Abstract
Objectives:
To compare resource utilization and costs among patients who used calcipotriene/betamethasone dipropionate topical suspension (Taclonex Scalp Topical Suspension, Leo Pharma A/S) vs those who used multiple body and scalp formulations for psoriasis.
Research design and methods:
A retrospective study using Truven Health MarketScan Commercial Database from 2006–2011 was performed to identify patients with psoriasis (ICD code 696.1x). Two study cohorts analyzed were cohort A (used body-only formulations for psoriasis and switched on the index date to using calcipotriene/betamethasone dipropionate topical suspension alone) and cohort B (used multiple body and scalp formulations for psoriasis). Patients were required to be continuously enrolled during 180-days pre- and post-index periods. Multiple regression analyses adjusting for baseline demographic and clinical covariates were performed.
Main outcomes measures:
Number of psoriasis-related outpatient visits, total healthcare costs, psoriasis-related costs, and use of systemic agents during post-index period.
Results:
A total of 1923 patients were identified with at least one prescription for calcipotriene/betamethasone dipropionate scalp topical suspension (cohort A = 367, cohort B = 1556). Patients using multiple medications (cohort B) were associated with 48% higher number of outpatient visits as compared with those who used a single formulation (cohort A) after controlling for baseline covariates (p < 0.001). A generalized linear model adjusting for baseline covariates showed significantly higher post-index total and psoriasis-related healthcare costs for cohort B as compared with cohort A (both p < 0.001). Patients in Cohort B also had twice the odds of using systemic agents as compared to patients in Cohort A (p < 0.001).
Conclusions:
Patients with body and scalp psoriasis using a single product had significantly lower overall and psoriasis-related healthcare costs, needed fewer psoriasis-related outpatient visits, and used less systemic agents during the post-index period. A lack of robust clinical measures to define the disease severity may have limited the interpretations from this study.
Introduction
Psoriasis is a chronic immune disease, affecting up to 7.5 million Americans with considerable sufferingCitation1. Plaque psoriasis, the most prevalent form of psoriasis, manifests as dry, scaly, red skin lesions that can be itchy and painful. Every part of the skin can be affected, and managing psoriasis can be extremely burdensome for patients and caregivers. Topical medications are commonly used to treat psoriasis, however they may be difficult and time-consuming to use. Adding to the difficulty of treating psoriasis is the need to use different products in different areas. The ointments that are typically used on the body are too messy for use in the scalp, and scalp psoriasis affects many patients with psoriasisCitation2,Citation3. The need for different topical formulations for different areas adds to the cost and complexity of treatment, and patients report that treatment of the disease is one of the worst aspects of having psoriasisCitation4. The resulting adherence to topical psoriasis treatments is poorCitation5.
The mainstays of topical psoriasis treatment include topical corticosteroids and topical vitamin D analogsCitation6. The combination of the two works better than either aloneCitation7, but use of multiple products results in a complex regimen. Simplifying treatments to one product has the potential to improve adherence and treatment outcomes. A fixed dose combination of calcipotriene, a vitamin D analog, and betamethasone dipropionate, a high potency corticosteroid, is available in an ointment formulation for psoriasis of the body (Taclonex Ointment, Leo Pharma A/S, Parsippany, NJ, USA) and in a gel/suspension formulation for scalp psoriasis (Taclonex Scalp Topical Suspension)Citation8. The American and European guidelines recommend the calcipotriene/betamethasone dipropionate fixed-dose combination products as a first line topical treatment for mild-to-moderate plaque psoriasis of the body and scalpCitation9.
To further simplify treatment, the gel/suspension formulation of calcipotriene with betamethasone dipropionate can be used for psoriasis lesions on both the body and the scalp. The potential advantages associated with using a single topical product for both scalp and body psoriasis are not well characterized. The objective of this study was to compare economic outcomes and resource utilization among patients who used single product (calcipotriene/betamethasone dipropionate topical suspension) vs those who used multiple body and scalp formulations for managing their psoriasis.
