Abstract
Background:
Guidelines from the Department of Health and Human Services in the US recommend ritonavir-boosted lopinavir (LPV/r) as a preferred protease inhibitor (PI) for HIV-positive antiretroviral-naїve pregnant women. These guidelines also cite ritonavir-boosted darunavir (DRV + RTV) as an alternative PI in this clinical scenario. The purpose of this analysis was to compare economic outcomes for regimens based on these two treatments.
Study design:
An existing discrete event simulation (DES) model was adapted to conduct a cost-minimization analysis comparing the two regimens in HIV-infected women of childbearing age (WOCBA), from the perspective of a healthcare payer in the US.
Methods:
The DES model was used to represent disease states, health events, healthcare encounters, pregnancy, and treatment choices in HIV-infected WOCBA starting treatment with regimens based on either LPV/r or DRV + RTV. It also incorporated parameters for individual patient characteristics, and for antiretroviral (ARV) treatment effectiveness, treatment sequencing, clinical progression, and resource use. Potential events included scheduled physician visits; viral suppression; viral rebound; AIDS-related complications; CHD events; treatment discontinuation and switching; ARV treatment side-effects (SE); and death. The primary outcomes were discounted 5-year and 10-year healthcare costs. Alternative scenarios considered different rates of switching from DRV + RTV to LPV/r upon conception.
Results:
Compared with DRV + RTV, LPV/r was associated with similar clinical outcomes while offering savings at the 5- and 10-year horizons (of $24,904 and $43,502 per patient, respectively), and in extensive sensitivity analyses. The main driver of the savings was the difference in cost between PIs.
Conclusions:
Starting HIV-infected ARV-treatment-naїve WOCBA on an LPV/r-based regimen is cost-saving and provides similar patient outcomes compared to a DRV + RTV-based regimen.
Background
Globally, HIV/AIDS is the leading cause of death in women of childbearing age (WOCBA)Citation1. Pregnancy in such individuals carries not only heightened risks for morbidity and adverse obstetric outcomesCitation2,Citation3, compared with uninfected womenCitation2, but also the threat of vertical (mother-to-child) transmission. Consequently, the possibility of pregnancy in HIV-infected WOCBA necessitates appropriate selection of ARV regimens to balance potentially competing goals, including treatment for maternal HIV disease, avoidance of teratogenic effects, prevention of vertical transmission, and control of costs.
In the US, such decision-making is supported by guidelines from the Department of Health and Human Services (DHHS)Citation4,Citation5. One suggested approach in these guidelines for HIV-infected treatment-naїve pregnant women is dual nucleoside reverse transcriptase inhibitor (NRTI) therapy plus a protease inhibitor (PI), a combination used more commonly in this group than dual NRTI therapy plus a non-nucleoside reverse transcriptase inhibitor (NNRTI)Citation4,Citation5. Specifically, such PI-based therapy obviates the teratogenic potential of the NNRTI efavirenz, which should not be started in the first trimesterCitation5. Moreover, the guidelines cite co-formulated lopinavir/ritonavir (LPV/r; Kaletra) as a preferred PI, as do other standard guidelinesCitation6–8.
Of note, however, the DHHS guidelines have recently re-classified ritonavir-boosted darunavir (DRV + RTV; Presista + Norvir) as an alternative PI for treatment-naїve pregnant women, having previously reported ‘insufficient data to recommend use’ in this settingCitation5. This new recommendation invites an examination of the rationale for originally preferring LPV/r. Trial evidence for treatment-naїve adults has indicated a non-inferior to superior short-term virologic response with DRV + RTV (viral load <50 copies/mL at 48 weeks in 84% vs 78% for LPV/r, p = 0.062; at 96 weeks in 79% vs 71% for LPV/r, p = 0.012)Citation9,Citation10. However, economic modeling suggests that these virologic efficacy outcomes translate into only a marginal, cost-ineffective long-term clinical difference (e.g., life expectancy 27.6 vs 27.4 years for LPV/r vs DRV + RTV, and similar lifetime rates of AIDS-related complications)Citation11,Citation12. Also, the basis for preferring LPV/r in treatment-naїve pregnant women on safety grounds is well defined. In particular, monitoring by the Antiretroviral Pregnancy Registry has ruled out an increase in the overall rate of birth defects with first-trimester exposures to LPV/r, but not to DRV + RTVCitation13, for which experience in pregnancy is limitedCitation5. Also, while boosted atazanavir (ATV + RTV) is cited in the guidelines as a preferred PI in pregnancy, clinical experience of it in this setting is much less than with LPV/r.
