Abstract
Objective:
The BFI (Bowel Function Index) is a 3-item questionnaire for assessing opioid-induced constipation (OIC). The aim of this study was to contribute to the validation of the psychometric properties of the BFI by confirming a constipation threshold, and through correlation with other validated tools: KESS (Knowles Eccersley Scott Symptom) score and generic (12-Item Short Form Health Survey, SF-12) and specific (Patient Assessment of Constipation–Quality of Life, PAC-QoL) quality-of-life scores.
† BFI Copyright 2002, Mundipharma GmbH, City, Country; BFI is subject of European Patent Application Publication No. EP 1,860,988 and corresponding patents and applications in other countries.
* PAC-QOL, Janssen Global Services, LLC, City, State, Country, USA.
Methods:
A survey on opioid-requiring cancer-patients was carried out in France. A questionnaire was filled out for all patients that recorded their demographic characteristics, an assessment of their constipation using BFI and KESS scores, and included a self-assessment of quality-of-life using PAC-QoL and SF-12. Correlation of BFI with KESS, PAC-QoL, and SF-12 was investigated.
Results:
Five hundred and twenty patients participated in the entire data collection with no loss. BFI was shown to be statistically correlated (r = 0.571; p < 0.0001) with the KESS score and matches up with PAC-QoL and to a lesser extent with the SF-12 generic quality-of-life questionnaire. A BFI threshold of 27–29 to discriminate constipated from non-constipated patients was confirmed.
Key limitations:
This cross-sectional study in a selected population of cancer pain patients has validated the psychometric properties of the BFI. Further confirmation of the validity of the BFI could be sought through the use of longitudinal studies, and larger populations, such as non-cancer pain patients treated with opioids.
Conclusion:
This study contributes to the validation of the psychometric properties of the BFI. It confirms the BFI as an easy-to-use tool to assess constipation and its impact on quality-of-life in chronic pain patients.
Introduction
Opioid-induced constipation (OIC) is an extremely frequent, persistent, and unpleasant side-effect endured by cancer patients who are obliged to take opioid pain relieversCitation1. Constipation is considered to greatly alter patients’ quality-of-life and is sometimes held to be an even greater source of discomfort than the pain of the underlying conditionCitation2. OIC also impacts on opioid analgesic effectiveness because constipation is painful, and this frequently leads to modifications in opioid analgesia prescriptions and intakeCitation3. A study of 2430 patients, who had been taking opioids for over 6 months for chronic pain, has shown that constipated patients, when compared with non-constipated patients, consulted their physician more frequently, felt less efficient at work, required sick leave more often, and were less inclined to pursue their daily activitiesCitation4. A questionnaire measuring the impact of constipation on quality-of-life has been validated: the Patient Assessment of Constipation Quality of Life (PAC-QoL)Citation5. This tool allows the discomfort caused by constipation to be measured in a symptom-related manner, in contrast with conventional quality-of-life questionnaires such as the 12-Item Short Form Health Survey (SF-12)Citation6.
The prevalence of constipation induced by opioids varies from one study to the next according to the population and the definitions used; this variation is compounded by the difficulty in determining the existence of constipationCitation7,Citation8. Two different studies report a constipation prevalence of 81% and 87%, respectively, in cancer patientsCitation3,Citation9. A meta-analysis of double-blind randomized controlled trials, involving patients treated with opioids for moderate-to-severe pain, shows that 41% of them suffered from constipationCitation10.
The difficulty for the physician lies in determining whether constipation exists or not, especially in patients taking strong opioids. The feeling of constipation is caused both by subjective criteria, such as ease of defecation or feeling of incomplete evacuation, and by objective data, such as the consistency and number of stools.
An easy-to-use questionnaire which allows a clear assessment of constipation would be useful for clinical studies and would significantly contribute to optimizing pain treatment in opioid-requiring patients in daily medical care.
