Abstract
Purpose:
Financial burden associated with providing healthcare to patients with pulmonary arterial hypertension (PAH) is poorly characterized. This study sought to quantify 3-year healthcare expenditures and determine whether expenditures differed between incident and prevalent PAH cases.
Methods:
This was a retrospective cohort study of Kaiser Permanente Colorado (KPCO) patients with confirmed diagnosis of PAH. Included patients were followed from study entry until 3 years, death, or termination of KPCO membership, whichever came first. All expenditures were reported in 2011 US dollars from the KPCO perspective.
Results:
In total, 157 patients were included: 44 (28%) prevalent and 113 (72%) incident cases. Mean age (prevalent vs incident cases) was 61 years vs 67 years and 13.6% vs 27.4% were males. The majority of patients (55%) were classified as WHO Group 1 PAH. Prevalent cases had less follow-up (843 vs 975 days; p = 0.033). Overall, median total per patient per day (PPPD) and 3-year total expenditures were $56 (interquartile range (IQR = $29–$166) and $50,599 (IQR = $25,958–$135,535), respectively. After adjustment for patient characteristics and chronic disease burden, median PPPD ($54 vs $56; p = 0.950) and 3-year ($37,340 vs $55,073; p = 0.111) total expenditures were equivalent between prevalent and incident cases; however, the risk of death during the 3-year follow-up was lower among incident cases (hazard ratio = 0.41, 95% CI = 0.18–0.91). No significant differences were detected in pharmacy, inpatient, medical office, emergency department, or other expenditures. Median PAH specialty medication PPPD expenditures were also equivalent, also ($226 vs $223 among specialty medication users; p = 0.861).
Conclusion:
Healthcare expenditures related to PAH represent substantial financial burden. Significant differences according to prevalent or incident case status appeared to be driven by median ED and inpatient expenditures; however, PAH specialty medication expenditures represented a substantial cost-driver overall. Future efforts should focus on optimizing care for patients with PAH to avoid unnecessary harm or waste.
Introduction
Pulmonary arterial hypertension (PAH) is a rare (estimated prevalence ranging from 15 per 1 million individuals worldwide to 1007 per 1 million individuals, depending on underlying etiology), fatal, and incurable disease characterized by chronically elevated pulmonary artery pressure, abnormal cell proliferation and remodeling, vasoconstriction, as well as thrombosisCitation1,Citation2. Patients with PAH suffer from chronic shortness of breath, limited exercise capacity, weakness and fatigue, along with progressive right heart failureCitation2. While long-term prognosis for patients with PAH is poor, survival rates have improved since the introduction of pharmacologic therapies including prostanoids, endothelin receptor antagonists (ERAs), and phosphodiesterase-5 (PDE-5) inhibitorsCitation3.
The currently available therapies are associated with symptomatic improvement, improved quality-of-life, delayed onset of clinical right heart failure, and longer survivalCitation4. Intravenous epoprostenol (Flolan®) was the first therapy introduced for PAH in 1995 and is considered to be the hallmark therapy for patients based on demonstration of improved survival compared to an historical cohort of PAH patients maintained by the World Health Organization (WHO) and the National Institutes of Health (NIH)Citation5. Subsequently other therapies with different pharmacologic mechanisms—ERAs and PDE-5 inhibitors—were approved. Bosentan (Tracleer®), a non-selective ERA, was the first oral PAH therapy approved in the US, with sildenafil (Revatio®), a PDE-5 inhibitor, closely following. Subsequently, ambrisentan (Letairis®) and tadalafil (Adcirca®) were approved in the US and represent incremental improvements over bosentan and sildenafil (mostly due to favorable pharmacokinetic or pharmacodynamic properties)Citation6,Citation7. Studies suggest that overall health and prognosis for patients with PAH is improving despite the potential expense of these therapies as well as financial burden they place upon health systemsCitation8.