This background suggests there might be advantages in pro-actively using LPV/r in HIV-infected treatment-naїve WOCBA with pregnancy potential, particularly those trying to conceive or with questionable adherence to contraception. Starting DRV + RTV instead, with a subsequent switch to LPV/r on conception, would be an alternative. However, this strategy would involve interruption of therapy, which is not recommended in the DHHS guidelines. In contrast, use of LPV/r in HIV-infected WOCBA offers the possibility of treatment continuity before, during, and after pregnancy; adherence to the DHHS guidelines; the support of extensive safety data; lower drug acquisition costs than for DRV + RTV (assuming the backbone treatments are identical for the two regimens); and avoidance of additional costs related to switching treatment during pregnancy. Because the health benefits of the two regimens over longer time horizons are considered to be similar, it is expected that the choice of the initial regimen will be determined largely by unit costs of LPV/r vs DRV + RTV, cost differences related to different side-effect profiles during pregnancy, and costs associated with the switching event (viral resistance testing, more frequent viral load/CD4+ testing, and liver function tests for DRV + RTV). However, it is not known whether initiating a regimen with LPV/r or, alternatively, with DRV + RTV and later switching confers economic advantages; nor is the potential magnitude of savings known.
The objective of this study, therefore, was to compare, from a US healthcare-payer perspective, costs associated with two therapeutic strategies for treatment-naїve, HIV-positive, WOCBA: initiating LPV/r + Truvada (TRV; a co-formulation of tenofovir and emtricitabine) or initiating DRV + RTV + TRV and then switching to LPV/r + TRV at time of pregnancy. Doses modeled were 800 mg DRV + 100 RTV daily and LPV/r 400 mg/100 mg BID. The analysis used 5- and 10-year horizons to permit a reasonable assessment of initial therapy selection and estimation of residual effects, respectively. It was based on the data from the ARTEMIS trial showing comparable efficacy of LPV/r + TRV and DRV + RTV + TRV (trial doses were same as those modeled) at 48 weeksCitation9. These similar health benefits of the two regimens argue for cost-minimization rather than cost-effectiveness or cost-utility analysis. We considered variations in assumptions related to the percentage of HIV-infected WOCBA who switch on conception, in adherence rates related to becoming pregnant, and in pregnancy rates.
Methods
Model overview
The WOCBA model simulates the journey of individual HIV-infected WOCBA through different disease states, health events, and healthcare encounters from initiation of ARV therapy, capturing the associated costs and health consequences. Discrete event simulation (DES) is a flexible modeling method characterized by the ability to represent complex behavior within, and interactions between individuals, populations, and their environmentsCitation14. Like the DES model from which it was derivedCitation12, the WOCBA model includes parameters for ARV treatment effectiveness, treatment sequencing, and clinical progression, as well as resource use. It also includes parameters for patients’ individual characteristics, for example, viral load and CD4 cell counts, treatment history, adherence behavior, resistance mutations, and risk of coronary heart disease (CHD). CHD risk is estimated using the Framingham equationCitation15, which incorporates total cholesterol, high-density lipoprotein and triglyceride concentrations, smoking status, systolic blood pressure, and presence of diabetes. Age, CD4 count, and viral load at baseline were estimated from ACTG trials 5102, 5142, and Abbott trial M05-730Citation1Citation2,Citation16–18. Model parameters determine the probabilities and timing of events for each patient including scheduled physician visits, viral suppressionCitation12, viral rebound, AIDS-related complications, CHD events, treatment discontinuation and switching, and ARV treatment side-effects (SE)Citation12. Death is an exit state in the model. When an event occurs, the patient is processed in the corresponding event module, which updates individual characteristics, health-related quality-of-life, and resource use, and re-calculates risks of future events based on updated patient characteristics. The DES model was implemented using ARENA (from Rockwell Software)Citation19.
