Abstract
Objective:
To evaluate the annual cost-utility of insulin degludec compared with glargine in patients with: type 1 diabetes (T1D), type 2 diabetes receiving basal-only therapy (T2D-BOT), and type 2 diabetes receiving basal-bolus therapy (T2B-BB) in Sweden.
Methods:
A cost-utility model was programmed in Microsoft Excel to evaluate clinical and economic outcomes. The clinical trials were designed as treat-to-target, with insulin doses adjusted in order to achieve similar glycemic control between treatments, thus long-term modeling is not meaningful. Basal and bolus insulin doses, incidence of hypoglycemic events, frequency of self-monitoring of blood glucose, and possibility for flexibility in timing of dose administration were specified for each insulin in three diabetes populations, based on data collected in Swedish patients with diabetes and a meta-analysis of clinical trials with degludec. Using these characteristics, the model estimated costs from a societal perspective and quality-adjusted life years (QALYs) in the two scenarios.
Results:
Use of degludec was associated with a QALY gain compared with glargine in T1D (0.31 vs 0.26 QALYs), T2D-BOT (0.76 vs 0.69 QALYs), and T2D-BB (0.56 vs 0.47 QALYs), driven by reduced incidence of hypoglycemia and possibility for flexibility around timing of dose administration. Therapy regimens containing degludec were associated with increased costs compared to glargine-based regimens, driven by the increased pharmacy cost of basal insulin, but partially offset by other cost savings. Based on estimates of cost and clinical outcomes, degludec was associated with incremental cost-effectiveness ratios of SEK 19,766 per QALY gained, SEK 10,082 per QALY gained, and SEK 36,074 per QALY gained in T1D, T2-BOT, and T2-BB, respectively.
Limitations:
The hypoglycemic event rates in the base case analysis were derived from a questionnaire-based study that relied on patient interpretation and recall of hypoglycemic symptoms. The relative rates of hypoglycemia with degludec compared to glargine were derived from a meta-analysis of phase III trials, which may not reflect the relative rates observed in real-world clinical practice. Both of these key limitations were explored in one-way sensitivity analyses.
Conclusions:
Based on reduced incidence of hypoglycemia and possibility for flexibility around timing of dose administration, use of degludec is likely to be cost-effective compared to glargine from a societal perspective in T1D, T2-BOT, and T2-BB in Sweden over a 1-year time horizon.
Introduction
Diabetes mellitus represents a significant challenge to healthcare service providers in Sweden, with a national prevalence of almost 6%Citation1. Whilst type 2 diabetes (T2D) comprises the majority of cases, the incidence of type 1 diabetes (T1D) in Sweden is 43.1 per 100,000, the second highest in the world behind FinlandCitation1,Citation2. The micro- and macrovascular complications associated with T1D and T2D result in the disease posing not only a significant clinical burden to patients with diabetes, but also a significant economic burden to healthcare payers. The estimated healthcare spending in Sweden as a result of diabetes mellitus and its related complications was estimated to be USD 3.8 billion in 2010, ∼8% of total healthcare expenditure, and this is expected to rise to USD 4.4 billion by 2030Citation3.
Maintaining tight glycemic control is the key clinical aim in reducing the incidence of diabetes-related complications in both T1D and T2D, and thereby controlling the clinical and economic burdenCitation4–6. In patients with T2D, oral agents form the first line therapy options for achieving normoglycemia for most patients. However, as the disease progresses the majority of patients require some form of insulin, either in addition to or replacing the preceding treatment. On the other hand, in patients with T1D, exogenous insulin replacement therapy is the only treatment optionCitation7,Citation8. There is a growing evidence base that, with careful titration and dose adjustment, all insulins can be used to achieve good glycemic control, and that alternative formulations can be considered equivalent in this regardCitation9–11. However, hypoglycemia (low blood sugar) remains a significant barrier to insulin use, as patients are reluctant to titrate to optimal doses due to the increased risk of potential unpleasant symptoms of hypoglycemia (sweating, pounding heart, confusion, headaches, and in severe cases unconsciousness, coma, or even death)Citation12. Different basal insulins are associated with different hypoglycemia risks, chiefly as a result of variability in absorption and duration profiles. Therefore, the clinical trials of the ultra-long acting insulin degludec have used a treat-to-target approach, with the insulin doses adjusted so as to achieve equivalent glycemic control with degludec and the comparator (i.e., insulin glargine), and focusing on hypoglycemia outcomes.