The KESS score (Knowles Eccersley Scott Symptom Score)Citation11,Citation12 is considered to be a standard score that allows discrimination between constipated and non-constipated individuals with a cut-off score of 9, but its length restricts use on a daily basis. An alternative score based on three questions only, the Bowel Function Index (BFI), was recently defined by Rentz et al.Citation13,Citation14 for evaluating the constipation caused by opioids over the past 7 days, using a numerical rating scale (NRS; 0–100). The psychometric properties of this index have been investigated in two studiesCitation13,Citation14, the most recent of which confirmed the external validity of the BFI for the symptoms reported using the 12-item PAC-SYM (Patient Assessment of Constipation Symptoms Questionnaire). The reference range for constipation for use with the BFI has recently been defined as 0–28.8 (in 95% of non-constipated patients with chronic pain)Citation15.
The aim of this article is to contribute to validating the psychometric properties of the BFI (using the data collected by an observational study of cancer patients in France who were taking strong opioids) by confirming a constipation threshold, and by investigating the correlation of the BFI with physicians’ subjective assessment of constipation and with validated tools: KESS score, and generic (SF-12) and specific (PAC-QoL) quality-of-life scores.
Methods
Data used to investigate the BFI scale were derived from an observational, cross-sectional, multi-centre study, the DYONISOS (DYsfonctiON Intestinales induiteS par les OpioïdeS forts) study, which took place from June 2009–June 2010 in 77 centres in France. The study centres involved were public or private hospitals and day care centres of oncologists, pain specialists, or specialists in palliative care. Adult patients presenting with pain induced by cancer, treated with opioids, followed up in the hospital or as outpatients, who had given informed consent, and who were not already included in another epidemiological study or clinical trial examining opioid effects or constipation treatment were eligible to be included into the study. The study comprised a two-part survey consisting of a patient self-administered questionnaire and a questionnaire to be filled out by the physician.
The questionnaire was filled out for all patients treated with opioids. The questionnaire recorded the patients’ demographic characteristics (age, gender, socio-economic status), their cancer (location, histology, metastases) and its treatment (surgery, chemotherapy, radiotherapy, palliative care), and their opioid treatment (duration, type, dosage). The physician also recorded on the form any bowel dysfunction (according to the physician’s subjective assessment), the patients’ symptoms, the assessment of constipation according to the KESS score and BFI score, and the previous prescription of preventive treatment using laxatives or opioid titration. Patients were asked to evaluate the impact of their bowel dysfunction on their quality-of-life using PAC-QoL and SF-12 self-questionnaires.
Other data on prevalence of bowel dysfunction and its treatment were collected and are published elsewhereCitation16.
Evaluation criteria
The main criterion used to assess constipation in this study was the BFI. The reference criteria for external validation consisted of the KESS constipation questionnaire, the specific quality-of-life scale (PAC-QoL), and the SF-12 generic quality-of-life scale.
The physician asked each patient to respond to the questions used to determine the BFI scoreCitation14. The BFI calculates constipation over the past 7 days using a NRS (0–100). The index is the average value of three items, as follows:
Ease of defecation (0 = easy/no difficulty–100 = severe difficulty);
Feeling of incomplete bowel evacuation (0 = not at all–100 = very strong); and
Personal judgement of the patient regarding constipation (0 = not at all–100 = very strong).
An index of over 28.8 indicates the existence of constipationCitation15.
The KESS constipation questionnaire is composed of 11 questions delivering scores between 0–39. A score of 9 or over indicates the existence of constipationCitation11.
The PAC-QoL questionnaire specifically examines quality-of-life altered by constipation. This questionnaire is composed of 28 questions, from which total impact of constipation on patient’s quality-of-life is calculated (0 = no impact, 4 = great impact). It also includes four other scores specifically determining how constipation affects worry, physical repercussions, social life, and patient satisfactionCitation5. The SF-12 questionnaire is a generic quality-of-life scaleCitation6. It is composed of 12 questions for calculating scores between 0–100 (0 = poor quality-of-life and 100 = excellent quality-of-life), for eight different aspects (physical function, role physical, bodily pain, general health, vitality, social function, role emotional, and mental health). These scores are also grouped into two overall dimensions: physical state and mental state.