To permit comparisons of different treatment regimens, the model generates cloned populations of women with identical individual characteristics at baseline to produce cohorts that differ only in the assigned initial regimen. The subsequent treatment pathway for these patients permits three switches of regimen in response to evolving clinical events (other than pregnancy). Additional model details including treatment pathways, disease progression, and risks of future AIDS-related events and drug-related SEs have been described previouslyCitation12. Model flow and treatment pathways are depicted in figures in the supplementary materials (see Supplementary Figures 1 and 2).
The model includes several WOCBA-specific features. Patients can become pregnant according to birth rates estimated from Arizona State Healthcare Cost and Utilization Project data using ICD-9 codes 042.xx or V08 in 2008 and 2009Citation11 (see ). The model simulates the possibility of only one pregnancy for each HIV-infected WOCBA, as data on response and patient behavior after multiple pregnancies are lacking. It is assumed that concern for the unborn child improves adherence to treatment, in keeping with evidence from cohort studies comparing adherence rates among pregnant and non-pregnant womenCitation20–22. Based on data published in Bardeguez et al.Citation20, a 15% increase in adherence among pregnant women was derived (75% of women with perfect adherence during pregnancy vs 65% post-partum) and, subsequently, was assumed to remain at the raised level for the current regimen, even after the child is born or the woman is past childbearing age. After any treatment switch, a woman is assigned an adherence based on the original level of adherence minus 10%. Another assumption is that, unlike other HIV-infected patients receiving ARV treatment, pregnant women do not switch regimens due to non-serious SEs and other non-virologic reasons, remaining instead on the current treatment as suggested in the DHHS guidelinesCitation5. The efficacy levels used in the model for the two regimens being compared were those seen in the ARTEMIS trial at 48 weeksCitation9.
Table 1. Demographic and baseline characteristics of WOCBA and selected key cost inputs.
In HIV-infected WOCBA on their first line of treatment who experience treatment failure (i.e., rebound in viral load) while pregnant, the model assumes that adherence rises to 100% as a result of clinicians’ efforts to improve adherence during pregnancy. In WOCBA who initiate treatment with DRV + RTV, a range of probabilities of switching to LPV/r is included in the model when the first pregnancy occurs (0%, 30%, 100%). No switch is implemented if women conceive while taking later lines of ARV therapy, however. The model assumes that vertical transmission (transmission from mother to child) of HIV is rare in the US and occurs primarily where the mother’s HIV-positive status has not been recognizedCitation23 and, therefore, ARV therapy not started. Also, there is no evidence of a difference in vertical transmission between LPV/r and DRV + RTV arms that would produce differences in cost or health outcomes. Similarly, birth defects and the method of delivery (such as Caesarean section) are not considered, in the absence of evidence on differences in these factors between the treatments.
Costs
Because analyses were conducted from a US payer perspective, only direct medical and pharmacy costs were considered, including those associated with ARV drugs, non-antiretroviral drugs, prophylactic treatment for opportunistic infection, routine medical care, and treatment for AIDS-related events and adverse effects. Indirect costs, such as those of caregiver time and productivity loss, were not considered. The daily drug cost of LPV/r + TRV was $56.59, while that of DRV + RTV + TRV was $73.89Citation24, referenced to wholesale acquisition cost (WAC). Other selected key cost inputs for the model are given in and have been detailed elsewhereCitation12. Costs were discounted at 3% per annum in accordance with US recommended standards for economic analysis. Discounting was performed from day one, including the first year, either by using discrete discounting for one-time costs or by using continuous discounting for daily costs. Costs are compared at 5 and 10 years to enable the reader to assess medium and longer-term economic consequencesCitation25.