After subcutaneous injection, insulin degludec forms soluble, stable multihexamers which slowly release insulin monomers into the bloodstreamCitation13. This mechanism allows for insulin degludec to have an ultra-long duration of action and a flat and stable glucose lowering profile with less variability in its day-to-day action than insulin glargineCitation14,Citation15. Since degludec is able to maintain a peakless insulin concentration for more than 42 h, patients have flexibility in when basal insulin doses are administered. As with any other insulin, a regular injection time is recommended; however, in situations where the patient needs to reschedule the dose, this can be done without compromising compliance or involving additional advice from healthcare professionalsCitation16.
Economic evaluation of new healthcare interventions is becoming increasingly important, as healthcare providers aim to maximize health outcomes with restricted budgets. The Dental and Pharmaceutical Benefits Agency (Tandvårds- och läkemedelsförmånsverket [TLV]) has recently confirmed that degludec will be reimbursed in Sweden for both patients with T1D and T2D, with a limitation in T2D to patients not achieving treatment targets due to hypoglycemiaCitation17. Whilst the majority of cost-utility analyses have evaluated the long-term impact of diabetes interventions, the equivalent glycemic control that can be achieved with alternative insulin products renders this approach unnecessary, as no outcomes measured in the degludec trial program would be expected to drive long-term differences between treatment arms. The present health economic model therefore focused on parameters which will be relevant already from the first year (and remain relevant in steady state) such as insulin doses, hypoglycemia rates, health-related quality-of-life, use of needles, and use of self-monitoring of blood glucose (SMBG) test strips. The aim of the present study was to evaluate the relative clinical and cost outcomes associated with use of insulin degludec and insulin glargine in therapy regimens for patients with type 1 diabetes (T1D), patients with type 2 diabetes receiving basal-only therapy (T2D-BOT), and patients with type 2 diabetes receiving basal-bolus therapy (T2D-BB) over an annual (steady state) time horizon in Sweden.
Methods
Model structure
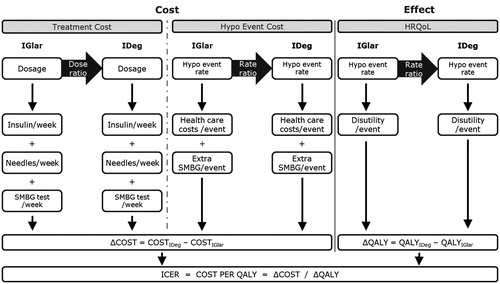
A cost-utility model was programmed in Microsoft Excel to evaluate the clinical and economic outcomes associated with use of degludec and glargine in T1D, T2D-BOT, and T2D-BB over an annual steady state time horizon. The basal and bolus insulin doses, incidence of non-severe and severe hypoglycemic events, frequency of SMBG, and timing of dose administration were specified for each insulin therapy in the three diabetes populations. Based on these characteristics, the model estimated the total costs associated with insulin use, SMBG, needles, hypoglycaemia, and lost productivity (optional), and the estimated change in quality-of-life (in terms of quality-adjusted life years [QALYs]) in the two scenarios. No discounting was applied as the analysis did not make predictions beyond a 1-year time horizon. Only statistically different parameters were used to minimize modeling uncertainty. A schematic of the model structure is shown in .
Rates of hypoglycemia
The rates of non-severe daytime, non-severe nocturnal, and severe hypoglycemia for patients receiving insulin glargine were taken from the 603 patients in Sweden enrolled in a multi-national study examining the frequency and consequences of hypoglycemia in patients with diabetesCitation18. This study enrolled a diverse range of patients with T1D and T2D, and weekly questionnaires were used to evaluate the frequency of hypoglycemic events, with data presented separately depending on therapy regimen (T1D, T2D-BOT, and T2D-BB) (). The study had very few exclusion criteria, requiring only an email address and ability to understand the survey. The study therefore captured a broad and representative sample of patients with diabetes in Sweden.
Table 1. Incidence of hypoglycemia in patients receiving insulin glargine and the rate ratio applied in the degludec arm.
A hypoglycemia meta-analysis of studies comparing degludec with glargine was conducted as part of the regulatory submission for degludec to the Food and Drug Administration (FDA). The meta-analysis received feedback from the FDA at two stages of the process, with adaptation of the protocol and methods as appropriate. This meta-analysis identified rate ratios to be applied in the degludec armCitation19. Rate ratios were calculated separately for T1D, T2D-BOT, and T2D-BB. A regression model including trial, treatment, previous therapy, gender, and region as fixed effects and age as a continuous covariate was used to calculate the rate ratios in the two arms of the study (). Hypoglycemia was considered in three categories: non-severe daytime, non-severe nocturnal, and severe. Whilst this approach differed from the pre-specified approach in the meta-analysis, this was required to prevent double counting of hypoglycemic events. It was assumed that the mortality rate following hypoglycemia was 0%.