Patient constipation was evaluated by the physician (four items: not at all, mild-to-moderate, very, extremely constipated).
Statistical analysis
For external validation of the BFI, a constipation threshold using a ROC curve (Receiver Operator Characteristics) analysis based on the KESS score (constipation exists if the score ≥9) was identified. Correlations between the BFI score and the KESS score were analysed. BFI sensitivity and specificity were calculated, taking the KESS score into account. The same analyses were done for physician’s subjective assessment of constipation.
Thereafter, and for further external validation of the BFI, the BFI score and the PAC-QoL and SF-12 quality-of-life scores were correlated. Similar analyses were undertaken to assess the correlations between the KESS score and these quality-of-life questionnaires. All statistical analyses were made using SAS version 9.2 software.
The sample was calculated based on the number required to determine the prevalence of constipation in cancer patients who are suffering pain and taking strong opioids. Data published in the literature show great variations from one study to the next, between 23%Citation8 and 87%Citation9.
Assuming a hypothesis of prevalence of constipation of 40% in patients, to describe this frequency with a 95% confidence interval, with an accuracy of 4% and a significance level of 0.05, 576 patients were required.
Results
Patient characteristics
Five hundred and twenty patients fulfilling the inclusion criteria were recruited in 77 study centres and participated in the entire data collection with no loss. Patient characteristics and information on underlying cancer disease is summarized in . Strong opioid treatment had been initiated on average 4.4 (SD = 6.7) months before study inclusion and consisted, in 77.4% of cases, of a single strong opioid (oxycodone 32.4%, morphine 30.9%, or fentanyl 13.7%) and, in 22.6% of cases, of a combination of two strong opioids which were essentially fentanyl and morphine. Before the visit, constipation and bowel dysfunction induced by opioids had led to laxative intake by 84.7% of patients. BFI values ranged from 0.0–100.0. Mean BFI values were 8.5 (SD = 19.3) in patients that the physicians subjectively assessed as non-constipated (n = 73) and 44.8 (SD = 27.0) in those considered to be constipated (n = 438) according to the physician’s subjective assessment. KESS values ranged from 0.0–33.0 and mean values were 4.5 (SD = 3.5) in patients that the physicians subjectively assessed as non-constipated and 12.5 (SD = 6.5) in those considered to be constipated, according to the physician’s subjective assessment.
Table 1. Characteristics of the study population (n = 520).
Identification of BFI constipation threshold
The BFI threshold value for indicating constipation in patients (0 = no constipation, 100 = severe constipation) was established using the ROC curve (). This method identifies the threshold where BFI sensitivity and specificity are at their peak compared to the KESS score (0–8 no constipation/9–39 constipation), which is considered here as the reference criterion.
Figure 1. ROC curve for choice of optimum threshold for BFI score (constipated patients according to the KESS score). BFI scores between 27–29 could be identified as constipation thresholds. The dot in the ROC curve that is closest to the top left-hand corner indicates the optimum threshold.

BFI scores between 27–29 show strictly comparable results in terms of sensitivity and specificity, and a BFI of 28.8 as described by Ueberall et al.Citation15 could be confirmed as the constipation threshold. Patients are considered to be constipated with a BFI score >28.8, a sensitivity score of 78.8% compared to the KESS score, a specificity score of 74.4%, a false-positive rate of 25.6%, and a false-negative rate of 21.2%.
With a BFI of over 28.8, prevalence of constipation observed in the study population reaches 58.5% (54.2–62.7).