Patient population
The model includes HIV-infected WOCBA aged at least 18 years starting ARV therapy with a PI-based regimen (DRV + RTV or LPV/r). This excludes those HIV-positive women who are ARV therapy-naїve and initiate ARV therapy because they become pregnant; and HIV-positive women taking another regimen such as efavirenz-based therapy who switch to a PI-regimen after conception owing to teratogenic risks.
Outcome measures
The primary outcomes were the discounted 5-year and 10-year healthcare costs of managing HIV-infected WOCBA. Secondary outcomes included quality-adjusted life years (QALYs), death rates, number of AIDS-related events, and rates of CHD. Secondary outcomes permitted confirmation of the assumption of similarity of clinical outcomes between LPV/r and DRV + RTV. The incremental cost-effectiveness ratios (ICERs) were calculated by dividing the differences in the sum of the discounted costs for the two treatments by the differences in the sum of the discounted QALYs for the regimens. QALY weights were defined according to the patient’s CD4+ count while utility decrements were chosen for the specific health states associated with opportunistic infection and cardiovascular diseaseCitation12.
Analyses
The base-case analysis consisted of comparing outcomes of women initiating LPV/r + TRV with those initiating DRV + RTV + TRV at 5-year and 10-year horizons and it assumed that none of them switch from DRV + TRV to LPV/r on conception. In addition to the base-case analysis, various alternative scenarios were considered to assess the importance of different rates of switching from DRV + RTV to LPV/r on conceptionCitation26; pregnancy rates; changes in adherence during pregnancy; baseline patient characteristics; ARV treatment sequences; and ARV discontinuations. Probabilistic sensitivity analyses (PSAs) were performed on the base case and on two other scenarios that assumed DRV switch rates of 30% and 100% by varying all unit costs ±20% according to a log-normal distribution, except ARV drug costs which were held fixed. In addition, the proportion of subjects in the moderate-adherence category was varied ±5% according to a beta distribution from 10% in the base case. Supplementary Table 2 gives the parameters and their distributions which were included in the PSA. One hundred replications of the model were performed to complete the PSA within each scenario. A further analysis varied the unit cost of DRV while fixing all other model parameters to determine the DRV break-even price (the DRV price at which all cumulated 5-year and 10-year HIV treatment and healthcare costs are equal for the LPV/r and DRV + RTV arms).
Results
Base case
reports health-related outcomes and discounted cost breakdown for the base-case scenario at 5 and 10 years. Total discounted costs over 5 years amounted to $107,790 per person when initiating ARV with LPV/r + TRV, compared to $132,694 when initiating ARV with DRV + RTV + TRV without a treatment switch on conception. For the 10-year horizon, the respective total costs were $192,862 compared to $238,854. ARV drugs were the main cost component for both treatment arms, representing 77–83% of total costs. Total discounted QALYs gained by patients initiating ARV with LPV/r + TRV were 3.32 years vs 3.33 years with DRV +RTV + TRV for the 5-year horizon, and 5.95 years vs 5.98 years, respectively, for the 10-year horizon (a difference of 11 days). Life-years and rates for death, AIDS-related events, SEs, and CHD at 5 and 10 years were similar for both treatment arms. The only notable differences were observed for nausea and diarrhea which were more common with LPV/r (, Supplementary Table 1).
Table 2. Health-related outcomes and discounted costs breakdown associated with the base case.