Insulin dosing
Mean daily doses of basal and bolus insulin in treatment regimens of T1D, T2D-BOT, and T2D-BB were taken from a meta-analysis of studies comparing degludec with glargine. The seven included trials were all randomized, controlled, open-label, parallel-group studies comparing degludec and glargine in patients with type 1 and type 2 diabetes. Trials comparing insulin degludec with alternative comparators were excluded.
Calculated mean doses were corrected for factors that may influence insulin requirements, including body weight, age, and gender. Degludec was associated with statistically significant lower insulin doses than glargine in T1D and T2D-BOT, but higher basal doses in T2D-BB. Additionally, for T1D patients, a statistically significant lower bolus insulin dose was observed ().
Table 2. Basal and bolus insulin use in patients receiving degludec and glargine.
Self-monitoring of blood glucose testing resource use
It is recommended that patients receiving glargine as basal insulin conduct a daily fasting blood glucose test to titrate and monitor dosing appropriatelyCitation20. Therefore, basal insulin glargine use was associated with seven SMBG tests per week. Insulin degludec has a longer plasma half-life than glargine, resulting in less day-to-day glycemic variability, and therefore SMBG can be carried out less frequently. During the initial titration period it is recommended that patients with T2D receiving insulin degludec conduct twice weekly SMBG to enable appropriate dose titration. At steady state, SMBG testing can be reduced to once weeklyCitation21. However, once daily testing is still recommended in patients with type 1 diabetes receiving degludec. It was assumed that receiving bolus insulin was associated with three SMBG tests per day in order to titrate doses appropriately, based on current guidelinesCitation8.
Based on these recommendations, the modeling analysis assumed that all T1D patients carried out 28 SMBG tests per week, T2D-BOT patients treated with degludec carried out one SMBG per week compared to seven per week when receiving glargine, and T2D-BB patients receiving degludec carried out 22 SMBG tests per week compared to 28 per week when receiving glargine.
As well as carrying out SMBG tests as part of standard care, SMBG tests are also carried out following hypoglycemic events. The survey used to inform the frequency of hypoglycemia also collected data on frequency of SMBG tests following hypoglycemic eventsCitation22. It was found that, on average, a hypoglycemic event was associated with 2.5, 1.8, and 2.8 extra SMBG tests in T1D, T2D-BOT, and T2D-BB, respectively, in Sweden.
Costs
In the base case, costs (2012 SEK) were accounted from a societal perspective, capturing the costs to a healthcare payer and the impact of lost workplace productivity as well as the direct costs of medical care. Insulin costs were based on the pharmacies’ selling price (PSP) in October 2012 (). Needle costs (based on one injection per administration of insulin), SMBG test strip costs, and SMBG lancet costs were assumed to be the minimum PSP as listed on the TLV website (accessed December 7, 2012).
Table 3. Unit costs used in the analysis.
The direct medical cost associated with severe hypoglycemia was taken from a costing study carried out in Sweden in 2006Citation23. The study used a ‘bottom-up’ approach to costing hypoglycemic events and captured the alternative management strategies that could be used in treatment of severe hypoglycemia, weighted by the frequency of their use: general practitioner (GP) visit, GP home visit, and emergency department visit (with or without ambulance transportation). The cost was inflated to a 2012 value, using inflation rates published by Statistics Sweden (Statistiska centralbyrån, [SCB])Citation24. The cost of severe hypoglycemia was assumed to be the same, irrespective of diabetes type or therapy regimen.
Costs associated with non-severe hypoglycemia were calculated based on the resource use reported by Geelhoed-Duijvestijn et al.Citation22. This survey suggested that non-severe hypoglycemic events were rarely reported to GPs, and this reporting was lowest in patients with type 1 diabetes. From the survey data the mean cost per non-severe hypoglycemic event was calculated, depending on the treatment regimen received, assuming that, when medical attention was sought, half of contacts were with a nurse and half with a doctor ().
Table 4. Mean direct and indirect cost of hypoglycemic events.
The survey conducted by Geelhoed-Duijvestijn et al.Citation22 also collected data on worker absenteeism as a result of hypoglycemic events in the 46% of patients that were employed. It was found that T2D-BB patients had the greatest time absent from work as a result of a non-severe hypoglycemic event, whilst T1D patients showed the least absenteeism. There was no significant difference in absenteeism following a severe hypoglycemic event. To calculate productivity losses associated with hypoglycemia, a mean hourly wage of SEK 279 was calculated based on the mean wage for employees within the private sector ()Citation25,Citation26.