External validation of BFI using KESS score and physician’s subjective assessment of constipation
There is a significant correlation between the BFI and the KESS score (r = 0.571; p < 0.0001).
The BFI is also correlated with severity of constipation, as subjectively assessed by the physician (r = 0.704; p < 0.0001). Taking the physicians’ clinical estimation of their patients’ constipation, a BFI at the 27, 28, 29 threshold shows a sensitivity of 66.4% and a specificity of 89.0%, a false-positive rate of 11.0% and a false-negative rate of 33.6%. When the physicians made a clinical assessment of severe or very severe constipation in their patients, the BFI showed a sensitivity of 92.6%, a specificity of 89.0%, a false-positive rate of 11.0%, and a false-negative rate of 7.4%.
External validation of BFI using quality-of-life scores
This second phase of BFI external validation is based on the hypothesis that, if the BFI score is considered to define constipation, this score will be correlated with an altered quality-of-life, particularly when caused by constipation (PAC-QoL). Therefore, that quality-of-life in constipated patients (classified as constipated according to their BFI score) will be significantly lower than in non-constipated patients.
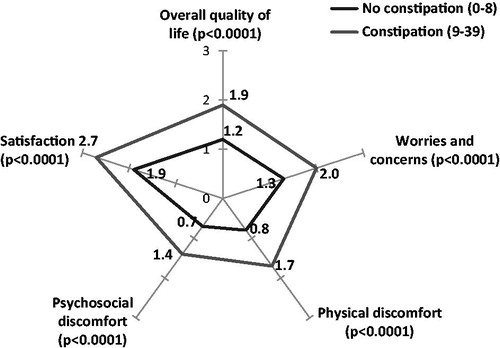
The impact of constipation on patients’ quality-of-life (evaluated by the PAC-QoL) was significantly greater in patients considered to be constipated according to the BFI, both for overall quality-of-life (1.2 (SD = 0.6) vs 1.9 (SD = 0.7), p < 0.0001) and for each of the specific dimensions such as: worries and concerns, physical discomfort, psychosocial discomfort, and satisfaction (p < 0.0001 for all) ().
Figure 2. Quality-of-life in constipated and non-constipated patients (BFI) compared using PAC-QoL. The scale for each axis ranges from 0 = no impact to 4 = high impact.
The results show a significant correlation between the BFI and impact of constipation on overall quality-of-life measured by the PAC-QoL (r = 0.537; p < 0.0001). This correlation also exists for the four dimensions contributing to the PAC-QoL score: Worries and concerns (r = 0.447; p < 0.0001), Physical discomfort (r = 0.455; p < 0.0001), Psychosocial discomfort (r = 0.421; p < 0.0001), and Satisfaction (r = 0.526; p < 0.0001) ().
Table 2. Comparison of correlation coefficients for the KESS score and the BFI, according to scores for different dimensions of quality-of-life.
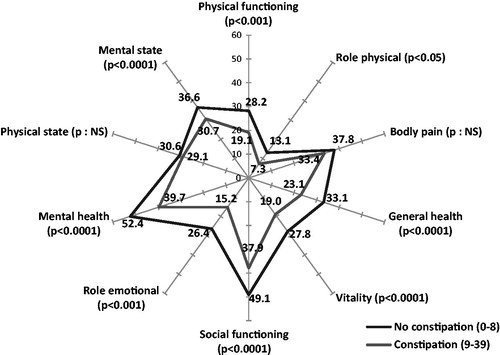
Patients’ quality-of-life evaluated by the SF-12 generic questionnaire was significantly poorer for patients who were classified as constipated according to the BFI, compared with non-constipated patients ().
Figure 3. Quality-of-life in constipated and non-constipated patients (BFI) compared using SF-12. The scale for each axis ranges from 0 = poor quality-of-life to 100 = excellent quality-of-life.
Correlations with the SF-12 quality-of-life score and its various aspects are all significant (), but the correlation coefficients are low at ∼−0.1 to −0.2.