While improvements in patient functional capacity and survival associated with these PAH therapies have been investigated, evidence of the financial burden associated with providing care to patients with PAH—particularly within integrated systems—is under-developed. One retrospective study that examined resource utilization and cost for managing patients with PAH who received either sildenafil or bosentan reported that these patients’ healthcare costs were significant with pharmacy expenditures as a primary driverCitation9. Wilkens et al.Citation10 similarly described the clinical and economic burden of PAH for patients in Germany. In addition, reports from Copher et al.Citation11 and Said et al.Citation12 examined costs related to patients with a PAH diagnosis in the US. Beyond recognizing that caring for patients with PAH is associated with economic and financial burden, these studies do not report or characterize the expenditures related to incident (diagnosis upon study entry) or prevalent diagnosis (diagnosis made prior to study entry). While it may be assumed that prevalent cases will be older and at a more advanced state of disease, thus incurring higher healthcare expenditures, incident cases may be more likely to utilize new technologies that may be more expensiveCitation13,Citation14. Little is known about the expenditure differences of these sub-groups of patients with PAH.
Because characterization of the financial burden associated with managing PAH patients within an integrated healthcare delivery system—particularly in the context of rapidly increasing healthcare costs—is currently incomplete, we sought to quantify 3-year healthcare expenditures for patients with PAH in Kaiser Permanente Colorado (KPCO) and to determine whether expenditures differed between prevalent and incident cases.
Methods
Study design
This was a retrospective cohort study characterizing the financial burden of PAH in patients with a confirmed diagnosis of PAH identified. This data-only study examined direct healthcare expenditures from the KPCO perspective. All patients meeting inclusion criteria were followed for 3 years or until death or loss of KPCO membership occurred. This study was reviewed and approved by the KPCO Institutional Review Board (IRB).
Study setting
Kaiser Permanente Colorado is a group model, not-for-profit, integrated healthcare delivery system serving more than 530,000 members at 26 medical offices in Colorado. Member visits for primary care and most specialties, including both cardiology and pulmonology, are provided by KPCO healthcare professionals. The vast majority of member hospitalizations occur at contract facilities. At KPCO, an electronic medical record (EMR) has been used since January 1, 1996. Data from the EMR, including diagnostic, laboratory, pharmacy, and imaging, along with claims and membership data, are stored in administrative databases. In addition, KPCO maintains a Decision Support System (DSS). The DSS gathers and compiles expenditures for healthcare encounters among KPCO members using both claims and internal expenditure algorithms. This system matches expenditures from the KPCO general ledger with member utilization to produce estimates that include both direct costs (e.g., provider time, medical supplies, and medications) and indirect costs (e.g., facilities, malpractice insurance, information technology, and other shared business costs). Expenditure data from the DSS are available from January 1, 2005 until the present.
Patient population
The International Classification of Diseases Ninth Revision code 416.0 (primary pulmonary hypertension) was used to query the KPCO electronic ambulatory diagnostic database between July 1, 2004 and June 30, 2008 to identify potential study patients. Potential study patient PAH diagnosis was confirmed with manual medical record chart review using the following criteria: (1) documentation that the patient was managed clinically by a PAH clinical specialist (i.e., cardiologist, pulmonologist) and underwent right heart catheterization at the time of PAH diagnosis; or (2) electronic prescription order for pulmonary vasodilator therapy including PDE-5 inhibitors (sildenafil and tadalafil), ERAs (ambrisentan and bosentan), prostanoids (epoprostenol, iloprost, and treprostinil), or any combination thereof.
All KPCO members ≥18 years of age with a confirmed PAH diagnosis recorded between July 1, 2004 and June 30, 2008 were included in the analysis. Patients whose initial confirmed PAH diagnosis was recorded in the EMR at any time prior to January 1, 2005 (i.e., a look-back period until January 1, 1996) were assigned to the prevalent case group. Patients whose initial confirmed PAH diagnosis was recorded in the EMR after January 1, 2005 and without a confirmed PAH diagnosis recorded at any time in the EMR prior to January 1, 2005 were assigned to the incident case group. Prevalent cases had continuous KPCO membership from January 1, 2004 until 4 years, KPCO membership termination, or death, whichever came first. Incident cases had at least 1 year of continuous KPCO membership prior to initial PAH diagnosis and then until 3 years of follow-up, KPCO membership termination, or death, whichever came first. Prevalent patients were followed from January 1, 2005 until 3 years, death, or KPCO membership termination, whichever came first. Incident patients were followed from date of initial, confirmed PAH diagnosis until 3 years, death, or KPCO membership termination, whichever came first.