Patients and methods
Data source
A retrospective observational study was carried out using the Truven Health MarketScan Commercial Database (Truven Health MarketScan Databases, Truven Health Analytics, Inc, Ann Arbor, MI). These claims data provide a nationally representative sample of the commercially-insured population in the US. These longitudinal data include pharmacy, inpatient, and outpatient claims, integrated at the patient level on over 110 million Americans. Data are available from 125 contributing employers, 13 health plans, and 11 state Medicaid programs. The database includes both inpatient and outpatient diagnoses (in ICD-9 format) and procedures (in CPT-4 and HCPCS formats), as well as both retail and mail order prescription records.
Available data on prescription records include the NDC code as well as quantity dispensed. Additional data elements include enrollees’ demographic variables (age, gender, geographic region), provider characteristics, payer type (e.g., commercial, self-pay), and start and end dates for plan enrollment. The current study used de-identified claims data and, hence, was determined not to require institutional review board review.
Sample selection
The database was queried from January 1, 2006 to March 31, 2011 to identify a cohort of psoriasis patients (ICD-9 code 696.1x). A sub-set of patients with at least one claim for calcipotriene/betamethasone dipropionate topical suspension was identified using NDC codes (00430323015, 00430323016, 50222022704, 50222022781, 00430324015, 50222050106) between January 1, 2007–March 31, 2010. This prescription identification window was selected to allow at least 1 year before and after the first service date for calcipotriene/betamethasone dipropionate topical suspension.
The first service date for a prescription of calcipotriene/betamethasone dipropionate topical suspension during this period was an index date. For this study, patients were required to be continuously enrolled during 180 days pre- and post-index dates. The following study cohorts were identified:
Cohort A: used multiple body and scalp formulations for psoriasis during 180 days prior to the index date, when they switched to using calcipotriene/betamethasone dipropionate scalp topical suspension alone. These patients did not have any claims for other psoriasis medications on or after the index date.
Cohort B: used multiple body and scalp formulations for psoriasis (e.g., calcipotriene/betamethasone dipropionate topical suspension and another medication) during 180 days before and after index date. Patients were required to be continuously enrolled during 180 days pre- and post-index periods. The sample attrition is described in .
Table 1. Sample attrition.
Study measures
Patients’ demographic characteristics were derived from the enrollment information in conjunction with information from the index claim. Characteristics related to index medications were also assessed using the information from the index claim. Psoriasis-related comorbidities were identified using diagnosis codes during the pre-index period. The specific study measures used can be found in .
Table 2. Demographic and clinical characteristics.
Since claims data do not have clinical measures to ascertain disease severity, the following algorithm was used as a proxy to assess psoriasis severity. Patients were categorized as having moderate–severe disease if they had ≥1 claims for systemic therapies or certain medical procedures in the pre-index period ()Citation10,Citation11.
Table 3. Algorithm for disease severity.
Patients with no pre-index claims for any systemic therapies or medical procedures mentioned above were categorized as having mild psoriasis.
Study outcomes
Number of psoriasis-related outpatient visits in the post-index period
This was defined as any outpatient visits with psoriasis diagnosis-ICD-9 code 696.1. Psoriasis-related outpatient visits were defined as outpatient claims with a diagnosis code of 696.1. Multiple claims on a single day were considered as a single visit to avoid any double-counting of visits on the same day.
Total healthcare costs in the post-index period
This includes pharmacy cost and cost of inpatient and outpatient services reported in the claims data (adjusted for 2011 US dollars using the medical component of the consumer price index).
Psoriasis-related healthcare costs in the post-index period
This includes pharmacy cost and cost of inpatient and outpatient services associated with a psoriasis claim identified using ICD-9 code 696.1 (adjusted for 2011 US dollars using the medical component of the consumer price index).
Use of systemic agents during post-index period
This was defined as at least one claim during the post-index period for any psoriasis-related systemic agent (including biologic agents, and non-biologic systemic agents).