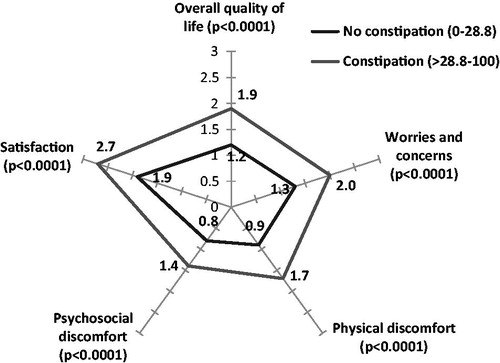
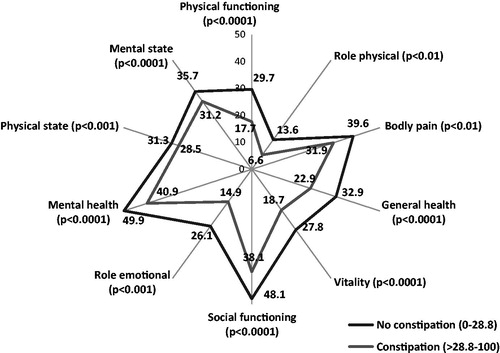
Similar analyses were undertaken on the correlations between the KESS score, the PAC-QoL, and the SF-12. The correlation coefficients match up well with those observed using BFI ().
Similarly, the differences observed between the mean values for the various dimensions of the PAC-QoL and the SF-12, for constipated and non-constipated patients as defined by the KESS score, are comparable to those observed for constipated and non-constipated patients using the BFI ( and ).
Discussion
Constipation has a significant impact on quality-of-life in patients requiring to take strong opioidsCitation2,Citation4. There is still a need for validated easy-to-use tools to assess OIC in clinical studies and in daily practice, in order to help in optimizing pain and constipation treatmentCitation17.
In our study, BFI values from 27–29 show strictly comparable results using the ROC curve, which is confirmation that these are threshold values that can be used to discriminate between constipated and non-constipated patients. This supports the results of the Ueberall et al.Citation15 study, which revealed a value of 28.8 as the upper limit of the confidence interval into which 95% of a reference population fell, composed of non-constipated patients suffering from chronic pain and taking Step I and II analgesia.
The study also aims to provide further validation of the psychometric properties of the BFI and its reproducibility, validity, and sensitivity to change have been demonstrated for patients suffering from opioid-induced constipationCitation13,Citation14.
The results show that the BFI is correlated with other means of constipation assessment. The BFI is statistically correlated with the KESS score. The KESS score is a well-recognized validated 11-item score ranging from 0 (no symptoms) to 39 (high symptom severity) that was originally developed to diagnose constipation and to discriminate among pathophysiological sub-groups. It can discriminate between constipated and non-constipated individuals with a cut-off score of 9.
In the study, the BFI was also shown to be correlated to the physician’s subjective assessment of constipation, underlining the ability of the BFI to assess constipation in real-life situations. The strong correlation is yet another proof of the external validity of the BFI score, and reflects the high clinical relevance of the BFI.
Correlation studies show that not only are BFI and KESS scores correlated, but they also match up with the PAC-QoL specific quality-of-life questionnaire, and to a lesser extent (but significantly) with the SF-12 generic quality-of-life questionnaire, even though the latter is not a constipation-specific questionnaire. However, this lower correlation with SF-12 was expected for patients suffering from cancer, which is the primary cause of their altered quality-of-life. With its significant correlation with KESS as well as with the quality-of-life scores, the BFI is proven to allow an assessment of objectively defined constipation, whilst also considering the patients’ subjective burden. Many patients do not suffer from an abnormal stool frequency or consistency but experience other symptoms such as straining and bloatingCitation3 which are a burden for the patients and are experienced as impairments in quality-of-life. A score that also records this aspect of quality-of-life is able to assess constipation in all its dimensions.