Patients were categorized according to World Health Organization (WHO) PAH classification (i.e., Group 1, idiopathic, inheritable, drug or toxin-induced, or associated PAH; Group 2, PAH due to left heart disease; Group 3, PAH due to lung disease and/or hypoxemia; Group 4, chronic thromboembolic PAH; and Group 5, PAH with unclear mechanisms)Citation2 and further characterized by a Chronic Disease Score (CDS). Verification of WHO PAH group classification was manually adjudicated through electronic medical record review by three individual investigators (SJ, PS, and ALB). The CDS is a validated risk measure for baseline health status and allows for the accounting of each patient’s risk of mortality and future healthcare utilization at the time of index event. The CDS ranges from 0–35, with higher scores suggesting a higher disease burden. After adjustment for age and sex, a patient with a CDS of 7 would have 9.8-times the risk of death compared to a patient with a CDS of 0Citation15,Citation16.
Outcomes
The primary outcome measure was the 3-year total healthcare expenditure in 2011 US dollars. Expenditures were stratified by inpatient, emergency department (ED), medical office, total ambulatory pharmacy, ambulatory specialty pharmacy, and other (e.g., ambulance, radiology, laboratory, durable medical equipment). Dental care was excluded as expenditure since KPCO does not provide this service. In addition, patients were stratified independently on incident/prevalent status and WHO PAH classification. Secondary outcomes included all-cause mortality and healthcare expenditures per patient per day (PPPD). Total healthcare expenditures, as opposed to disease-specific, were described since most patients were being treated for multiple chronic diseases during the study period. Per patient per day expenditures are provided since patients had variable lengths of follow-up. Additionally in order to assess the impact of end-of-life care on expenditures, expenditures from the last 90 days of the study period were calculated and compared between patients who died and did not die during the study period. Finally, to assess the immediate impact of a PAH diagnosis on expenditures, expenditures from the first year of the study period were calculated.
Data collection
Information on patient medical encounters, medication purchases, and characteristics were obtained from queries of the electronic, administrative records, and claims databases. Information on type of PAH was verified with manual chart review and discrepancies were resolved by a third reviewer. Additionally, information on PAH medication purchases was verified with manual medical chart review. Patient age was determined as of study entry. Date of death was determined from the administrative membership database.
Queries of the KPCO’s DSS database were made using place of service, revenue, procedure, laboratory, and radiologic codes to identify encounter expenditures during year of service performed. Expenditures were adjusted for inflation to 2011 US dollars with the medical care price index.
Data analysis
Results are reported as percentages, means and standard deviations, or medians and interquartile ranges (IQR). Per patient per day expenditures were calculated by dividing each individual patient’s accumulated expenditures during follow-up by his/her length of follow-up. Median cohort and group PPPD expenditures with their interquartile ranges are reported as expenditure data in toto were non-normally distributed. Mean cohort and group PPPD expenditures are reported, also. Categorical variables were compared between groups using the chi-square test of association or Fisher’s exact test, as applicable. Interval-level variables were compared using two-sample t-tests and ANOVAs between and across groups, respectively, for parametric data or Wilcoxon rank-sum and Kruskal-Wallis tests between and across groups, respectively, for non-parametric data.
Expenditures between incident and prevalent groups were compared further using linear regression analysis with log-transformed expenditures as the dependent variable and grouping indicator as the independent variable. Expenditure data were log transformed owing to the non-normal distributions of the group expenditures. Regression models were adjusted for age, sex, race, CDS, and histories of diagnosis for hypertension and diabetes mellitus. Cumulative incidence of all-cause mortality was compared between incident and prevalent groups using a Nelson-Allen cumulative hazard function graph and Cox proportional hazards modeling with adjustment for age, sex, race, CDS, and histories of diagnosis for hypertension and diabetes mellitus. Adjusted analyses were not conducted across WHO Groups owing to the small sample sizes in some groups.