Utilities
Health-related quality-of-life data for the baseline diabetes state and the impact of hypoglycemia were taken from the Swedish respondents in a multinational survey examining the preferences of the general populationCitation27. Descriptions of health states were derived from a survey of 247 patients with diabetes, which were then valued by 1635 members of the general population in Sweden using the time trade-off method. The health-related quality-of-life decrement associated with each SMBG test was based on the Diabetes Glycaemic Education and Monitoring (DiGEM) study, where patients undergoing intensive SMBG reported worse health-related quality-of-life scores based on EQ-5D dataCitation28. To reflect the possibility for flexibility in timing of dose administration with insulin degludec, a disutility of 0.015 was applied in the glargine arm in each population evaluated to reflect the lack of flexibility, based on a time trade-off study carried out in Sweden, Canada, and the UKCitation29. The utilities used in the analysis are summarized in .
Table 5. Utilities and disutilities used in the modeling analysis.
Sensitivity analyses
To assess the sensitivity of model outcomes to changes in key input parameters, a series of one-way sensitivity analyses were performed. To investigate the importance of the difference in insulin dosing between the alternative therapies, basal and bolus (where appropriate) insulin doses were assumed to be equal in the degludec and glargine arms of the study. Insulin glargine is often administered twice daily rather than once daily and, therefore, a scenario was investigated where an extra needle was used each day in the glargine arm. Conservatively, it was assumed that twice daily administration would not be accompanied by an increased dose of glargine. The importance of SMBG was evaluated by setting SMBG use as equivalent in the degludec and glargine arms, and by removing the disutility associated with SMBG, in two separate analyses. The impact of hypoglycemia was investigated in three scenarios: one in which the cost of hypoglycemia was reduced by 20%, one in which the hypoglycemia rates were equal in both arms, and one in which the frequency of hypoglycemia was based on events confirmed by a blood glucose measurement of less than 3.1 mmol/LCitation18. A further analysis was conducted in which a mortality rate of 1.7% was applied following severe hypoglycemic events, based on a prospective, population-based study evaluating mortality arising from severe hypoglycemia that resulted in an emergency callCitation30. The importance of flexible dose timings to patients was evaluated by removing the disutility associated with lack of flexibility in the glargine arm. A scenario was explored where degludec was compared with neutral protamine Hagedorn (NPH) insulin, as this is a lower cost alternative basal insulin, but associated with a higher rate of hypoglycemia in T1D and T2D-BOT. Hypoglycemia rate ratios for NPH were based on an indirect comparison, using insulin glargine as a bridge from degludec to NPH. In T2D-BB, NPH was assumed not to lead to increased rates of hypoglycemia, based on a meta-analysis carried out for Canadian Agency for Drugs and Technologies in Health (CADTH) which identified no evidence pertinent to T2D-BBCitation31. A comparison with NPH was also conducted in patients that were not experiencing hypoglycemic events. An analysis was also conducted with the costs of lost productivity excluded, to evaluate cost-effectiveness from a healthcare payer perspective. Probabilistic sensitivity was conducted, in which the stochastic parameters were sampled with mean (and standard error) from 1000 runs of the model reported. From this data, cost-effectiveness scatterplots and cost-effectiveness acceptability curves were plotted in each of the analyzed diabetes populations.
Results
Base case results
Use of degludec was associated with increased mean quality-adjusted life years compared with glargine in T1D (0.306 QALYs vs 0.261 QALYs), T2D-BOT (0.764 QALYs vs 0.685 QALYs), and T2D-BB (0.560 QALYs vs 0.470 QALYs) over 1 year of treatment (). Improvements in health-related quality-of-life in all three populations were driven chiefly by reduced incidence of hypoglycemia in the degludec arm and possibility for flexibility around timing of dose administration. In the type 2 diabetes populations, improvements in quality-of-life were also driven by the reduced need for SMBG testing.
Table 6. Base case results.
Therapy regimens containing degludec were associated with increased costs compared to glargine-based regimens in all three populations, driven by the increased pharmacy cost of basal insulin. However, this was partially offset by cost savings made in other areas. In T1D, savings were made as a result of reduced basal and bolus insulin doses, reduced direct costs following non-severe nocturnal hypoglycemic events, and reduced productivity losses following non-severe nocturnal hypoglycemic events. In T2D-BOT, costs were partially recouped as a result of reduced basal insulin dose, reduced SMBG costs, reduced direct costs following non-severe nocturnal and severe hypoglycemic events, and reduced productivity losses following non-severe nocturnal and severe hypoglycemic events. In T2D-BB, cost savings were made as a result of reduced SMBG use, reduced non-severe daytime and non-severe nocturnal hypoglycaemia, and reduced productivity losses following hypoglycemia.