Statistical analyses
Descriptive analyses (frequencies and percentages) of baseline characteristics and univariate (one-way analysis of variance and chi-squared) analyses were performed. Differences in number of psoriasis-related outpatient visits and total healthcare costs during pre- and post-index periods were compared using t-tests. Use of any systemic agents (yes/no) in the post-index period was compared using chi-squared tests. Multiple regression analyses were performed to study the association between type of treatment (single agent vs multiple agents) and study outcomes controlling for demographic and clinical characteristics, comorbidities, and psoriasis severity. The Adjusted Poisson regression model was used to study the association between the number of psoriasis-related outpatient visits and type of treatment. The coefficients obtained from the model were then exponentiated to obtain the differences in actual number of visits. The association between post-index healthcare costs and type of treatment was examined using the adjusted generalized linear model (GLM) with gamma distribution and log-link function. The adjusted logistic regression model was used to study the association of use of any systemic agents (yes/no) in the post-index period and type of treatment. For all multivariate models, cohort A was used as a reference category. Thus, positive coefficients in Poisson regression indicated that cohort B had higher logs of expected counts of outcomes (psoriasis-related visits) as compared to cohort A. Because of the difficulty in interpreting arithmetic mean ratios, we calculated the average marginal effects for costs and psoriasis-related outpatient visits by treatment types.
Results
A total of 1923 patients were identified with at least one prescription for calcipotriene/betamethasone dipropionate topical suspension during the study period from January 1, 2007 to March 31, 2010 [cohort A (single treatment) = 367; cohort B (multiple treatments) = 1556). The median age of patients was ∼47 years, with more than half of the patients being females (57.5%). Significantly more females than males used a single treatment to treat their psoriasis (62.9% vs 56.2%, p = 0.02). No significant differences in comorbidities were found between study cohorts except for psoriatic arthritis, the rate of which was significantly higher among cohort B as compared to cohort A (3% vs 6.2%, p = 0.02). Significantly more patients in cohort B also had severe disease and a higher number of psoriasis-related outpatient visits during the pre-index period ().
In bivariate analyses, there were no statistically significant differences in the psoriasis-related outpatient visits prior to index date (1.92 ± 4.03 vs 1.87 ± 4.17; p = 0.84, ). During the post-index period (180 days), the mean number of psoriasis-related outpatient visits in cohort A was significantly lower than that in cohort B (1.51 ± 3.91 vs 2.49 ± 6.06, p = 0.0001). A lower percentage of patients in cohort A used systemic agents in the post-index period as compared to patients in cohort B (10.9% vs 22.4%, p < 0.0001). Mean total healthcare costs were also significantly lower during the post-index period in cohort A ($4013.71 ± $7634.33, median = $1645.48) as compared with cohort B ($5873.19 ± $10,020.64, median = $2865.84, p < 0.0001) (). While pre-index psoriasis related costs did not differ significantly between the study cohorts, the post-index costs were almost twice among cohort B as compared with cohort A ($1296.92 ± $2859.98; median = $630.95 vs $2634.87 ± $4042.35; median = $1223.92).
Figure 1. Association between study outcomes and treatment type (bivariate analyses). (a) Association between treatment type and post-index number of psoriasis-related outpatient visits. (b) Association between treatment type and post-index total healthcare costs. (c) Association between treatment type and post-index psoriasis-related costs. (d) Association between treatment type and post-index use of systemic agents. Cohort A: used body-only formulations for psoriasis and switched on the index date to using one drug (calcipotriene/betamethasone scalp topical suspension). Cohort B: used multiple body and scalp formulations for psoriasis (calcipotriene/betamethasone scalp topical suspension and another medication). * t-tests were used to study the association between type of treatment and number of psoriasis-related outpatient visits and total healthcare costs in the post-index period. ** Chi-squared test was used to study the association between type of treatment and use of systemic agents in the post-index period.