Results
In total, 263 patients were identified with a diagnosis of PAH. Of these, 106 were excluded because they did not have a confirmed diagnosis of PAH. Thus, 157 patients were included in the analysis; 113 (72%) and 44 (28%) patients in the incident and prevalent case groups, respectively. Overall, patients were predominately elderly with mean age of 65.4 (SD = ±14.4) years, female (76.4%), and white (70.1%), and with a mean CDS of 5.8 (). The most frequent PAH classification was WHO Group 1. Incident cases were more likely to have had hypertension than prevalent cases (p = 0.013). All other characteristics were equivalent between prevalent and incident cases (p > 0.05).
Table 1. Baseline characteristics overall and by prevalence/incidence status.
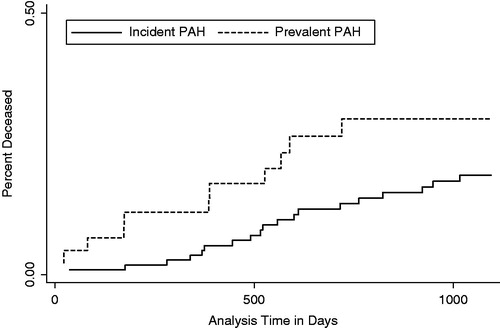
Patients were followed for a mean of 843 (±377) vs 975 (±265) days in the prevalent and incident case groups, respectively (p = 0.033) (). A total of 30 (19%) patients died from any cause during their 3-year follow-up (). In unadjusted analysis, mortality rates were equivalent between prevalent and incident cases and across WHO classes (all p > 0.05). However, in adjusted Cox proportional hazards modeling where time to death is taken into consideration, incident cases had a lower risk of death (hazards ratio = 0.41, 95% CI = 0.18–0.91).
Table 2. Study outcomes overall and by prevalent/incident status.
Overall, the 3-year median total healthcare expenditures were $50,559 (). The median total expenditures were $56 PPPD. Median ‘other’ expenditures contributed most to the overall total expenditures at $16 PPPD. The overall median pharmacy expenditures were $5 PPPD and median PAH medications (i.e., pulmonary vasodilator therapy) expenditures were $226 PPPD for those patients who received PAH medications (n = 18; prevalent cases n = 6 (14%) and incident cases n = 12 (11%); p = 0.591).
Incident cases had higher median total ED expenditures ($1250 vs $357; p = 0.009) and inpatient expenditures ($7313 vs $980; p = 0.028) over the 3-year follow-up compared to prevalent cases in unadjusted analyses (). However, in adjusted analysis, these expenditures were no longer statistically significantly different. Median PPPD total expenditures, ambulatory pharmacy expenditures, PAH specialty medication expenditures (among specialty medication users only), and median total expenditures were equivalent between prevalent and incident cases (all p > 0.05 in unadjusted and adjusted analyses). Median PPPD total expenditures for patients who did and did not receive a PAH medication were $435 (mean = $430, IQR = $292–$561) and $49 (mean = $102, IQR = $26–$115), respectively (unadjusted p = 0.287, adjusted p = 0.099).
During the last 90 days of life, prevalent and incident cases who died had median total expenditures of $7621 (mean = $19,050, IQR = $1407–$46,113) and $13,229 (mean = $28,831, IQR = $7276–$34,570), respectively (unadjusted p = 0.149, adjusted p = 0.087). During the last 90 days of the study period, prevalent and incident cases who did not die had median total expenditures of $2546 (mean = $11,179, IQR = $1257–$20,205) and $3066 (mean = $10,191, IQR = $1417–$12,309), respectively (unadjusted p = 0.940, adjusted p = 0.803). Overall, cases who died and survived had 90-day end-of-study-period median total expenditures of $12,776 (mean = $25,245, IQR = $5030–$34,570) and $2810 (mean = $10,448, IQR = $1417–$13,296), respectively (unadjusted p < 0.001, adjusted p < 0.001).
During the first year of the study period, prevalent and incident cases had median total expenditures of $15,693 (mean = $42,077, IQR = $7852–$28,986) and $17,706 (mean = $33,041, IQR = $9926–$39,391), respectively (unadjusted p = 0.317, adjusted p = 0.289). Prevalent and incident cases had median PPPD total expenditures of $43 (mean = $115, IQR = $22–$79) and $49 (mean = $91, IQR = $27–$108), respectively (unadjusted p = 0.350, adjusted p = 0.301).