The BFI not only allows constipation and severity to be assessed, but allows the physician to monitor the effects of his/her therapeutic decisions regarding choice and dosage of strong opioids, and the associated decisions that aim to reduce the adverse effects of opioids on gut function. A difference more than or equal to 12 points, achieved by an intervention, is considered to be clinically significant, while a variation under 7.5 is unlikely to be clinically pertinentCitation14.
The different analyses undertaken in this study support the external validity of the psychometric properties of the BFI in relation to the KESS score, the physician’s subjective assessment of constipation, and the specific and generic quality-of-life scores. This study also confirms the existence of a constipation threshold at ∼27–29, with which all studies undertaken so far with the BFI concur. The advantage of the BFI is that it has the appropriate complexity to cover symptoms of constipation and their impact on quality-of-life, but involves only three questions, which makes it more practical than the KESS score to use on a daily basis, and means that assessment is simple and quick.
Transparency
Declaration of funding
This study was designed and financed by Mundipharma SAS France, and conducted under the sponsorship of Mundipharma SAS France.
Declaration of financial/other interests
LA and SP were principal co-ordinators of this study and have received fees from Mundipharma: LA was a short-term board member for Mundipharma SAS France and SP was a board member for Mundipharma SAS. NB participated as an investigator in this study. NB has received fees as a speaker for a Mundipharma symposium. BC was an employee of Mundipharma SAS France. CC is an employee of Mundipharma SAS France. All authors had full access to the data and were involved in the development and writing of the manuscript. All authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; critically reviewed the manuscript and have provided final approval of the version to be published. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgements
The authors thank all those involved in conducting this study. Editorial support was provided by Catharina Buschmann-Kramm of Mundipharma Research GmbH & Co. KG.
Notes
† BFI Copyright 2002, Mundipharma GmbH, City, Country; BFI is subject of European Patent Application Publication No. EP 1,860,988 and corresponding patents and applications in other countries.
* PAC-QOL, Janssen Global Services, LLC, City, State, Country, USA.
References
- World Health Organization. Cancer pain relief. Geneva: WHO, 1996
- Fallon MT. Constipation in cancer patients: prevalence, pathogenesis, and cost-related issues. Eur J Pain 1999;3:3-7
- Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35-42
- Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag 2009;5:137-44
- Marquis P, De La Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540-51
- Ware JEJK, M. Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33
- McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control 2004;11:3-9
- Meuser T, Pietruck C, Radbruch L, et al. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001;93:247-57
- Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliative Med 1998;12:375-82
- De Schepper HU, Cremonini F, Park MI, et al. Opioids and the gut: pharmacology and current clinical experience. Neurogastroenterol Motil 2004;16:383-94
- Knowles CH, Eccersley AJ, Scott SM, et al. Linear discriminant analysis of symptoms in patients with chronic constipation: validation of a new scoring system (KESS). Dis Colon Rectum 2000;43:1419-26
- Knowles CH, Scott SM, Legg PE, et al. Level of classification performance of KESS (symptom scoring system for constipation) validated in a prospective series of 105 patients. Dis Colon Rectum 2002;45:842-3
- Rentz AM, van Hanswijck de Jonge P, Leyendecker P, et al. Observational, nonintervention, multicenter study for validation of the Bowel Function Index for constipation in European countries. Curr Med Res Opin 2011;27:35-44
- Rentz AM, Yu R, Muller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ 2009;12:371-83
- Ueberall MA, Müller-Lissner S, Buschmann-Kramm C, et al. The Bowel Function Index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. The Journal of International Medical Research 2011;39:41-50
- Abramowitz L, Béziaud N, Labreze L, et al. Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: a cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J Med Econ 2013;16:1423-33
- McCrea GL, Miaskowski C, Stotts NA, et al. Review article: self-report measures to evaluate constipation. Aliment Pharm Therap 2008;27:638-48