Based on these estimates of cost and clinical outcomes, degludec was associated with incremental cost effectiveness ratios (ICERs) of SEK 19,766 per QALY gained, SEK 10,082 per QALY gained, and SEK 36,074 per QALY gained in T1D, T2D-BOT, and T2D-BB, respectively. Based on the commonly quoted willingness-to-pay threshold of SEK 500,000 per QALY gained, degludec is likely to be cost-effective in Sweden in all three patient groups analyzed.
Sensitivity analyses
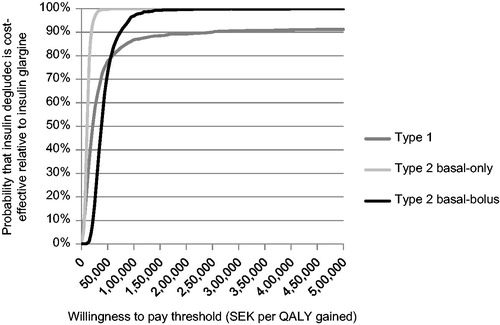
Across all three patient groups evaluated, cost-utility outcomes were highly sensitive to hypoglycemia rates (). When hypoglycemia rates in the degludec arm were assumed to be equal to the glargine arm, ICERs were increased by SEK 44,825 per QALY gained, SEK 11,400 per QALY gained, and SEK 61,921 per QALY gained compared to base case values in in T1D, T2D-BOT, and T2D-BB, respectively. However, ICERs remained below a willingness-to-pay threshold of SEK 500,000 per QALY gained, indicating that degludec is likely to be cost-effective even if no benefit in terms of reduced hypoglycemia is achieved.
Table 7. Results of sensitivity analyses.
In T2D-BB, cost-utility outcomes were highly sensitive to the use of NPH insulin, rather than glargine, but this was not the case in T1D and T2D-BOT. This was as a result of the differing hypoglycemic profiles of NPH in the three patient groups, in comparison to glargine. In T1D and T2D-BOT, NPH was associated with a significant increase in hypoglycemia, over glargine and degludec. Therefore, whilst costs were lower with NPH than degludec (or glargine), use of NPH was associated with poorer clinical outcomes, and therefore the ICER for degludec only varied slightly from the comparison with glargine. In T2D-BB, NPH is not associated with increased hypoglycemia rates compared to glargine, and therefore the incremental clinical benefit with degludec remained unchanged, but the increase in costs was larger than in the comparison with glargine. Therefore, degludec was associated with an ICER of SEK 86,117 per QALY gained compared with NPH, which remains below the commonly quoted cost-effectiveness threshold in Sweden. When degludec was compared with NPH in patients not experiencing hypoglycemia, ICERs were higher in all three patient populations, but remained below a willingness-to-pay threshold of SEK 500,000 per QALY gained.
Different SMBG use associated with degludec and glargine was a key driver of outcomes in both type 2 diabetes populations evaluated. When this difference was abolished, ICERs increased to SEK 36,600 per QALY gained and SEK 71,669 per QALY gained in T2D-BOT and T2D-BB, respectively. This was predominantly as a result of reduced cost savings through avoided SMBG tests, but also due to the abolition of health-related quality of benefit as a result of avoided testing.
Assuming that the dose required to achieve equivalent glycemic control, in terms of international units, was the same in the degludec and glargine arms resulted in notable increases of ICERs in T1D and T2D-BOT. This was not the case in T2D-BB, since degludec was associated with an increased dose in the base case analysis.
Plotting incremental cost and effectiveness results for the 1000 iterations on the cost-effectiveness plane found that the majority of points fell in the upper right quadrant in all three analyzed populations (). This indicates that use of degludec is likely to be more effective (in terms of quality-adjusted life expectancy) and more costly than glargine, over a 1-year time horizon. These scatterplots were then used to develop cost-effectiveness acceptability curves for T1D, T2D-BOT, and T2D-BB (). The analysis showed that, assuming a willingness-to-pay threshold of SEK 500,000 per QALY gained, there was a 91.2% probability that use of degludec would be cost-effective compared to glargine in T1D, and a 100% probability of being cost-effective in T2D-BOT and T2D-BB.