Patients in WHO Group 2 had the highest mean age (p < 0.008). Classes were equivalent in proportion of males, comorbidities, CDS, race, and incident case status (all p > 0.05) (). While median total PPPD expenditures were numerically highest for patients in the WHO Group 5 group at $115 PPPD, this did not reach statistical significance compared to any other group (). No other statistically significant differences were detected in expenditures across WHO groups (all p > 0.05).
Table 3. Patient characteristics by World Health Organization (WHO) PAH class status.
Table 4. Study outcomes by World Health Organization (WHO) PAH class status.
Discussion
In this financial analysis of 157 patients with chart-review confirmed prevalent and incident PAH, we identified that median all-cause, total PPPD expenditures were $56 or ∼$1680 per patient per month (PPPM) in 2011 dollars. We identified that total PPPD expenditures were equivalent between these groups. Expenditures for prevalent cases may provide the most salient information for healthcare payers since these expenditures identify immediate impacts on budgets. However, expenditures for incident cases, where new, more expensive treatment technologies are more likely employed, may not be estimated accurately when using only the expenditures from prevalent cases. In addition, newly diagnosed patients may require more frequent ED and inpatient visits, for example for episodes of decompensation, if they are more symptomatic. Interestingly, we did not find statistical differences in total expenditures between prevalent and incident cases during the first year of study inclusion. Unfortunately, published studies that examined differences in expenditures between incident and prevalent cases of patients of PAH with which to compare our findings were not identified. Even so, previous publications provide a rationale and explanation for comparing incident and prevalent cases; namely, that this reduces the healthy user effect (i.e., prevalent users are likely to have survived early periods of pharmacotherapy), which increases bias substantially if risk varies with time, as it does in patients with PAHCitation13,Citation14.
Two recent US studies, however, have reported that mean all-cause, total PPPM ‘costs’ in patients with a PAH diagnosis in the US range from ∼$6200–$9300. Our mean all-cause, total PPPM expenditures (∼$4200) appear to be lower. This is especially evident when considering that we report our expenditures in 2011 dollars; whereas Copher et al.Citation11 report their costs in 2008 dollars and, while not explicitly stated, Said et al.Citation12 report their costs from the year in which the costs were accumulated (2004–2009). Our reported expenditures may be lower due to our study being performed in an integrated healthcare system, including both prevalent and incident cases, and using the perspective of the insurer in our analysis.
Another US study of patients who received either sildenafil or bosentan reported that mean all-cause, total PPPM costs were ∼$6500 in 2008 dollarsCitation9. We identified an apparently higher mean all-cause, total PPPM expenditure of ∼$12,900 for our patients with PAH who received a PAH medication. Our expenditures for patients who received a PAH medication may be higher due to our inclusion of all PAH therapies (e.g., epoprostenol, iloprost, treprostinil) and our expenditures being in 2011 dollars. It is interesting to note that the majority of our patients with PAH had not received a PAH therapy; thus, possibly restraining our cohort’s total expenditures.
Overall, expenditures for our patients with PAH were driven by ‘other’ expenditures. Other expenditures included items such as ambulance transportation, radiologic assessments, laboratory measurements, and durable medical equipment. Conversely for patients who received a PAH medication, expenditures were driven by PAH medications. Our findings supplement findings from the studies by Wilkens et al.Citation10 and Angalakuditi et al.Citation9 that suggested the economic burden of clinical PAH management was driven primarily by therapy utilization.
Mortality was high overall amongst patients in our study. However, prevalent patients had a higher risk of death. Despite their younger age, the higher percentage of females, and lower comorbidity burden compared to incident cases, their increased risk may have been related to their having had PAH for a longer time at study start. As would be expected, patients who died during our 3-year follow-up had ∼3-fold higher 90-day, end-of-study expenditures than patients who survived the entire follow-up. These findings suggest that end-of-life care for patients with PAH can add an extra financial burden on healthcare payers.