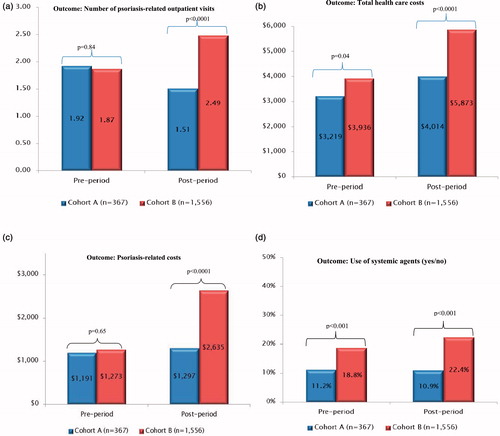
The study outcomes were also examined separately among patients with moderate-to-severe psoriasis vs those with mild psoriasis. Among moderate-to-severe psoriasis patients, there were no statistically significant differences in the mean number of psoriasis-related outpatient visits during pre- and post-index periods (4.34 ± 7.9 vs 3.73 ± 7.03; p = 0.49 in the pre-period and 3.73 ± 7.03 vs 4.55 ± 9.03; p = 0.07 in the post-periods). A lower percentage of patients in cohort A used systemic agents in the post-index period as compared to patients in cohort B (38.4% vs 53.3%, p < 0.017). Mean total healthcare costs were also significantly lower during the post-index period in cohort A ($4810.62 ± $5303.06, median = $2579.7) as compared with cohort B ($9672.25 ± $14,680.56, median = $5518.46, p < 0.001). Similarly, psoriasis-related costs during the post-index period were significantly lower among cohort A as compared with cohort B ($5043.90 ± $6031.54, median = $2487.65 vs $2657.44 ± $3952.78, median = $881.3). For mild psoriasis patients, all study outcomes except for total healthcare costs were significantly lower among cohort A as compared with cohort B ( and ).
Table 4. Study outcomes by disease severity (bivariate analyses) for mild psoriasis.
Table 5. Study outcomes by disease severity (bivariate analyses) for moderate-to-severe psoriasis.
describes a summary of regression analyses. Poisson regression analyses indicated that, after controlling for baseline covariates, patients using multiple treatments had 48% (95% CI = 34–63%) more psoriasis-related outpatient visits in the post-index period as compared with those using a single treatment (p < 0.0001). The adjusted post-index total healthcare costs were $986 higher in cohort B as compared with cohort A (p = 0.002). The adjusted psoriasis-related costs were $999 higher in cohort B as compared with cohort A (p < 0.0001). Finally, multiple logistic regression analyses indicated that patients in cohort B had twice the odds of using systemic agents (biologic and non-biologic) in the post-index period as compared to patients in cohort A (OR = 2.31, p < 0.001).
Table 6. Estimates from regression models.
Discussion
Medication non-adherence to topical treatments in psoriasis is associated with poor clinical and economic outcomesCitation12. The most common patient-reported reasons for poor adherence to topical therapies are low efficacy, time consumption, and poor cosmetic characteristics of topical agentsCitation13. A growing body of literature supports using simplified treatment regimens for improving adherence in chronic skin conditionsCitation12. For the treatment of scalp psoriasis, where the drug application is particularly cumbersome and the treated areas are visible, a once daily treatment regimen could potentially improve adherence compared with two or more separate applications, which may in turn lead to higher treatment efficacyCitation14. Our results suggested there may also be economic advantages of using calcipotriene/betamethasone suspension in psoriasis management, particularly involving psoriasis of both body and scalp. These results are consistent with data reported by Devaux et al.Citation15. Further studies are required to examine medication adherence rates among the study cohorts and its impact on healthcare utilization and costs.
In this study, more females were managed on calcipotriene/betamethasone topical suspension alone (cohort A) than with multiple products for body and scalp psoriasis (cohort B). Psoriasis patients who were switched to calcipotriene/betamethasone topical suspension alone had a lower number of psoriasis-related outpatient visits in the pre-index period than those managed with multiple products. In addition, these patients appeared to have milder psoriasis (based on the use of systemic therapies in the pre-index period) than patients managed by multiple products.
Although it is possible that patients with milder psoriasis can be managed on one product and that more severe disease requires the use of multiple therapies, nonetheless patients using one product required less systemic agents and had fewer psoriasis-related outpatient visits, perhaps indicating better management of disease following the treatment with calcipotriene/betamethasone topical suspension alone. In order to examine the potential impact of severity on study outcomes, we ran the outcomes analyses stratified by disease severity and also added disease severity as an independent variable in the regression models. A higher percentage of patients with more severe disease used multiple agents to manage their psoriasis as compared to those with milder disease (30% vs 20%). However, more interestingly, among patients with severe psoriasis, those who switched to calcipotriene/betamethasone topical suspension alone had significantly lower use of systemic agents in the post-index period as compared to those who continued to use multiple products. Although not statistically significant, the mean number of psoriasis-related outpatient visits was also lower in the cohort that used a single agent to treat their body and scalp psoriasis. Total and psoriasis-related costs remained higher for those who used multiple products. Similar trends were seen among patients who had mild psoriasis. Further studies with a robust indicator for disease severity would be needed to examine the impact of disease severity on prescription patterns in patients with both body and scalp psoriasis.
