with standard conventional antipsychotics in chronic schizophrenia. J Med Econ 2001;4:137-56
- Revicki DA, Shakespeare A, Kind P. Preferences for schizophrenia-related health states: a comparison of patients, caregivers and psychiatrists. Int Clin Psychopharmacol 1996;11:101-8
- ATC/DDD index 2012. Oslo: WHO Collaborating Centre for Drug Statistics Methodology. http://www.whocc.no/atc_ddd_index/. Accessed December 2, 2012
- The World Fact Book: Finland. https://www.cia.gov/library/publications/the-world-factbook/geos/fi.html. Accessed December 2, 2011
- National Institute for Health and Clinical Excellence (NICE). http://www.nice.org.uk/. Accessed December 5, 2011
- Yahoo Finance Currency Converter. http://ca.finance.yahoo.com/currencies/converter/#from=GBP;to=EUR;amt=1. Accessed December 4, 2011
- Koskinen H, Martikainen JE, Maljanen T. Antipsychotics and antidepressants: an analysis of cost growth in Finland from 1999 to 2005. Clin Ther 2009;31:1469-77
- Niskanen P, Pihkanen TA. A comparative study of home treatment and hospital care in the treatment of schizophrenic and paranoid psychotic patients. Acta Psychiatr Scand 1971;47:271-7
- Einarson TR, Zilbershtein R, Vicente V, et al. Pharmacoeconomic analysis of paliperidone palmitate for treating schizophrenia in Greece. Ann Gen Psychiatry 2012;11:18
- All Wales Medicines Strategy Group. Final appraisal report: Olanzapine depot (ZypAdhera®), Lilly UK. Advice No: 1510. Penarth, Wales: All Wales Medicines Strategy Group. 2010. http://www.wales.nhs.uk/sites3/Documents/371/olanzapine%20depot%20(ZypAdhera)%20schizophrenia.pdf. Accessed October 10, 2011
- Farahati F, Boucher M, Moulton K, et al. Atypical antipsychotic monotherapy for schizophrenia: clinical review and economic evaluation of first year of treatment [Technology report number 91]. Ottawa: Canadian Agency for Drugs and Technologies in Heath, 2007
- Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the United States: a micro-simulation economic decision model. Curr Med Res Opin 2011;27:713-30
- Suokas JT, Perala J, Suominen K, et al. Epidemiology of suicide attempts among persons with psychotic disorder in the general population. Schiz Res 2010;124:22-8
- Alaraisanen A, Miettunen J, Rasanen P, et al. Suicide rate in schizophrenia in the Northern Finland 1966 Birth Cohort. Soc Psychiatr Psychiatr Epidemiol 2009;44:1107-10
- Honkonen T, Henriksson M, Koivisto AM, et al. Violent victimization in schizophrenia. Soc Psychiatry Psychiatr Epidemiol 2004;39:606-12
- Laajasalo T, Hakkanen H. Excessive violence and psychotic symptomatology among homicide offenders with schizophrenia. Crim Behav Ment Health 2006;16:242-53