Alternative scenarios
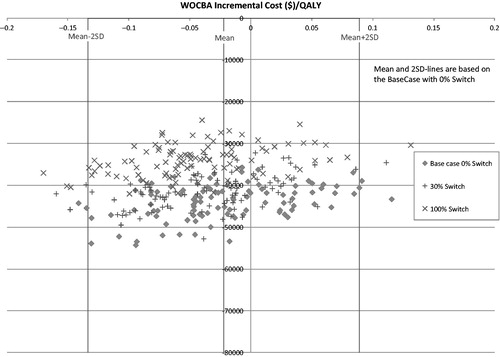
Total healthcare costs across all the alternative scenarios (, ) indicated that LPV/r + TRV represents a cost-saving option compared to DRV + RTV + TRV, irrespective of pregnancy or DRV + RTV switch rates, or baseline CD4 or viral load assumptions. However, a high proportion of poorly adherent patients was associated with reduced savings ($12,788 at 5 years and $23,102 at 10 years). The cost savings are indicated by most of the data points being located in quadrant 3. This location of these points also indicates that cost savings are accompanied by an apparent loss of QALYs. However, the reader should note that the scale for the potential loss of QALYs in is very small, being in every case less than 0.06 QALYS, which equates to less than 22 days of quality-adjusted survival lost over the 10-year horizon.
Figure 1. Scatterplot showing incremental costs and QALYs at 10 years for alternative scenarios. Currencies are in US dollars.
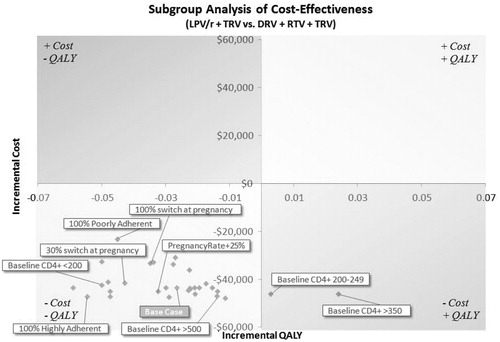
Table 3. Total healthcare costs for base-case and alternative scenarios.
Probabilistic sensitivity analyses
shows three clouds of points representing incremental costs and QALYs for the different scenarios for DRV switch rates at pregnancy included in the PSA. Cost savings ranged between $24,360 with a 100% switch, and $54,260 with no DRV switching, over the 10-year horizon. The plots in also show that probabilistic sensitivity analysis of the base case resulted in variations of estimates ranging from an approximate loss of 0.15 QALYs to a gain of ∼0.12 QALYs. As represented in , the variation is less at 2 SD. However, variations in model parameters did not result in large changes in cost savings for the LPV/r regimen. The figure also indicates that slight decreases in cost savings may result if model assumptions for regimen switching are changed. However, under none of the scenarios was there loss of the cost savings related to the LPV/r regimen.
DRV breakeven price
In supplementary analyses, the analysis of the DRV price reduction that would be required for there to be no difference in total healthcare costs for the two treatment arms over 10 years was examined. This reduction was 45% (Supplementary Figure 3).
Discussion
Our economic analysis involved a cost minimization comparison of LPV/r and DRV + RTV, in combination with TRV, among HIV-infected WOCBA. The overall results showed that there was little difference in key clinical outcomes between LPV/r and DRV + RTV. There were, however, significant differences in patient management costs, with LPV/r being associated with cost savings compared to DRV + RTV, at both the 5-year and 10-year horizons ($24,904 and $43,502, respectively). The main driver of these savings was the cost of the ARV drugs. In addition, LPV/r remained cost-saving across multiple sensitivity analyses (although the savings were considerably reduced if patients were assumed to be poorly adherent to therapy).
While other published studies have undertaken health economic comparisons of LPV/r and DRV + RTV, ours is the first to do so for HIV-infected WOCBA. In general, previous studies have addressed different populations and employed different methodologies. For example, an analysis of treatment-naїve patients concluded that DRV + RTV was cost-effectiveCitation27. However, this study’s relatively short time horizon (1 year) and its primary outcome (incremental cost per additional patient with a virologic response at 48 weeks) do not conform with current guidelines which recommend that economic evaluations should use a clinically relevant time-horizon and clinical end-points such as life-years, QALYs, or deathsCitation28,Citation29. Other studies suggesting cost-effectiveness of DRV + RTV have focused on special populations, including patients with extensive treatment experienceCitation30; treatment-experienced patients previously exposed to PI therapyCitation31; and treatment-experienced, LPV/r-naїve patients who were PI-resistantCitation31. These groups are of limited direct relevance to our study. Another important point is that, typically, these other studies have employed Markov modeling. Such an approach is challenged by the complexity of HIV and its management. In particular, Markov models have limited capacity to capture all relevant clinical events over an extended time horizon, which requires incorporating the contributing inter-relationships (e.g., adherence, resistance mutations, the association between immunological and virologic status, treatment switching and sequencing, and potential side-effects of ARV therapy) and to allow for how key elements such as disease history and prior treatment/response influence the subsequent clinical course. In contrast, the current DES model can take into account the possibility of multiple events with competing risks and to adjust hazards when rate-changing events occur.