Figure 2. Cost-effectiveness scatterplot for degludec vs glargine in patients with type 1 diabetes, patients with type 2 diabetes receiving basal-only therapy, and patients with type 2 diabetes receiving basal-bolus therapy. Incremental costs are expressed in SEK and incremental effectiveness in QALYs. Each point represents a run of the model (with sampling from distributions) from a total of 1000 simulations. QALY, quality-adjusted life years; SEK, 2012 Swedish Krona.
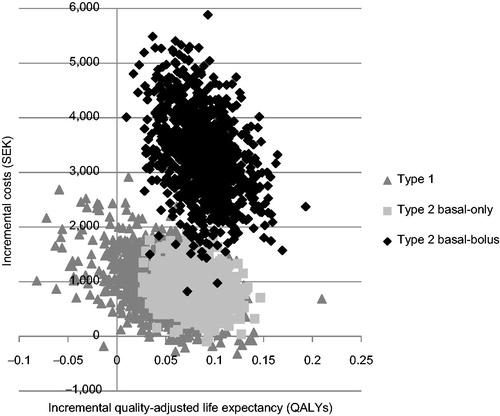
Figure 3. Cost-effectiveness acceptability curves for degludec vs glargine in patients with type 1 diabetes, patients with type 2 diabetes receiving basal-only therapy, and patients with type 2 diabetes receiving basal-bolus therapy. The curve represents the proportion of 1000 points (see ) associated with incremental cost-effectiveness ratios lower (and therefore considered cost-effective) than the range of willingness-to-pay thresholds described on the x-axis. QALY, quality-adjusted life years; SEK, 2012 Swedish Krona.
Discussion
Based on a simple, transparent, short-term cost-utility analysis, use of insulin degludec is highly likely to be cost-effective compared with insulin glargine from both a societal and healthcare payer perspective in T1D, T2D-BOT, and T2D-BB in Sweden over an annual (steady state) time horizon. Improvements in clinical outcomes were driven by reduced incidence of hypoglycemia, reduced frequency of SMBG testing, and the possibility for flexibility of timing of dose administration. Use of degludec was associated with increased costs, driven by the higher acquisition cost of degludec compared to glargine. However, this was partially offset by reduced expenditure as a result of fewer SMBG tests, reduced cost of treating hypoglycemia, and reduced productivity losses following hypoglycemia. Sensitivity analyses indicated that the conclusion that use of degludec is likely to be cost-effective compared to glargine was robust to changes in model parameters, with all calculated ICERs falling below a willingness-to-pay threshold of SEK 500,000 per QALY gained. The analysis represents one of the first cost-utility evaluations of degludec and indicates that use of the ultra-long-acting insulin is likely to be cost-effective in Sweden.
A key advantage of the present analysis is the simplicity and transparency of the model used to evaluate cost-utility. The majority of published cost-utility analyses of both type 1 and type 2 diabetes interventions have taken a long-term perspective, evaluating clinical and cost outcomes over patient lifetimesCitation32–35. A long-term modeling approach is consistent with the progression of diabetes, with diabetes-related complications in the future having a significant impact on quality-of-life and medical costs. Achieving glycemic control is the key treatment objective in minimizing the risk of complications over patient lifetimes, and there is growing evidence that, with appropriate dose titration, alternative insulin formulations can be used to achieve good glycemic controlCitation9–11. Therefore, the present analysis has evaluated cost-utility over an annual steady state time horizon, focusing on the day-to-day management of patients with diabetes following the initial titration period, through insulin and SMBG testing, and acute events such as hypoglycemia.
However, as with all modeling studies, the limitations of the study should be noted when putting the findings in context. The estimation of hypoglycemia rates in the glargine arm of the study came from a large-scale study of insulin-using patients with diabetes in SwedenCitation18. Whilst this represents a key source of real world country-specific data, the formulation of insulin used was not recorded in this study. Furthermore, the study methodology required patients to recall symptoms of hypoglycemia or a confirmed blood glucose measurement of <3.1 mmol/L over the previous week. While the study authors selected an optimum recall period based on data from a previous study, symptoms such as sweating, shaking, and headache may be incorrectly interpreted as hypoglycemia or, conversely, patients may attribute hypoglycemic conditions to another underlying cause. The underlying uncertainty around hypoglycemia rates arising from these factors was explored in a number of sensitivity analyses. When only hypoglycemic events confirmed by SMBG tests were used and when no difference in hypoglycemia rates between the two arms was assumed, use of degludec remained cost-effective in all three populations. Whilst hypoglycemia rates are a key driver of cost-effectiveness outcomes, the conclusion that degludec is cost-effective in all three analyzed patient groups was robust to applying alternative values.