This study, however, should be considered as an initial exploration of these issues, and caution should be exercised in interpreting these findings due to a number of study limitations. First, the observational study design does not permit causal inference of our results. Second, the study has inherent limitations associated with using the retrospective claims data, thus limiting randomization of the study cohorts. Due to a lack of clinical measures, the study used a proxy to ascertain severity of psoriasis in the study population. A clinical measure may be more robust in identifying psoriasis severity. Finally, since the study used claims data, any untreated scalp psoriasis or patients who used over-the-counter (OTC) scalp psoriasis medications were not captured in the analyses.
Conclusions
In this retrospective claims data analyses, patients using calcipotriene/betamethasone topical suspension alone had significantly lower overall and psoriasis-related healthcare costs, needed fewer psoriasis-related outpatient visits, and used less systemic agents during the post-index period as compared to patients who used multiple psoriasis medications to manage their psoriasis. The analyses controlled for baseline demographic and clinical covariates including pre-index disease severity (ascertained by the use of systemic agents during the pre-index period) in the multivariate regression models. A significantly lower healthcare utilization in the follow-up period may have resulted in the overall reduction of healthcare costs among these patients. This study suggests that using only one product suitable for both body and scalp psoriasis (such as calcipotriene/betamethasone topical suspension) could be a simpler and more economical regimen for psoriasis patients.
Transparency
Declaration of funding
This study was supported by LEO Pharma, Inc.
Declaration of financial/other relationships
Dr Feldman is a consultant for LEO Pharma, Inc. Outcomes, Inc. provided editorial support. Dr Levi is an employee of Leo Pharma. Mr Pathak and Ms Kakatkar are paid consultants at Outcomes, Inc. Dr Balkrishnan is Principal Consultant to Outcomes, Inc. and provided research support for this study. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Previous Presentation: Poster Presentation at 2013 Winter Clinical Dermatology Conference, January 18–23, 2013, Grand Hyatt Kauai, Koloa, Hawaii.
References
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999;41:401-7
- National Scalp Psoriasis (NPF). Scalp Psoriasis. 2008. http://www.psoriasis.org/document.doc?id=155. Accessed June 6, 2013
- van de Kerkhof PC, de Hoop D, de Korte J, et al. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology 1998;197:326-34
- Rapp SR, Exum ML, Reboussin DM, et al. The physical, psychological and social impact of psoriasis. J Health Psychol 1997;2:525-37
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol 2004;51:212-6
- Pearce DJ, Stealey KH, Balkrishnan R, et al. Psoriasis treatment in the United States at the end of the 20th century. Int J Dermatol 2006;45:370-4
- Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol 1996;35:268-9
- Girolomoni G, Vena GA, Ayala F, et al. Consensus on the use of the fixed combination calcipotriol/betamethasone dipropionate in the treatment of plaque psoriasis. G Ital Dermatol Venereol 2012;147:609-24
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009;60:643-59
- Kimball AB, Robinson D, Jr Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US Healthcare Databases, 2001–2002. Dermatology 2008;217:27-37
- Yu AP, Tang J, Xie J. Economic burden of psoriasis compared to the general population and stratified by disease severity. Curr Med Res Opin 2009;25:2429-38
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improving adherence to topical therapy. J Am Acad Dermatol 2008;59:1009-16
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012;26:61-7
- Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol 2004;140:408-14
- Devaux S, Castela A, Archier E, et al. Topical vitamin D analogues alone or in association with topical steroids for psoriasis: a systematic review. J Eur Acad Dermatol Venereol 2012;26:52-60