Given that such methodological challenges are better handled using DESCitation31 than with Markov modelsCitation32, it is notable that, in a previous study using a DES model with a lifetime horizon, we showed that initiating with LPV/r rather than DRV + RTV was very likely to be cost-saving, while producing very similar clinical outcomesCitation12. We see the use of DES as a major strength of our cost comparison in HIV-infected WOCBA, particularly given the added challenges posed when attempting to model ARV treatment choices and consequences in the context of pregnancy.
A few limitations in our analysis should be noted, and these relate primarily to missing data. For example, data for estimating the likelihood of various resistance mutations are not publically available and so could not be reproduced in the model. Instead, we estimated the rates of selecting resistance mutations by drug class as observed in clinical trials. There is also a lack of information on regimen-based adherence and treatment efficacy, the impact of ARV regimen history on subsequent efficacy, the impact of adherence on viral suppression, and the impact of drug class-based resistance mutations on viral suppression. It is important to note, however, that many factors pertaining to patient baseline characteristics, resource and management costs, pregnancy rates, and adherence were tested in the sensitivity analyses, results of which suggested the robustness of our findings.
In conclusion, we believe that our model, through offering a comprehensive simulation of 5-year and 10-year horizon of ARV management, indicates that starting HIV-infected WOCBA on LPV/r can be cost-saving and provide similar patient outcomes compared to DRV + RTV. These results may be influential for US health systems providing care in this patient demographic.
Transparency
Declaration of funding
This research was funded by Abbvie.
Declaration of financial/other relationships
RB, BD, OV and KG are employees of Abbvie and own shares in the company. KS received funding from Abbvie as a consultant. KD and JM are employees of Evidera (previously UBC) which received consultancy fees from Abbvie for the completion of this research.
Supplementary Material
Download PDF (368.9 KB)Acknowledgment
The authors would like to acknowledge Ike Iheanacho for medical writing services.
References
- Women and health: today’s evidence, tomorrow’s agenda. Switerzland: World Health Organization, 2009
- Kourtis AP, Bansil P, McPheeters M, et al. Hospitalizations of pregnant HIV-infected women in the USA prior to and during the era of HAART, 1994–2003. Aids 2006;20:1823-31
- Bansil P, Jamieson DJ, Posner SF, et al. Hospitalizations of pregnant HIV-infected women in the United States in the era of highly active antiretroviral therapy (HAART). J Womens Health 2007;16:159-62
- Senise JF, Castelo A, Martinez M. Current treatment strategies, complications and considerations for the use of HIV antiretroviral therapy during pregnancy. AIDS Rev 2011;13:198-213
- Panel on treatment of HIV-infected pregnant women and prevention of perinatal transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed September 2, 2012
- BHIVA Writing Group. Guidelines for the management of HIV infection in pregnant women 2012. http://www.bhiva.org/documents/Guidelines/Treatment/2012/120430PregnancyGuidelines.pdf. Accessed September 2, 2012
- European Aids Clinical Society. EACS Guidelines Version 6.1 - November 2012. http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/EACSGuidelines-v6.1-English-Nov2012.pdf. Accessed December 14, 2012
- World Health Organization (WHO). Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. Accessed September 2, 2012
- Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS 2008;22:1389-97
- Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. Aids 2009;23:1679-88
- Möller J, Desai K, Simpson K, et al. Cost of Switching Darunavir + Ritonavir (DRV + RTV) to Lopinavir/Ritonavir (LPV/r) in HIV-1-infected Treatment-naïve Women of Child-bearing Age (WOCBA). Poster presented at HIV-11, Glasgow, Scotland, November 11-12, 2012
- Simpson KN, Pei PP, Moller J, et al. Lopinavir/ritonavir versus darunavir plus ritonavir for HIV infection: a cost-effectiveness analysis for the United States. PharmacoEconomics 2013;31:427-44
- Antiretroviral Pregnancy Registry Steering Committee. The Antiretroviral Pregnancy Registry - Interim report. 2012. http://www.apregistry.com/forms/interim_report.pdf. Accessed September 2, 2012
- Karnon J, Stahl J, Brennan A, et al. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–4. Value Health: J Int Soc Pharmacoecon Outcomes Res 2012;15:821-7
- Anderson KM, Odell PM, Wilson PW, et al. Cardiovascular disease risk profiles. Am Heart J 1991;121:293-8
- Gathe J, da Silva BA, Cohen DE, et al. A once-daily lopinavir/ritonavir-based regimen is noninferior to twice-daily dosing and results in similar safety and tolerability in antiretroviral-naive subjects through 48 weeks. JAIDS 2009;50:474-81
- Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008;358:2095-106
- Henry K, Katzenstein D, Cherng DW, et al. A pilot study evaluating time to CD4 T-cell count <350 cells/mm(3) after treatment interruption following antiretroviral therapy +/− interleukin 2: results of ACTG A5102. JAIDS 2006;42:140-8
- Kelton WD, Sadowski RP, Sadowski DA. Simulation with Arena. New York: McGraw-Hill, 1998
- Bardeguez AD, Lindsey JC, Shannon M, et al. Adherence to antiretrovirals among US women during and after pregnancy. JAIDS 2008;48:408-17
- Mellins CA, Chu C, Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care 2008;20:958-68
- Vaz MJ, Barros SM, Palacios R, et al. HIV-infected pregnant women have greater adherence with antiretroviral drugs than non-pregnant women. Int J STD & AIDS 2007;18:28-32
- American Academy of Pediatrics Committee on Pediatric A. HIV testing and prophylaxis to prevent mother-to-child transmission in the United States. Pediatrics 2008;122:1127-34
- AnalySource®. AnalySource® Online. http://www.firstdatabank.com/Products/analysource.aspx. Accessed May 20, 2011
- Lipscomb MW, Torrance GW. Time preference. In: Gold MR, ed. Cost-effectiveness in health and medicine. New York; Oxford: Oxford University Press, 1996
- Huntington SE, Bansi LK, Thorne C, et al. Treatment switches during pregnancy among HIV-positive women on antiretroviral therapy at conception. Aids 2011;25:1647-55
- Brogan AJ, Mrus J, Hill A, et al. Comparative cost-efficacy analysis of darunavir/ritonavir and other ritonavir-boosted protease inhibitors for first-line treatment of HIV-1 infection in the United States. HIV Clin Trials 2010;11:133-44
- National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. 2008. Article ref: 1618
- Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health: J Int Soc Pharmacoecon Outcomes Res 2003;6:9-17
- Mauskopf J, Brogan A, Martin S, et al. Cost effectiveness of darunavir/ritonavir in highly treatment-experienced, HIV-1-infected adults in the USA. PharmacoEconomics 2010;28(1 Suppl):83-105
- Moeremans K, Annemans L, Lothgren M, et al. Cost effectiveness of darunavir/ritonavir 600/100 mg bid in protease inhibitor-experienced, HIV-1-infected adults in Belgium, Italy, Sweden and the UK. PharmacoEconomics 2010;28(1 Suppl):107-28
- Jaime Caro J, Ozer Stillman I, Danel A, et al. Cost effectiveness of rimonabant use in patients at increased cardiometabolic risk: estimates from a Markov model. J Med Econ 2007;10:239-54