While WHO Group 1 patients are commonly prescribed PAH medications and these medications can contribute the most to overall expenditures in patients with PAH, we found that total expenditures were equivalent across WHO GroupsCitation17. However; we found no statistical differences in total PPPD expenditures despite wide variation in expenditures across WHO groups. The most likely reason for the lack of statistical significance is the limited power we had due to the individual groups’ small sample sizes.
We were able to distinguish prevalent from incident cases and collect and analyze expenditures across the spectrum of health services, but our study is not without limitations. Our sample size was confined to objectively confirmed PAH cases, and this may have limited our ability to detect statistically significant differences in expenditures between groups. Our expenditures for clinical services and medications were based on a single health plan. The expenditures we report may not be representative of other plans or settings. However, evidence to confirm or refute this is not readily available. We were unable to quantify and include patient out-of-pocket expenses (e.g., co-payment, co-insurance) in our analysis. The likelihood that these expenditures would substantially alter our findings is low, as patient contributions to direct medical costs tend to be a minor component of total expenditures. We did not identify PAH-related expenditures since our patients, typically, had multiple chronic conditions and PAH-related expenditures are not distinguished readily from expenditures for the clinical management of concomitant chronic diseases. Finally, we were not able to match patients between groups based upon baseline characteristics (e.g., age or gender) due to the small number of patients enrolled; however, this is typical of studies of patients with PAH given its low penetrance in the general population.
Conclusions
Findings from our investigation suggest that clinical management of patients with PAH, regardless of incident or prevalent case status, represents a substantial financial burden to health insurers. With rapidly increasing healthcare costs, future clinical and administrative efforts should focus on optimizing care for patients with PAH by minimizing waste and strategically aligning expenses with areas of greatest evidence-based benefit. Future research could be conducted among a larger sample of patients to assess if differences in expenditures exist across WHO groups.
Transparency
Declaration of funding
The authors received no funding in preparation of this manuscript.
Declaration of financial/other relationships
SJ is a recipient of a $25,000 IOM Anniversary Fellowship Grant (2012–2014) and a $5000 ASHP Research and Education Foundation Residents Research Grant (2013–2014). Neither of these supported this work. CZ is an employee and has received sponsorship from Eli Lilly. TD, AB, and PS declare no conflicts of interest. CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
References
- Kirson NY, Birnbaum HG, Ivanova JI, et al. Prevalence of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension in the United States. Curr Med Res Opin 2011;27:1763-8
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-619
- Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142:448-56
- Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164-72
- Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002;40:780-8
- Falk JA, Philip KJ, Schwarz ER. The emergence of oral tadalafil as a once-daily treatment for pulmonary arterial hypertension. Vasc Health Risk Manag 2010;6:273-80
- Hrometz SL, Shields KM. Role of ambrisentan in the management of pulmonary hypertension. Ann Pharmacother 2008;42:1653-9
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376-87
- Angalakuditi M, Edgell E, Beardsworth A, et al. Treatment patterns and resource utilization and costs among patients with pulmonary arterial hypertension in the United States. J Med Econ 2010;13:393-402
- Wilkens H, Grimminger F, Hoeper M, et al. Burden of pulmonary arterial hypertension in Germany. Respir Med J 2010;104:902-10
- Copher R, Cerulli A, Watkins A, et al. Treatment patterns and healthcare system burden of managed care patients with suspected pulmonary arterial hypertension in the United States. J Med Econ 2012;15:947-55
- Said Q, Martin BC, Joish VN, et al. The cost to managed care of managing pulmonary hypertension. J Med Econ 2012;15:500-8
- Hoyle M, Anderson R. Whose costs and benefits? Why economic evaluations should simulate both prevalent and all future incident patient cohorts. Med Decis Making 2010;30:426-37
- Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915-20
- Clark DO, Von Korff M, Saunders K, et al. A chronic disease score with empirically derived weights. Med Care 1995;33:783-95
- Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol 1992;45:197-203
- Ventetuolo CE, Klinger JR. WHO Group 1 pulmonary arterial hypertension: current and investigative therapies. Prog Cardiovasc Dis 2012;55:89-103