The meta-analysis used to inform the relative risk of hypoglycemia and the doses of insulin received in the degludec and glargine arms is an important and high-quality data source, capturing alternative event rates across the three different populations, through pooled analysis of seven clinical trials. However, the present analysis assumes that the data collected in the clinical trials is replicated in routine clinical practice. In the treat-to-target trials included in the meta-analysis, insulin doses were titrated until glycemic control was achieved with alternative insulins. Whilst clinical practice aims to optimize glycemic control in all patients with diabetes, this may not always be possible due to a variety of factors, such as non-adherence to medications or missed appointments. How use of degludec and glargine in clinical practice would differ from clinical trials and how this would affect the cost-effectiveness of insulin degludec is unclear. However, in the present analysis, extensive sensitivity analysis suggests that conclusions are resilient to a variety of alternative modeling assumptions, and that degludec is highly likely to be cost-effective in Sweden.
The flat, stable pharmacokinetic profile of degludec is associated with lower day-to-day glycemic variability than glargine, and therefore the frequency of SMBG testing can be reducedCitation21. Therefore, the present analysis has assumed that, in patients with T2D, degludec is associated with a reduced frequency of SMBG tests compared to glargine. The reduced expenditure as a result of less frequent use of SMBG tests partially offsets the increased acquisition cost of degludec, but avoidance of tests is also associated with a quality-of-life benefit. Due to clinical inertia, the reduced frequency of SMBG tests may not be seen in clinical practice in the short-term, and these cost and clinical benefits may take some time to materialize. However, sensitivity analysis in which SMBG use was assumed to be equivalent in the two arms of the study found that degludec remained cost-effective in both type 2 diabetes populations evaluated. Patients with diabetes may also measure their blood sugar for reasons other than dose titration, such as before driving or after exercise. The present analysis assumes that the frequency of these non-scheduled SMBG tests will not differ between the treatment arms and, therefore, would not drive incremental differences between degludec and glargine.
The TLV has confirmed that degludec will be reimbursed in Sweden for both patients with T1D and T2D. However, as part of the appraisal process it was decided that reimbursement in T2D would be limited to patients not achieving treatment targets due to hypoglycemiaCitation17. The present analysis suggests that use of insulin degludec is cost-effective across the diabetes population as a whole. Moreover, the sensitivity analyses in which hypoglycemia benefit associated with degludec was abolished suggested that, whilst ICERs were increased, use of degludec remained cost-effective in all three analyzed populations.
Conclusions
The present study represents one of the first cost-utility evaluations of the ultra-long acting insulin degludec to be published in the peer-reviewed literature. Clinical trials have shown that insulin degludec is associated with a reduction in hypoglycemic events and the pharmacokinetic profile allows less frequent SMBG testing in comparison with glargine. Based on these favorable characteristics, degludec is highly likely to be cost-effective in patients with type 1 diabetes, patients with type 2 diabetes receiving basal-only therapy, and patients with type 2 diabetes receiving basal-bolus therapy from a societal perspective in Sweden.
Transparency
Declaration of funding
This study was supported by funding from Novo Nordisk Scandinavia AB.
Declaration of financial/other relationships
Åsa Ericsson is an employee of Novo Nordisk Scandinavia AB. Richard Pollock, Barnaby Hunt, and William Valentine are employees of Ossian Health Economics and Communications. Ossian received funding from Novo Nordisk to support the present study. JME Peer Reviewer 1 has in the past been a recipient of research/grant funding and has been a consultant to numerous pharmaceutical companies, including NovoNordisk. Peer reviewer 2 has no relevant financial or other relationships to disclose.
References
- International Diabetes Federation. IDF Diabetes Atlas, 5th edn. Brussels, Belgium: International Diabetes Federation, 2011. http://www.idf.org/diabetesatlas. Accessed September 3, 2013
- Norberg M, Danielsson M. Overweight, cardiovascular diseases and diabetes: health in Sweden: The National Public Health Report 2012. Scand J Public Health 2012;40(9 Suppl):135-63
- Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:293-301
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86
- ; Action to Control Cardiovascular Risk in Diabetes Study GroupGerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59
- ; ADVANCE Collaborative GroupPatel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- American Diabetes Association. Standards of medical care in diabetes 2010. Diabetes Care 2010;33(1 Suppl):S11-S61
- Farcasiu E, Ivanyi T, Mozejko-Pastewka B, et al. Efficacy and safety of prandial premixed therapy using insulin lispro mix 50/50 3 times daily compared with progressive titration of insulin lispro mix 75/25 or biphasic insulin aspart 70/30 twice daily in patients with type 2 diabetes mellitus: a randomized, 16-week, open-label study. Clin Ther 2011;33:1682-93
- Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716-30
- Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;2:CD005613
- Child BP, Grothe JM, Greenleaf PJ. Strategies to limit the effect of hypoglycemia on diabetes control: identifying and reducing the risks. Clin Diabetes 2012;30:28-33
- Wang F, Surh J, Kaur M. Insulin degludec as an ultralong-acting basal insulin once a day: a systematic review. Diabetes Metab Syndr Obes 2012;5:191-204
- Heise T, Nosek L, Bøttcher SG, et al. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab 2012;14:944-50
- Heise T, Hermanski L, Nosek L, et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab 2012;14:859-64
- Jonassen IB, Havelund S, Ribel U, et al. Insulin Degludec is a new generation ultra-long acting basal insulin with a unique mechanism of protraction based on multi-hexamer formation. Abstract presented at the American Diabetes Association 70th scientific sessions, Orlando, Florida, USA, June 25-29, 2010.
- Tandvårds- och läkemedelsförmånsverket. Stockholm, 2013; http://www.tlv.se/Upload/Beslut_2013/bes130618-tresiba.pdf. Accessed September 3, 2013
- Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, et al. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med 2013; published online 26 July 2013, doi: 10.1111/dme.12261
- Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab 2013;15:175-84
- Sanofi-Aventis. Structure titration to target fasting glucose. Bidgewater, New Jersey, USA, 2007; http://www.lantus.com/hcp/dosing-titration/titration-guide.aspx Accessed September 3, 2013
- Philis-Tsimikas A, Brod M, Niemeyer M, et al. Insulin Degludec Once-Daily in Type 2 Diabetes: Simple or Step-Wise Titration (BEGIN: Once Simple Use). Adv Ther 2013;30:607-22
- Geelhoed-Duijvestijn PH, Pedersen-Bjergaard U, Jensen MM, et al. Effects of patient-reported non-severe hypoglycaemia on healthcare resource use and work-time loss in seven European Countries. Abstract presented at ISPOR 15th Annual European Congress, Berlin, Germany, November 3-7, 2012.
- Jönsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with Type 2 diabetes in Sweden. Value Health 2006;9:193-8
- Statistiska centralbyrån. Konsumentprisindex (KPI). Stockholm, 2013; http://www.scb.se/Pages/Product____33769.aspx. Accessed September 3, 2013
- Statistiska centralbyrån. Arbetskostnadsindex för arbetare och tjänstemän inom privat sektor (AKI). Stockholm, 2013; http://www.scb.se/Pages/TableAndChart____248027.aspx. Accessed September 3, 2013
- Statistiska centralbyrån. Arbetskostnadsindex för arbetare och tjänstemän inom privat sektor (AKI). Stockholm, 2013; http://www.scb.se/Pages/TableAndChart____248029.aspx. Accessed September 3, 2013
- Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes 2013;11:90
- Simon J, Gray A, Clarke P, et al. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ 2008;336:1177-80
- Evans M, Jensen HH, Bøgelund M, et al. Flexible insulin dosing improves health-related quality of life (HRQoL) in a basal only treatment regimen: a time trade-off survey. Abstract presented at the American Diabetes Association 73rd scientific sessions, San Francisco, California, June 21–25, 2013
- Holstein A, Plaschke A, Vogel MY, et al. Prehospital management of diabetic emergencies–a population-based intervention study. Acta Anaesthesiol Scand 2003;47:610-5
- COMPUS. Optimal therapy recommendations for the prescribing and use of insulin analogues. Optimal Therapy Report CADTH 2009;3:(6)
- Pfohl M, Schädlich PK, Dippel FW, et al. Health economic evaluation of insulin glargine vs NPH insulin in intensified conventional therapy for type 1 diabetes in Germany. J Med Econ 2012;15(2 Suppl):14-27
- Kamble S, Schulman KA, Reed SD. Cost-effectiveness of sensor-augmented pump therapy in adults with type 1 diabetes in the United States. Value Health 2012;15:632-8
- Pollock RF, Curtis BH, Valentine WJ. A long-term analysis evaluating the cost-effectiveness of biphasic insulin lispro mix 75/25 and mix 50/50 versus long-acting basal insulin analogs in the United States. J Med Econ 2012;15:766-75
- Yang L, Christensen T, Sun F, et al. Cost-effectiveness of switching patients with type 2 diabetes from insulin glargine to insulin detemir in Chinese setting: a health economic model based on the PREDICTIVE study. Value Health 2012;15(1 Suppl):S56-9